Abstract
Essential oils are liquids containing non-toxic compounds that are unfavorable to the growth of microorganisms. They are sold globally at affordable or very high prices, depending on the availability and type of plant, the scale of production, the extraction method, costs associated with logistics and electricity consumption, among other variables. Each year, the quantity of research dedicated to the antimicrobial potential of essential oils in poultry farming is expanding. Researchers consensually relay that this increase is due to the growing resistance of microorganisms to traditional antimicrobials and concerns about the toxicity of these products. This review proposes an analysis of the antimicrobial feasibility of using essential oils to address microbial challenges in poultry farms, aiming to ensure the production and supply of microbiologically safe hatching eggs. Based on the findings in the literature, in addition to following other necessary precautions in the daily routines of poultry farming practices, developing an antimicrobial control program with essential oils that integrates poultry facilities, poultry and hatching eggs, adapted to the particularities of each context seems to be extremely effective.
1. Introduction
Microbial communities are not limited to just colonizing poultry facilities, they also colonize poultry until the moment of slaughter and beyond [1]. Microbial colonization comes from the environment, maternal transmission, transmission between poultry during the consumption of feed and water, as well as human transmission [2,3,4,5,6]. Microbiological damage that compromises the health and well-being of poultry can occur much sooner than expected, resulting in widespread complications such as production cessation and significant repair expenses, requiring immediate solutions.
Poultry farms that follow quality sanitary principles make it possible to raise poultry both in confined and unconfined environments, with due restrictions on contamination of their products, including hatching eggs. Poultry feeding and watering systems, egg collection systems, ventilation and refrigeration systems, materials and equipment storage rooms and egg storage rooms, as well as parking areas, transport trucks and circulation spaces of people and vehicles, must comply with high sanitary standards. Typically, professional staff at poultry farms perform a variety of tasks to mitigate the risk of uncontrolled contamination in poultry facilities and prevent the development of avian infections [7]. Adopting appropriate work attire, following procedures using microbiologically safe materials, and strictly controlling access to facilities, are some measures adopted. However, concern about the abusive use of synthetic antimicrobials in the poultry industry, aiming to maintain high sanitary standards, has led researchers to recommend updating prevention strategies [7,8,9,10,11].
The global dissemination of essential oils as sanitizers in poultry farming [7,8,12,13,14,15] promotes the innovative “Healthy Sanitization of Poultry Farms” concept. This paradigm aims to implement effective avian health control protocols, adapted to ideal spatial conditions, with the responsibility of reducing microbial levels in the air and on contaminated surfaces and preserving the integrity of animal, human and environmental health, considering possible failures during handling and repetitive daily work. Furthermore, it is a concept that aims to encompass standards established by regulatory authorities or government bodies to ensure the supply of hatching eggs with acceptable microbiological parameters. Furthermore, the synergy between indirect antimicrobial treatment (in the poultry farming environment, as mentioned above) and direct antimicrobial treatment (in the poultry itself) [7,8,9,16] can be a way to maximize the antimicrobial benefits in poultry products. Notably, the administration of antimicrobials formulated with essential oils through diets or water to poultry provided impressive results in the production of eggs without conventional antibiotic residues and with lower microbial loads in the shell [17,18].
This review proposes an analysis of the antimicrobial feasibility of using essential oils to address microbial challenges in poultry farms, aiming to ensure the production and supply of microbiologically safe hatching eggs.
2. Paper Search Strategy
For this review, papers (research and review), book chapters and conference papers available on Google Scholar written in Portuguese or English up to 2 January 2024 were examined. The search terms were organized into six distinct groups, covering investigations on topics such as “microbial contamination in poultry farms”, “poultry antimicrobial management”, “essential oils”, “antimicrobial function of essential oils”, “application of essential oils in poultry farming” and “essential oils and poultry products”. The papers were thoroughly researched until each topic was comprehensively understood. Papers meeting the criteria defined for each group were chosen for inclusion, while those that did not meet the specified criteria were excluded.
3. Poultry Farms Are an Ideal Environment for Undesirable Microorganisms
Floors, fans, vents, feed loaders, feeders, drinkers, and wall crevices of poultry farms can be persistently contaminated with Salmonella spp., Campylobacter spp., Escherichia coli and/or Staphylococcus aureus [4]. Likewise, feed contaminated with Salmonella spp. and Escherichia coli can be fed to poultry, contributing to systemic contamination of the farm [5]. Furthermore, fungal contamination by Aspergillus flavus, Aspergillus niger, Aspergillus fumigatus, Mucor spp., Penicillium spp. and/or Fusarium spp. Can be observed in water lines, cooling pad water, fans, and floors of broiler farms [19]. These factors compromise the quality of water and air in poultry facilities. Bacterial (e.g., by Salmonella spp. and Escherichi coli) and fungal (e.g., by Aspergillus spp.) contamination in poultry farms harms the poultry health and the quality and viability of poultry products.
In a study carried out by Kemmett et al. [20], the bacterium Escherichia coli was identified in several pathological changes present in broiler chickens during the first week of life, including pericarditis, perihepatitis, abnormal liver color, ascites, cellulitis, and abnormal yolk sac. These changes are particularly concerning, as it suggests that approximately 70% of poultry mortality in the first week can be attributed to these complications [20]. Muna et al. [21] reported that young broilers contaminated with Salmonella spp., mainly Salmonella enterica subsp. enterica serovar Enteritidis and Salmonella enterica subsp. enterica serovar Typhimurium developed septicemia due to systemic changes and injuries in vital organs, such as the liver, intestine, spleen, heart, and brain. These changes include hepatomegaly, splenomegaly, inflammation of the intestinal mucosa, necrotic foci in the spleen, liver, and brain, as well as degeneration of the myocardial muscle fiber [21]. An outbreak of fungal infections of the respiratory tract of poultry naturally caused by Aspergillus spp. was reported in a poultry house [22]. These infections have resulted in significant complications, such as alveolar emphysema, atelectasis, thrombosis, and pneumonic lung with granulomatous tissue and granulomatous encephalitis [22]. These complications, in turn, contributed to the mortality of 200 approximately two-week-old broiler chickens [22].
