Abstract
Background: The accurate estimation of the prevalence of mpox-induced ophthalmic lesions will enable health departments to allocate resources more effectively during the ongoing mpox pandemic. The aim of this meta-analysis was to estimate the global prevalence of ophthalmic manifestations in mpox patients. Methods: A systematic search was carried out in seven databases—Pub Med, Scopus, Web of Science, EMBASE, ProQuest, EBSCOHost, and Cochrane—for studies published on or before 12 December 2022. The pooled prevalence of ophthalmic manifestations was estimated by the random effects model. Risk of bias assessment of the studies and sub-group analysis to explain heterogeneity were undertaken. Results: Overall, 12 studies were included, with 3239 confirmed mpox cases, among which 755 patients reported ophthalmic manifestations. The pooled prevalence of ophthalmic manifestations was 9% (95% confidence interval (CI), 3–24). Studies from Europe reported a very low prevalence of ocular manifestations of 0.98% (95% CI 0.14–2.31), compared to studies from Africa with a substantially higher prevalence of 27.22% (95% CI 13.69–43.26). Conclusions: A wide variation in the prevalence of ocular manifestations among mpox patients was observed globally. Healthcare workers involved in mpox-endemic African countries should be aware of ocular manifestations for early detection and management.
1. Introduction
The ongoing multi-country outbreak of mpox (formerly known as monkeypox) has been declared a “Public Health Emergency of International Concern” (PHEIC) by the World Health Organization (WHO). Monkeypox virus (MPXV) causes mpox, a zoonosis. The origin of mpox traces back to 1958, when MPXV was identified in monkeys at a laboratory in Denmark. Historically, in 1970, the first mpox patient was identified from the Democratic Republic of the Congo (DRC) [1]. From 1970 to 1990, there were sporadic disease outbreaks in Central and West African countries. Post 1990, mpox cases were on the rise, and the majority of the cases were from the DRC. Presently, mpox is endemic in countries of Central and West Africa [2]. The first mpox outbreak outside Africa occurred in the United States of America (USA). The current multi-country outbreak was first noted in the United Kingdom, followed by Portugal, Canada, and Spain. Following this, many other countries reported mpox outbreaks [2], most of which were not epidemiologically related to the mpox-endemic countries. As of 11 December 2022, the WHO reported 82,624 confirmed mpox cases spanning 110 countries. Although the overall risk assessment at the global level is moderate, the Americas are at high risk [3].
Mpox is largely a self-limiting disease, with a maximum incubation period of 21 days. However, mpox has been responsible for considerable fatalities. Historically, the mortality rate has varied from 1% to 10% in endemic countries [4,5], while it has been between 3% and 6% in the ongoing multi-country break. The genetic clade of MPXV also impacts the transmission and severity of the mpox disease. The Congo basin clade has been shown to have higher transmissibility and cause more severe disease than the West African clade [6]. Rash (93%) and fever (72%) are the most common clinical features reported among mpox patients [7]. The clinical manifestations of mpox are similar to smallpox [2], with lymphadenopathy being the differentiating feature in mpox [8]. The epidemiological profile of mpox is evolving, showing variations in risk profile, clinical characteristics, and disease outcomes [9,10]. This transition is under study during the ongoing multi-country outbreak [7]. Atypical manifestations involving other systems, such as the oral, neurological, and respiratory systems, have been reported among patients with mpox [7,11]. Similarly, ocular signs and symptoms have also been reported among mpox patients. The manifestations have ranged from mild rash and focal lesions in the peri-ocular areas to visual loss [12]. Conjunctivitis, peri-ocular vesicular rash, photophobia, and oedema have been reported as the most common ocular manifestations (>20%) [12]. Among these, conjunctivitis was reported among children less than ten years old [13]. Atypical presentations of mpox are not uncommon. An mpox case from Spain had lacrimation, eye pain, and photophobia as the initial and presenting symptoms [14]. Conjunctivitis has been associated with an increased rate of bed riddance among mpox patients [13]. Conjunctivitis has also been hypothesised as a potential route of human-to-human transmission of mpox [14]. As sequelae, corneal ulcerations (1–4%), keratitis (3.6–7.5%), and, finally, vision loss (5–10%) have been recorded in previous studies [15,16]. Thus, the morbidity associated with ophthalmic lesions in patients with mpox is substantial.
Ocular manifestations and complications are more prevalent among unvaccinated (mpox/small pox vaccine) individuals [12,15]. Considering the current scenario where vaccine protection against mpox in older adults is also waning due to time lapse, ocular manifestations may lead to severe complications. Therefore, it becomes essential to quantify the prevalence of ophthalmic manifestations in mpox. This will inform healthcare planners in anticipating and mobilising resources to suspect, identify, and manage ocular manifestations at the earliest opportunity and prevent complications. Based on our current evidence, no meta-analysis of ocular manifestations among mpox patients could be found while searching electronic databases. Hence, the following systematic review with meta-analysis was conducted to explore the ocular features of mpox and estimate the pooled prevalence of mpox-associated ocular manifestations, globally and regionally.
2. Materials and Methods
2.1. Research Question and Selection Criteria
The present systematic review and meta-analysis were carried out based on the following research question: “What is the prevalence of ocular lesions in mpox patients?”. The “preferred reporting standard of systematic reviews and meta-analysis” (PRISMA) checklist was adhered to in the index meta-analysis (Table S1). The systematic search and identification of eligible studies were centred on the PICO criteria elaborated in (Table S2. The current systematic review and meta-analysis protocol has been registered with the International Prospective Register of Systematic Reviews (PROSPERO), with reference ID CRD42022383265.