In production systems, eggs can be horizontally contaminated by Salmonella enterica subsp. enterica serovar Typhimurium, present in poultry feces [3]. Thus, the concern arises because the eggshell is an access portal for microorganisms and is close to internal structures. In addition to Salmonella spp., pathogens from other genera such as Clostridium, Enterococcus, Staphylococcus, Alcaligenes, Enterobacter, Escherichia, Klebsiella, Pseudomonas, Shigella, Aspergillus, Candida, Fusarium and Penicillium can also lodge in the eggshell (reviewed by Oliveira et al. [23]), exposing the embryo to a more intense microbial load during critical stages of development, where the embryo’s period of vulnerability is more evident. Due to this concern, some studies have explored the severity of microbial infections during embryonic development in poultry [24,25,26]. Embryonic mortality appears to be the most common consequence [27], becoming a detriment to the productive balance of the poultry chain.
It is important to clarify that the proliferation and dissemination of high rates of microbial contamination and mortality on poultry farms is not something expected and common on farms that adopt a rigorous and correct routine in health management at all stages of the production chain.
4. Essential Oils and Their Bacterial and Fungal Functions
In the industrial processing of natural products, large volumes of essential oils can be extracted from aromatic plants. Conventional and green processes can extract these oils from plants, but the conventional process by steam distillation stands out among them all [28]. These essential oils, volatile liquids, have aromas similar to those of the original plant and are loaded with functional components. Studies on the chemical analysis of essential oils have revealed that oil can contain more than 20 functional compounds [29]. The heterogeneity of the chemical composition of essential oils requires chemical analysis to determine the essential oils suitable for use as an antimicrobial agent. Depending on the essential oil, the main compound may be a monoterpene, phenol, aldehyde, ketone, alcohol, hydrocarbons, or another compound (Table 1). Phenols, alcohols, and aldehydes were found to be the most effective against Gram-negative and Gram-positive bacteria, while hydrocarbons were the least effective [30]. This finding agrees with El-Baroty et al. [31], who stated that antimicrobial activity gradually decreases from phenols (with greater activity) to hydrocarbons (with lower activity).

Table 1.
Main compound of different essential oils.
Therefore, the chemical composition of essential oils may explain their antimicrobial functions, including effectiveness against bacteria and fungi isolated or not from poultry (Table 2). This occurs because the interaction of these compounds with the cell wall and membrane of microorganisms promotes an increase in the permeability of these structures, resulting in leakage or alteration of microbial homeostasis [39,40]. Although some essential oils have been tested effectively to combat microorganisms on poultry farms, recent studies have warned that the effectiveness of these oils depends on the dose [13,15]. Zingiber Officinalis essential oil reduced the bacterial growth of Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 11622) strains in a dose-dependent manner (400–5 µg/mL), as evidenced by Galgano et al. [41]. In agreement, Boukhatem et al. [42] reported that Eucalyptus globulus essential oil also inhibited, depending on the dose (20, 40 and 60 µL/disc), the growth of foodborne and/or food spoilage pathogens such as Enterobacter sakazakii, Klebsiella ornithinolytica, Escherichia coli, Bacillus cereus, Staphylococcus aureus, Candida albicans, Candida parapsilosis, Saccharomyces cerevisiae, Trichosporon spp. and Aspergillus niger. Therefore, it is essential to carefully evaluate essential oils when programming an antimicrobial formulation that meets the specific demands of each poultry farm sector. For example, the appropriate sanitizing formula for a poultry house may not be the same as that recommended for application to poultry or for sanitizing hatching eggs. Furthermore, the formulation must simultaneously act to reduce Gram-negative and Gram-positive bacteria, as well as fungi, to levels that are considered safe. Carrying out in vitro antimicrobial tests is an initial direction for developing antimicrobial formulations in poultry farming. This is because the results obtained in vitro generally reflect directly on in vivo tests [12].

Table 2.
Summary of essential oils’ antibacterial and antifungal capacity against bacteria and fungi isolated or not from poultry.
5. Managing Poultry Farms with Essential Oils to Obtain Microbiologically Safe Hatching Eggs
5.1. Poultry House
Improving the relationship between poultry farms and the application of essential oils can mark substantial poultry production progress, as the antimicrobial efficacy of these oils can effectively align with management practices in poultry production sheds. An efficient sanitization plan for poultry sheds using essential oils must cover all structural and non-structural elements necessary to guarantee high-quality poultry production. Essential oils as sanitizers have proven efficiency in poultry sheds. An investigation into daily aerosol air sanitization in a poultry house during broiler farming revealed that sanitization for 60 min with a formulation containing different compounds, including 0.3% thyme, eucalyptus, and fir essential oils, in a dose of 50 mL/m3 of the room reduced the bacterial load in the air by 99%, without presenting toxicity to chickens [14]. In addition to broilers exposed to sanitization having a higher average body weight, than those not exposed, their blood tests indicated a significant increase in the amount of haemoglobin, lysozyme levels and bactericidal activity [14]. The nebulization of 0.5 mL of an aqueous solution of Mentha piperita or Thymus vulgaris essential oil at a concentration of 1:500 to 1:250 in poultry houses was proven effective in reducing the bacterial and fungal load in the air, drinkers, walls and/or litter [7,8]. Similarly, the combined application every three hours of Pinus silvestris and Eucalyptus polybractea essential oils at a concentration of 1:500 proved to be an efficient protocol for improving bacterial and fungal quality in the poultry air environment [16].
5.2. Poultry
Hatching eggs, subjected to an effective sanitization process, do not absolutely guarantee that the poultry resulting from hatching will be free of pathogens. Furthermore, even with inefficiently clean and sanitized poultry environments, this poultry can still be colonized by microorganisms present in the environment in which they live. As a result of this scenario, poultry constitutes a potential source of contamination for derived products. The main concern is centered on assessing the microbiological quality of poultry, aiming to ensure that it does not pose harmful risks to the final product or consumers. Given this need, it is recommended to subject poultry to antimicrobial therapies to guarantee both their microbiological quality and that of their final products within acceptable parameters. Studies have reported interesting results from antimicrobial treatments with essential oils via feed or water in poultry (Figure 1). Denli et al. [9] demonstrated that laying hen diets plus 150 mg/kg of Origanum vulgare essential oil reduced the contamination of total coliforms by 0.61 log10 CFU/mL and Escherichia coli by 1.09 log10 CFU/mL in eggshells. An antimicrobial treatment for layers via water-drinkers using cinnamaldehyde essential oil (diluted in a proportion of 1:8000 in drinking water) reduced the bacterial count in the cecum and eggshells [18]. Laying hens (89%) naturally infected with Mycoplasma synoviae (pathogen normally transmitted from breeding poultry to eggs) recovered after consuming diets supplemented with 100 mg/kg of Melaleuca alternifolia essential oil [17]. These authors reinforced the importance of these poultry eggs being free of conventional antibiotic residues [17]. Dietary supplementation with a blend of essential oils (containing 25% thymol and 25% carvacrol as active components, 37% silicon dioxide as caking inhibitor, and 13% glycerides as stabilizing agents; 120 mg/kg of feed) significantly reduced mortality associated with necrotic enteritis, inhibited the transport of Enterobacteriaceae in the liver and improved the intestinal integrity of broiler chickens [58]. On the other hand, diets containing 150 ppm of Lippia origanoides essential oil improved the feed conversion rate of layers [59].
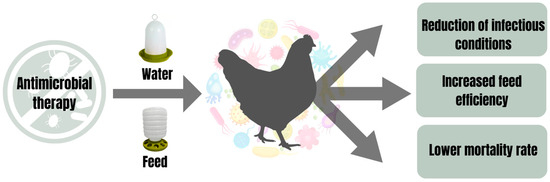
Figure 1.
Benefits of antimicrobial therapy via water or feed in poultry.
The effects of supplying feed and water with essential oils on digestibility, feed consumption, feed conversion and, mainly, on maintaining the integrity of the intestinal health of poultry were also investigated. Barbarestani et al. [60] reported that providing feed supplemented with 600 mg of Lavandula angustifolia essential oil per kg of feed improved the growth performance of broilers. These improvements were mainly attributed to promoting intestinal microbiota balance, improving intestinal structure, and increasing antioxidant capacity. Abdel-Wareth and Lohakare [61] observed that the inclusion of Mentha piperita essential oil in the diet of laying hens at different concentrations (0, 74, 148, 222 and 296 mg/kg of feed) resulted in notable improvements in the feed intake and feed conversion ratio. Furthermore, there was a linear increase in the digestibility of crude protein, ether extract and phosphorus. These findings were directly correlated with significant improvements in the poultry laying performance. Providing drinking water for broilers enriched with 0.4 mL/L of Lavandula angustifolia essential oil [62] or 400 mg/L of Satureja khuzistanica essential oil [63] resulted in significant improvements in performance indices, including feed conversion. This improvement was also observed when laying hens received drinking water containing 0.2 to 0.3 mL/L of a mixture of essential oils from Origanum vulgare, Mentha piperita and Pimpinella anisum [64]. Diet supplemented with 15 mg/kg of e Origanum vulgare essential oil plus 2.4 g/kg of attapulgite demonstrated significant benefits on the height of ileal villi and the composition of the intestinal microbiota of broilers [65].
5.3. Hatching Eggs
Sanitizing eggs for hatching is also a poultry standard to ensure eggs have fewer pathogens. A bibliographical survey by Oliveira et al. [6] reported that the sanitization of hatching eggs proved viable to reduce the microbial load of the eggshell in 85–86% of protocols carried out at the experimental level. Sanitization offers an immediate reduction in the microbial load of the shell and internal contents of the eggs, lower chances of recontaminated eggs, a better hatchability rate, microbiologically safer embryos and chicks, and healthier and more viable poultry [12,13,66,67,68,69,70]. However, in some cases, sanitization did not reduce microbial contamination of the eggshell and/or caused complications such as malformations and failure to hatch [71,72,73,74]. Most of these complications require corrections in the sanitization protocol, as they may be due to poor application and the level of toxicity of the sanitizers.
Plants naturally provide many of the active ingredients for preparing sanitizers. Some of the sanitizers currently available and tested for a commercial application are based on essential oils produced by plants. In poultry farming, microbiological tests are progressively carried out to evaluate the viability of essential oils in sanitizing hatching eggs [12,15,75]. This intensifies practices that use ecological principles for antimicrobial protection in the poultry sector and de-intensifies environmental externalities caused by environmentally harmful practices. Many trees, including Citrus aurantifolia, Ocimum basilicum, and Allium sativum, harbor essential oils of interest to global research centers thanks to dedicated researchers who consistently share experimental results that advance the characterization of these essential oils [11,76,77,78]. Because they are (1) active against bacteria and fungi, (2) safe for humans and animals (dose-dependent), (3) sourced from readily available plants, and they have (4) positive cost–benefit ratio and (5) application versatility, essential oils need to be continually validated to redefine poultry farming, seeking to move it away from its conventional approach and cultivate an image deeply rooted in sustainability, where natural and ecologically responsible practices are the main guide.
Antimicrobial therapy on poultry farms with essential oils requires a comprehensive approach. In addition to focusing on microbial control of the air, physical structure, materials, and poultry, it is necessary to integrate the sanitization of hatching eggs. This is a therapeutic complement to the cleaning activities that must be included in the management plan of poultry farms, aiming, through methods such as spraying, to reinforce the natural antimicrobial barrier of eggshells (Figure 2) [79]. This therapy may involve the use of essential oils to obtain a series of benefits that favor poultry production within appropriate microbiological quality standards (Figure 2) [15]. Mustafa et al. [80] observed that spraying Lavandula angustifolia essential oil significantly reduced the total count of aerobic bacteria on the eggshell surface of hatching eggs by 1.42 log10. Before the eggs hatched, this reduction was still significantly 0.52 log10 [80]. Likewise, Oliveira et al. [12] highlighted that after 1 h of spraying on hatching eggs, Syzygium aromaticum essential oil (0.39%) significantly reduced the total count of aerobic mesophilic bacteria and Enterobacteriaceae in eggshells by 1.19 log10. In addition to essential oils (1%) demonstrating the ability to reduce the bacterial load in eggshells after collection, a significant fungal reduction of 0.55 log10 and 0.45 log10 was also evidenced after immersing the eggs for 10 s in the essential oil of Cymbopogon flexuosus and Lippia rotundifolia, respectively [81].
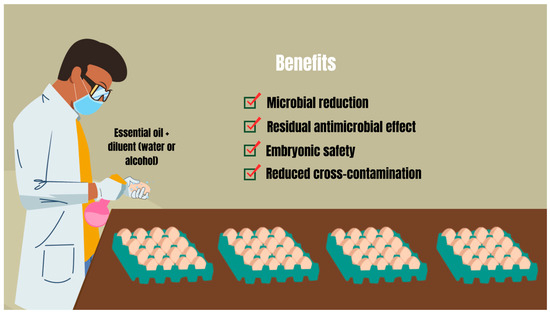
Figure 2.
Spraying hatching eggs with essential oils and some of their benefits for poultry production. Source: Adapted from Oliveira et al. [6].
Eggs subjected to sanitization with essential oils of Citrus aurantifolia, Ocimum basilicum and Allium sativum demonstrated significantly lower mean counts for total aerobic mesophilic bacteria (2.41 log10 CFU/mL) and Enterobacteriaceae (0.34 log10 CFU/mL) compared to non-sanitized eggs (5.12 ± 0.10 and 3.25 ± 0.75 log10 CFU/mL, respectively) (Table 3). The sanitizer based on Allium sativum essential oil demonstrated the greatest efficiency in reducing the bacterial load of the eggshell, resulting in a significant reduction of 3.25 log for total aerobic mesophilic bacteria and Enterobacteriaceae (Table 3). The three essential oils are comparable to formaldehyde (Table 3; unpublished data). Therefore, the essential oils used to date to sanitize eggs meet the recommendations of previous studies to balance microbiological efficiency with environmental responsibility and health preservation, as they are biodegradable, healthy, available, and efficient antimicrobial materials without serious impacts on the environment.

Table 3.
The bacterial count of eggshells sanitized with Citrus aurantifolia, Ocimum basilicum and Allium sativum 1 essential oils *.
The hatchability rates of an incubation cycle may be associated with the toxicity and antimicrobial profile of the compounds used to sanitize hatching eggs [12,82]. Oliveira et al. [12] reported that the greatest hatchability success was observed in eggs sprayed with Syzygium aromaticum essential oil at 0.6 mg/mL (92.37 ± 3.25%) and paraformaldehyde (94.44 ± 4.54%), which were statistically similar. However, the grain alcohol treatment resulted in a lower hatching success (85.00 ± 2.20%) compared to the paraformaldehyde treatment while the propolis treatment resulted in an approximate 43–48% lower hatchability than the other treatments. It was demonstrated that day-old chicks from eggs sprayed with 0.39% Syzygium aromaticum essential oil did not exhibit morphological changes in their tissues [15]. The authors suggested that this indicates no or negligible topical toxicity of Syzygium aromaticum essential oil to ensure the hatching of healthy chicks [15]. Bekhet and Khalifa [82] showed that immersing eggs in a solution of 0.5% Origanum vulgare or Cuminum cyminum essential oil showed a better hatchability rate (96.21 ± 0.56% and 95.76 ± 0.94%, respectively) than eggs sanitized with alcohol (88.66 ± 1.54%), formaldehyde (82.05 ± 0.56%) and non-sanitized eggs (84.06 ± 1.54%). However, due to their oily nature, the use of essential oils in high concentrations can be disadvantageous [13], as this can result in the formation of an artificial layer that occludes the pores and potentially affects gas exchange of embryos until hatching, leading to reduced hatchability rates [83]. This argument is supported by results from several studies on table egg coatings that have proven the efficient contribution of essential oils in minimizing water and gas loss from eggs [52,53,54,55,56,84]. No negative effects were reported on the timing or hatch window of chicks from eggs sanitized with 0.39% Syzygium aromaticum essential oil, 0.2–0.4% Cuminum cyminum, or 0.2–0.4% Origanum vulgare [69,85].
6. Conclusions
In summary, the antimicrobial effects of essential oils bring significant benefits to poultry farming, contributing to the reduction of pathogen load in poultry houses and promoting positive effects on digestibility and feed consumption, improving feed conversion and the health of the poultry intestinal tract. Additionally, they help reduce the microbiota on eggshells and improve hatchability rates. It is important to highlight that essential oils are an antimicrobial treatment option that has been accepted for administration in ovo. This in ovo delivery device is a carefully researched topic in poultry farming mainly to overcome challenges of post-hatch poultry vaccination, to improve poultry production efficiency and to protect or treat poultry from pathogenic microbial infections [86,87]. Therefore, future studies also need to focus especially on the use of essential oils to prevent the growth of pathogenic bacteria in the embryonic development microenvironment and their effects on productivity. Implementing interconnected therapies using essential oils via feed, drinking water and sanitation (depending on the production stage) can be an effective strategy to combat primary and secondary contamination on poultry farms, generating synergistic effects and optimizing the results of systemic treatment. This approach needs to involve the integrated application of several therapies with essential oils, from entry into the farm until the transport of eggs to the hatcheries, contributing to maintaining an environment with safe microbiological levels throughout the process. Ensuring the availability of microbiologically safe eggs for the hatchery represents the first step to generating healthy chicks destined for farms. It is proposed to use essential oils as a microbial control agent in the poultry sector, suggesting their integrated application as follows: sanitization of poultry sheds with Thymus vulgaris essential oil in a proportion of 1:500–1:250 and incorporation of 150 mg/kg of Origanum vulgare in poultry feed. After laying, sanitize the hatching eggs with Syzygium aromaticum essential oil 0.39%.
Author Contributions
Conceptualization, G.d.S.O. and V.M.d.S.; writing—original draft preparation, G.d.S.O.; writing—review and editing, G.d.S.O., C.M., I.R.R.V. and V.M.d.S.; visualization, G.d.S.O., C.M. and V.M.d.S.; supervision, C.M. and V.M.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) grant number 001. It received support from the Fundação de Amparo à Pesquisa do Distrito Federal (FAPDF) for scientific publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the granted scholarship to the doctoral student G.d.S.O.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schäfer, D.F.; Steffens, J.; Barbosa, J.; Zeni, J.; Paroul, N.; Valduga, E.; Junges, A.; Backes, G.T.; Cansian, R.L. Monitoring of contamination sources of Listeria monocytogenes in a poultry slaughterhouse. LWT Food Sci. Technol. 2017, 86, 393–398. [Google Scholar] [CrossRef]
- Cox, N.A.; Richardson, L.J.; Maurer, J.-J.; Berrang, M.E.; Fedorka-Cray, P.J.; Buhr, R.J.; Byrd, J.A.; Lee, M.D.; Hofacre, C.L.; O’Kane, P.M. Evidence for Horizontal and Vertical Transmission in Campylobacter Passage from Hen to Her Progeny. J. Food Prot. 2012, 75, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Pande, V.V.; Devon, R.L.; Sharma, P.; McWhorter, A.R.; Chousalkar, K.K. Study of Salmonella Typhimurium infection in laying hens. Front. Microbiol. 2016, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Gulla, K.; Sattlegger, E.; Mutukumira, A.N. Persistent contamination of Salmonella, Campylobacter, Escherichia coli, and Staphylococcus aureus at a broiler farm in New Zealand. Can. J. Microbiol. 2020, 66, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Ezemba, C.C.; Obi, C.P.; Eleanya, L.C.; Udoye, I.; Ezemba, A.S.; Osuala, O.J.; Archibong, E.J. The incidence of Salmonella and E. coli in poultry feeds. J. Curr. Biomed. Res. 2021, 1, 45–56. [Google Scholar]
- Oliveira, G.d.S.; McManus, C.; Salgado, C.B.; dos Santos, V.M. Effects of Sanitizers on Microbiological Control of Hatching Eggshells and Poultry Health during Embryogenesis and Early Stages after Hatching in the Last Decade. Animals 2022, 12, 2826. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, D.; Sowińska, J. The effectiveness of peppermint and thyme essential oil mist in reducing bacterial contamination in broiler houses. Poult. Sci. 2013, 92, 2834–2843. [Google Scholar] [CrossRef]
- Witkowska, D.; Sowińska, J.; Żebrowska, J.; Mituniewicz, E. The antifungal properties of peppermint and thyme essential oils misted in broiler houses. Braz. J. Poult. Sci. 2016, 18, 629–638. [Google Scholar] [CrossRef][Green Version]
- Denli, M.; Vural, A.; Alp, S.Y. The Influence of Oregano Essential Oil on Egg Quality and Egg Shell Contamination of Laying Hens Kept in Furnished Cages. Sci. Pap. Ser. D Anim. Sci. 2019, 62, 48–52. [Google Scholar]
- Toghyani, P.; Shahzamani, S.; Gholami Ahangaran, M.; Ali Mousavi Firouzabadi, S. Comparison of Eucalyptus Extract and Formaldehyde on Hatchability and Survival Rate of Chicks in Disinfection of Fertile Eggs. Int. J. Pharm. Res. Allied Sci. 2020, 9, 105–109. [Google Scholar]
- Oliveira, G.D.S.; McManus, C.; Sousa, H.A.D.F.; Santos, P.H.G.D.S.; dos Santos, V.M. A Mini-Review of the Main Effects of Essential Oils from Citrus aurantifolia, Ocimum basilicum, and Allium sativum as Safe Antimicrobial Activity in Poultry. Animals 2024, 14, 382. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; Nascimento, S.T.; dos Santos, V.M.; Silva, M.G. Clove Essential Oil in the Sanitation of Fertile Eggs. Poult. Sci. 2020, 99, 5509–5516. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; dos Santos, V.M.; Nascimento, S.T. Essential Oils as Sanitisers for Hatching Eggs. Worlds Poult. Sci. J. 2021, 77, 605–617. [Google Scholar] [CrossRef]
- Ponomarenko, G.V.; Kovalenko, V.L.; Balatskiy, Y.O.; Ponomarenko, O.V.; Paliy, A.P.; Shulyak, S.V. Bactericidal efficiency of preparation based on essential oils used in aerosol disinfection in the presence of poultry. Regul. Mech. Biosyst. 2021, 12, 635–641. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; de Araújo, M.V.; de Sousa, D.E.R.; de Macêdo, I.L.; de Castro, M.B.; dos Santos, V.M. Sanitizing hatching eggs with essential oils: Avian and microbiological safety. Microorganisms 2023, 11, 1890. [Google Scholar] [CrossRef]
- Bakutis, B.; Baliukoniene, V.; Mickiene, R. The use of essential oils to improve of environment quality in poultry houses. In Animal Hygiene and Sustainable Livestock Production, Proceedings of the XVth International Congress of the International Society for Animal Hygiene, Vienna, Austria, 3–7 July 2011; Tribun EU: Brno, Czech Republic, 2011; Volume 2, pp. 643–645. [Google Scholar]
- Puvača, N.; Lika, E.; Tufarelli, V.; Bursić, V.; Pelić, D.L.; Nikolova, N.; Petrović, A.; Prodanović, R.; Vuković, G.; Lević, J.; et al. Influence of Different Tetracycline Antimicrobial Therapy of Mycoplasma (Mycoplasma synoviae) in Laying Hens Compared to Tea Tree Essential Oil on Table Egg Quality and Antibiotic Residues. Foods 2020, 9, 612. [Google Scholar] [CrossRef]
- Patil, V.; Hedau, M.; Kaore, M.; Badar, S.; Kadam, M.; Chaudhari, S.; Rawool, D.; Barbuddhe, S.; Vergis, J.; Kurkure, N. Potential of cinnamaldehyde essential oil as a possible antimicrobial against fowl typhoid in layers. Trop. Anim. Health Prod. 2023, 55, 126. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.N.; Khalefa, H.S.; Aboul-Ella, H.; Mubarak, S.T. Efficacy of disinfection on airborne and waterborne fungal load in broiler chicken houses. J. Anim. Health Prod. 2021, 9, 296–302. [Google Scholar] [CrossRef]
- Kemmett, K.; Williams, N.J.; Chaloner, G.; Humphrey, S.; Wigley, P.; Humphrey, T. The contribution of systemic Escherichia coli infection to the early mortalities of commercial broiler chickens. Avian Pathol. 2014, 43, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Muna, E.A.; Salih, M.H.; Zakia, A.M.; Halima, M.O.; Abeer, A.M.; Ameera, M.M.; Ali, H.O.; Idris, S.B. Pathology of Broiler Chicks Naturally Infected with Salmonella enteritidis (S. Enteritidis) & Salmonella typhimurium (S. Typhimurium) during an Outbreak in Sudan. J. Sci. Res. Rep. 2016, 10, 1–8. [Google Scholar] [CrossRef]
- Hamid, F.F.A.; Reduan, M.F.H.; Jasni, S.; Chung, E.L.T.; Nordin, M.L.B.; Jesse, F.F.A.; Rajdi, N.I.Z.M.; Kamaruzaman, I.N.A.B.; Shaharulnizim, N. Aspergillosis concurrent with secondary bacterial infection in broiler chicks: A case report. Comp. Clin. Path. 2021, 30, 341–345. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; dos Santos, V.M.; McManus, C. Propolis: Effects on the Sanitisation of Hatching Eggs. Worlds Poult. Sci. J. 2022, 78, 261–272. [Google Scholar] [CrossRef]
- Kabir, S.M.L. Avian colibacillosis and salmonellosis: A closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health 2010, 7, 89–114. [Google Scholar] [CrossRef]
- Kalita, N.; Pathak, N.; Saikia, G.; Ahmed, M. Prevalence and pathology of dead-in-shell embryos of Vanaraja egg. Indian J. 2013, 37, 104–105. [Google Scholar]
- Hananeh, W.M.; Al-Natour, M.Q.; Alaboudi, A.R.; Abo-Shehada, M.N.; Ismail, Z.A.B. Congenital abnormalities in dead-in-shell chicks associated with mixed bacterial infections. Heliyon 2021, 7, e06272. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Murase, K.Y. Virulence of Escherichia coli Isolates Obtained from Layer Chickens with Colibacillosis Associated with Pericarditis, Perihepatitis, and Salpingitis in Experimentally Infected Chicks and Embryonated Eggs. Avian Dis. 2018, 62, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Stratakos, A.C.; Koidis, A. Methods for extracting essential oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 31–38. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, N.; Fu, Y.J.; Wang, W.; Luo, M.; Zhao, C.J.; Zu, Y.G.; Liu, X.L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J.; Suschke, U.; Schneele, J.; Geiss, H.K. Antibacterial activity and irritation potential of selected essential oil components—Structure-activity relationship. Nat. Prod. Commun. 2006, 1, 1003–1012. [Google Scholar] [CrossRef]
- El-Baroty, G.S.; El-Baky, H.H.A.; Farag, R.S.; Saleh, M.A. Characterization of Antioxidant and Antimicrobial Compounds of Cinnamon and Ginger Essential Oils. Afr. J. Biochem. Res. 2010, 6, 167–174. [Google Scholar]
- Silveira, S.M.; Cunha, A., Jr.; Scheuermann, G.N.; Secchi, F.L.; Verruck, S.K.M. Composição química e atividade antibacteriana dos óleos essenciais de Cymbopogon winterianus (citronela), Eucalyptus paniculata (eucalipto) e Lavandula angustifolia (lavanda) [Chemical composition and antibacterial activity of essential oils from Cymbopogon winterianus (citronella), Eucalyptus paniculata (eucalyptus) and Lavandula angustifolia (lavender)]. Rev. Inst. Adolfo Lutz 2012, 71, 471–480. [Google Scholar]
- Penteado, A.L.; Eschionato, R.A.; de Souza, D.R.C.; Queiroz, S.D.N. Avaliação in vitro de atividade antimicrobiana de óleos essenciais contra Salmonella Typhimurium e Staphylococcus aureus [In Vitro Evaluation Of Antimicrobial Activity Of Essential Oils Against Salmonella Typhimurium and Staphylococcus aureus]. Hig. Aliment. 2021, 35, e1060. [Google Scholar] [CrossRef]
- Parreira, D.S.; Alcántara-de la Cruz, R.; Leite, G.L.D.; Ramalho, F.D.S.; Zanuncio, J.C.; Serrão, J.E. Quantifying the harmful potential of ten essential oils on immature Trichogramma pretiosum stages. Chemosphere 2018, 199, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Jugreet, B.S.; Mahomoodally, M.F. Essential oils from 9 exotic and endemic medicinal plants from Mauritius shows in vitro antibacterial and antibiotic potentiating activities. S. Afr. J. Bot. 2020, 132, 355–362. [Google Scholar] [CrossRef]
- Reis, J.B.; Figueiredo, L.A.; Castorani, G.M.; Veiga, S.M.O.M. Avaliação Da Atividade Antimicrobiana Dos Óleos Essenciais Contra Patógenos Alimentares [Evaluation of antimicrobial activity of essential oils against food pathogens]. Braz. J. Health Rev. 2020, 3, 342–363. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Fontaine, V.; Mathieu, V.; Zhiri, A.; Baudoux, D.; Stévigny, C.; Souard, F. Antibacterial and cytotoxic activities of ten commercially available essential Oils. Antibiotics 2020, 9, 717. [Google Scholar] [CrossRef]
- Hayatgheib, N.; Fournel, C.; Calvez, S.; Pouliquen, H.; Moreau, E. In vitro antimicrobial effect of various commercial essential oils and their chemical constituents on Aeromonas salmonicida subsp. salmonicida. J. Appl. Microbiol. 2020, 129, 137–145. [Google Scholar] [CrossRef]
- D’Agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; Debourgogne, A. Essential Oils and Their Natural Active Compounds Presenting Antifungal Properties. Molecules 2019, 24, 3713. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Galgano, M.; Capozza, P.; Pellegrini, F.; Cordisco, M.; Sposato, A.; Sblano, S.; Camero, M.; Lanave, G.; Fracchiolla, G.; Corrente, M.; et al. Antimicrobial Activity of Essential Oils Evaluated In Vitro against Escherichia coli and Staphylococcus aureus. Antibiotics 2022, 11, 979. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Boumaiza, A.; Nada, H.G.; Rajabi, M. Eucalyptus globulus essential oil as a natural food preservative: Antioxidant, antibacterial and antifungal properties in vitro and in a real food matrix (orangina fruit juice). Appl. Sci. 2020, 10, 5581. [Google Scholar] [CrossRef]
- Souza, D.S.; Almeida, A.C.; Andrade, V.A.; Marcelo, N.A.; Azevedo, I.L.; Martins, E.R.; Figueiredo, L.S. Antimicrobial Activity of Lippia origanoides and Lippia rotundifolia Oils against Enterobacteria Isolated from Poultry. Arq. Bras. Med. Vet. Zootec. 2015, 67, 940–944. [Google Scholar] [CrossRef][Green Version]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Giovanelli, S.; Rocchigiani, G.; Pistelli, L.; Mancianti, F. Antibacterial and antifungal activity of essential oils against some pathogenic bacteria and yeasts shed from poultry. Flav. Frag. J. 2016, 31, 302–309. [Google Scholar] [CrossRef]
- Ebani, V.V.; Najar, B.; Bertelloni, F.; Pistelli, L.; Mancianti, F.; Nardoni, S. Chemical Composition and In Vitro Antimicrobial Efficacy of Sixteen Essential Oils against Escherichia coli and Aspergillus fumigatus Isolated from Poultry. Vet. Sci. 2018, 5, 62. [Google Scholar] [CrossRef]
- Munda, S.; Dutta, S.; Pandey, S.K.; Sarma, N.; Lal, M. Antimicrobial Activity of Essential Oils of Medicinal and Aromatic Plants of the North East India: A Biodiversity Hot Spot. J. Essent. Oil-Bear. Plants 2019, 22, 105–119. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Plaza-Diaz, J.; Gomez-Llorente, C.; Lucas Gómez, E.; Sabés Alsina, M.; Gil, Á. In vitro examination of antibacterial and immunomodulatory activities of cinnamon, white thyme, and clove essential oils. J. Funct. Foods 2021, 81, 104436. [Google Scholar] [CrossRef]
- Seyedtaghiya, M.H.; Fasaei, B.N.; Peighambari, S.M. Antimicrobial and Antibiofilm Effects of Satureja hortensis Essential Oil against Escherichia coli and Salmonella Isolated from Poultry. Iran. J. Microbiol. 2021, 13, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Fazio, M.L.S.; Bazan, J.R.; Geromel, M.R. Ação De Óleos Essenciais Cítricos Sobre Algumas Bactérias [Action of Citrus Essential Oils on Some Bacteria]. Rev. InterCiência-IMES Catanduva 2020, 1, 11–16. [Google Scholar]
- Oliveira, G.D.S.; McManus, C.; Pires, P.G.D.S.; dos Santos, V.M. Combination of Cassava Starch Biopolymer and Essential Oils for Coating Table Eggs. Front. Sustain. Food Syst. 2022, 6, 957229. [Google Scholar] [CrossRef]
- Campos, R.C.; Peres, C.D.; de Almeida, V.S.; Virgolin, L.B.; Geromel, M.R.; Fazio, M.L.S. Ação Antimicrobiana De Óleos Essenciais De Pimenta Preta, Salsa E Manjericão Doce [Antimicrobial Action of Essential Oils from Black Pepper, Parsley and Sweet Basil]. In Segurança Alimentar e Nutricional [Food and Nutrition Security], 2nd ed.; Treptow, T.C., Ed.; Atena: Ponta Grossa, Brazil, 2022; pp. 167–172. [Google Scholar] [CrossRef]
- Vale, I.R.R.; Oliveira, G.d.S.; McManus, C.; de Araújo, M.V.; Salgado, C.B.; Pires, P.G.d.S.; de Campos, T.A.; Gonçalves, L.F.; Almeida, A.P.C.; Martins, G.D.S.; et al. Whey Protein Isolate and Garlic Essential Oil as an Antimicrobial Coating to Preserve the Internal Quality of Quail Eggs. Coatings 2023, 13, 1369. [Google Scholar] [CrossRef]
- de Araújo, M.V.; Oliveira, G.d.S.; McManus, C.; Vale, I.R.R.; Salgado, C.B.; Pires, P.G.d.S.; de Campos, T.A.; Gonçalves, L.F.; Ameida, A.P.C.; Martins, G.d.S.; et al. Preserving the Internal Quality of Quail Eggs Using a Corn Starch-Based Coating Combined with Basil Essential Oil. Processes 2023, 11, 1612. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; McManus, C.; Salgado, C.B.; Pires, P.G.d.S.; de Figueiredo Sousa, H.A.; da Silva, E.R.; dos Santos, V.M. Antimicrobial Coating Based on Tahiti Lemon Essential Oil and Green Banana Flour to Preserve the Internal Quality of Quail Eggs. Animals 2023, 13, 2123. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.d.S.; McManus, C.; Salgado, C.B.; Pires, P.G.d.S.; dos Santos, V.M. Rice flour coating supplemented with rosemary essential oil to preserve the internal, microbiological, and sensory quality of quail eggs. Acta Aliment. 2023, 52, 294–304. [Google Scholar] [CrossRef]
- Oliveira, A.F.M.; da Silva, F.L.; da Silva, R.T.; Morais, F.M.; dos Santos, R.R.L.; da Silva, L.L.W.V.; Morais, C.C. Atividade antifúngica de óleos essenciais frente a cepa de Candida albicans [Antifungal activity of essential oils against Candida albicans strain]. Res. Soc. Dev. 2022, 11, e04111435696. [Google Scholar] [CrossRef]
- Abed, A.H.; Radwan, I.A.; El-Aziz, M.M.A.; Ali, A. Antifungal activity of natural essential oils against molds and yeasts associated with respiratory problems in broiler chickens. Adv. Anim. Vet. Sci. 2021, 9, 348–355. [Google Scholar] [CrossRef]
- Du, E.; Guo, Y. Dietary Supplementation of Essential Oils and Lysozyme Reduces Mortality and Improves Intestinal Integrity of Broiler Chickens with Necrotic Enteritis. Anim. Sci. J. 2021, 92, e13499. [Google Scholar] [CrossRef]
- Ramirez, S.Y.; Peñuela-Sierra, L.M.; Ospina, M.A. Effects of oregano (Lippia origanoides) essential oil supplementation on the performance, egg quality, and intestinal morphometry of Isa Brown laying hens. Vet. World 2021, 14, 595–602. [Google Scholar] [CrossRef]
- Yarmohammadi Barbarestani, S.; Jazi, V.; Mohebodini, H.; Ashayerizadeh, A.; Shabani, A.; Toghyani, M. Effects of dietary lavender essential oil on growth performance, intestinal function, and antioxidant status of broiler chickens. Livest. Sci. 2020, 233, 103958. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.A.A.; Lohakare, J.D. Productive performance, egg quality, nutrients digestibility, and physiological response of bovans brown hens fed various dietary inclusion levels of peppermint oil. Anim. Feed Sci. Technol. 2020, 267, 114554. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D.; Zych, S. The use of lavender (Lavandula angustifolia) essential oil as an additive to drinking water for broiler chickens and its in vitro reaction with enrofloxacin. Animals 2021, 11, 1535. [Google Scholar] [CrossRef]
- Khosravinia, H. Mortality, production performance, water intake and organ weight of the heat stressed broiler chicken given savory (Satureja khuzistanica) essential oils through drinking water. J. Appl. Anim. Res. 2016, 44, 273–280. [Google Scholar] [CrossRef]
- Karadagoglu, O.; Ozsoy, B.; Olmez, M.; Aydin, O.D.; Sahin, T. The Effects of Drinking Water Supplemented with Essential Oils on Performance, Egg Quality and Egg Yolk Fatty Acid Composition in Laying Hens. Acta Vet. Eurasia 2018, 44, 85–92. [Google Scholar]
- Skoufos, I.; Tzora, A.; Giannenas, I.; Tontis, D.; Bartzanas, T.; Kittas, C.; Panagakis, P. Effects of oregano essential oil and attapulgite on growth performance, intestinal microbiota and morphometry in broilers. S. Afr. J. Anim. Sci. 2016, 46, 77–88. [Google Scholar] [CrossRef]
- Shafey, T.M.; Hussein, E.O.S.; Al-Batshan, H.A. Effects of Ultrasonic Waves on Eggshell Strength and Hatchability of Layer-Type Breeder Eggs. S. Afr. J. Anim. Sci. 2013, 43, 56–63. [Google Scholar] [CrossRef]
- Shahein, E.H.A.; Sedeek, E.K. Role of Spraying Hatching Eggs with Natural Disinfectants on Hatching Characteristics and Eggshell Bacterial Counts. Egypt. Poult. Sci. J. 2014, 34, 213–230. [Google Scholar] [CrossRef]
- Cantu, K.; Archer, G.S.; Tucker, Z.S.; Coufal, C.D. Effectiveness of Duck Hatching Egg Sanitization with the Combination of Hydrogen Peroxide and Ultraviolet Light. J. Appl. Poult. Res. 2019, 28, 301–306. [Google Scholar] [CrossRef]
- Oliveira, G.S.; Nascimento, S.T.; Dos Santos, V.M.; Dallago, B.S.L. Spraying Hatching Eggs with Clove Essential Oil Does Not Compromise the Quality of Embryos and One-Day-Old Chicks or Broiler Performance. Animals 2021, 11, 2045. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.C.B.; Brito, D.A.P.; Soares, S.C.P.; Gomes, K.S.; Saldanha, G.K.M.S.; Soares, V.S. Maintenance of quality of eggs submitted to treatment with propolis extract and sanitizers. Acta Sci. Anim. Sci. 2022, 44, e53584. [Google Scholar] [CrossRef]
- Hrnčár, C.; Prachárová, S.; Bujko, J. The Effect of Disinfection of Hatching Eggs on Hatchability of Oravka Chickens. Sci. Pap. Anim. Sci. Biotechnol. 2012, 45, 411–414. [Google Scholar]
- Zeweil, H.S.; Rizk, R.E.; Bekhet, G.M.; Ahmed, M.R. Comparing the Effectiveness of Egg Disinfectants against Bacteria and Mitotic Indices of Developing Chick Embryos. J. Basic Appl. Zool. 2015, 70, 1–15. [Google Scholar] [CrossRef]
- Wlazlo, L.; Drabik, K.; Al-Shammari, K.I.A.; Batkowska, J.; Nowakowicz-Debek, B.; Gryzińska, M. Use of Reactive Oxygen Species (Ozone, Hydrogen Peroxide) for Disinfection of Hatching Eggs. Poult. Sci. 2020, 99, 2478–2484. [Google Scholar] [CrossRef]
- Oliveira, G.S.; Santos, V.M.; Nascimento, S.T.; Rodrigues, J.C. Alternative sanitizers to paraformaldehyde for incubation of fertile eggs. Poult. Sci. 2020, 99, 2001–2006. [Google Scholar] [CrossRef]
- Yildirim, I.; Ozsan, M.; Yetisir, R. The Use of Oregano (Origanum vulgare L.) Essential Oil as Alternative Hatching Egg Disinfectant versus Formaldehyde Fumigation in Quails (Coturnix coturnix japonica) Eggs. Rev. Med. Vet. 2003, 154, 367–370. [Google Scholar]
- Poonkodi, K. Chemical composition of essential oil of Ocimum basilicum L. (Basil) and its biological activities-an overview. J. Crit. Rev. 2016, 3, 56–62. [Google Scholar]
- Jain, S.; Arora, P.; Popli, H. A comprehensive review on Citrus aurantifolia essential oil: Its phytochemistry and pharmacological aspects. Braz. J. Nat. Sci. 2020, 3, 354. [Google Scholar] [CrossRef]
- Ezeorba, T.P.C.; Chukwudozie, K.I.; Ezema, C.A.; Anaduaka, E.G.; Nweze, E.J.; Okeke, E.S. Potentials for health and therapeutic benefits of garlic essential oils: Recent findings and future prospects. Pharmacol. Res. Mod. Chin. Med. 2022, 3, 100075. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; McManus, C.; dos Santos, V.M. Garlic as Active Principle of Sanitiser for Hatching Eggs. Worlds Poult. Sci. J. 2022, 78, 1037–1052. [Google Scholar] [CrossRef]
- Mustafa, A.A.; Mirza, R.A.; Aziz, H.I. Lavender Essential Oil in Sanitation on Fertile Egg. Passer J. Basic Appl. Sci. 2023, 5, 377–381. [Google Scholar] [CrossRef]
- Nogueira, W.C.L.; Pena, A.C.S.; de Souza, C.N.; Azevedo, I.L.; Fariafilho, D.E.; Almeida, A.C. Disinfection of Fertile Eggs of Free-Range Poultry with Essential Oils. Rev. Bras. Saude Prod. Anim. 2019, 20, e0822019. [Google Scholar] [CrossRef]
- Bekhet, G.; Khalifa, A.Y.Z. Essential Oil Sanitizers to Sanitize Hatching Eggs. J. Appl. Anim. Res. 2022, 50, 695–701. [Google Scholar] [CrossRef]
- Tebrün, W.; Motola, G.; Hafez, M.H.; Bachmeier, J.; Schmidt, V.; Renfert, K.; Reichelt, C.; Brüggemann-Schwarze, S.; Pees, M. Preliminary Study: Health and Performance Assessment in Broiler Chicks Following Application of Six Different Hatching Egg Disinfection Protocols. PLoS ONE 2020, 15, e0232825. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; dos Santos, V.M. Essential oils and propolis as additives in egg coatings. World’s Poult. Sci. J. 2022, 78, 1053–1066. [Google Scholar] [CrossRef]
- Zeweil, H.; Rizk, R.; Bekhet, G.; Ahmed, R. Effect of Egg Disinfection on Hatching Performance for Bandarah Chicken Strain. Egypt. Poult. Sci. J. 2013, 33, 289–307. [Google Scholar]
- Oliveira, G.d.S.; McManus, C.; dos Santos, V.M. Control of Escherichia coli in Poultry Using the In Ovo Injection Technique. Antibiotics 2024, 13, 205. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; McManus, C.; Salgado, C.B.; dos Santos, V.M. Bibliographical Mapping of Research into the Relationship between In Ovo Injection Practice and Hatchability in Poultry. Vet. Sci. 2023, 10, 296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).