2.2. Databases Included and Search Strategy
The search was carried out in the following seven databases on 12 December 2022: PubMed, Scopus, Web of Science, EMBASE, ProQuest, EBSCOHost, and Cochrane (Table S3). We also searched pre-print servers, such as medRxiv, arXiv, bioRxiv, BioRN, ChiRxiv, ChiRN, and SSRN. Furthermore, studies obtained by hand search in the references of eligible primary research papers and reviews, which met our eligibility criteria, were also included in the data extraction. The search keywords included were “mpox”, “MPXV”, “Monkeypox”, “ophthalmic”, “eye”, and “ocular”. The database-wise search strategy was applied, and the results obtained are enumerated in Supplementary Table S2. The identified articles were managed using Mendeley Desktop V1.19.5 software.
2.3. Selection of Studies
2.3.1. Title Abstract Screening
The title abstracts of the articles found via the afore-mentioned systematic search were individually examined by two investigators (TKS and SM) by applying the eligibility criteria, and articles were identified for full-text screening. The co-investigators discussed the issue and came to a decision if there was a dispute about whether to include an article for full-text review.
2.3.2. Full-Text Screening and Data Extraction
Eligible full-text articles were reviewed for suitability of data extraction by two investigators, and extraction of the data was performed independently (AGP and SM). In a consensus conference conducted after the independent extraction, discrepancies in the data extraction between the investigators were eliminated. The third investigator (BKP) adjudicated any discrepancies that could not be resolved. A final table was formulated that included information such as the name of the first author, publication year, the geography of the study where it was undertaken, design of the study, total mpox positive patients, and patients with ocular manifestations. PRISMA flow chart was used to report the general search, screening, data extraction, systematic review, and meta-analysis conducted to ensure scientific precision (Figure 1).
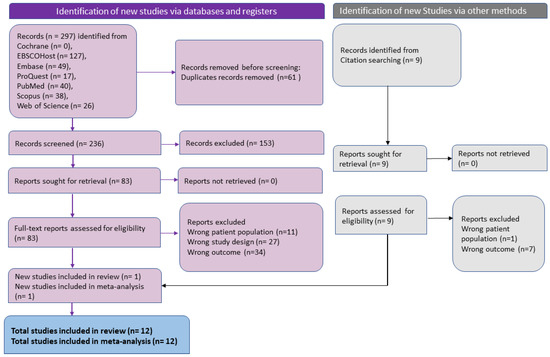
Figure 1.
PRISMA flowchart for included studies in systematic review and meta-analysis of ophthalmic manifestations of mpox.
2.3.3. Quality Assessment
Two investigators (AGP and SM) used the “National Heart, Lung and Blood Institute” (NHLBI) quality assessment method for case series and cross-sectional studies to independently evaluate the risk of bias in the included studies.
2.4. Data Analysis
The pooled estimate of the ocular lesion prevalence, along with the 95% confidence interval (CI), was estimated by collating the total number of mpox patients and those with ocular manifestations. A sensitivity analysis was planned to account for the risk of bias in the studies by including only the studies rated as fair or good quality. Another sensitivity analysis was conducted to account for the potential overlap of the cases between various studies reporting from the same place and time. The pooled estimate was calculated after removing the potential studies with overlapping data if their country/region was the same and there was even a slight overlap in their period of data collection. To determine the cause of heterogeneity, we undertook the following subgroup analyses: geographical factors (according to the continent of the study), MPXV endemicity (endemic vs. non-endemic nations), and 2022 studies vs. pre-2022 studies. Heterogeneity between the studies was assessed by Q-statistics and I2 test. If the included studies were homogenous, then the Q value would be same as that of the degree of freedom (df). Depending on the I2 value, heterogeneity can be declared low (25%), moderate (25–50%), or high (>50%). Since there was high heterogeneity among the studies, a random effects regression model (DerSimonian and Laird estimator) was applied to determine the pooled estimate [17]. Prediction interval was calculated based on the Tau2 statistics [18]. Baujat plot, influence diagnostics, and leave-one-out analysis were applied to identify and address the outliers among the studies. A Doi plot was used to evaluate the publication bias. A trim-and-fill test was undertaken if there was a publication bias. Small study effects were assessed by the Eggers test. A p-value of <0.05 was interpreted as statistically significant. Comprehensive meta-analysis v4 software was used to conduct all the statistical analyses [19].
3. Results
3.1. Eligible Studies
Figure 1 shows the selection process of the article as a PRISMA flow chart. The systematic search yielded 236 articles after removing 61 duplicates. After the title and abstract screening, 83 articles were included for full-text review. In the full-text review, 73 articles were found to be ineligible due to incorrect outcomes (35), incorrect study design (27), and incorrect patient population (11). Finally, 12 studies were found to be eligible for data extraction and meta-analysis.
3.2. Study Characteristics
The studies that are included were carried out between 1987 and 2022. Among the twelve studies included, six were cross-sectional studies [13,16,20,21,22,23], two were case series [24,25], two were retrospective studies [15,26], one was a prospective observational study [27], and one study was mentioned as a prospective cross-sectional study [28]. The studies had sample sizes between 21 [23] and 1057 [20]. Most of the included studies were conducted in the Democratic Republic of the Congo (six out of twelve studies (50%) (Table 1).

Table 1.
Baseline characteristics of mpox patients presenting with ophthalmological manifestations (N = 12 studies).
The highest prevalence of ocular manifestations (51%) was reported by a study from the DRC [20], while the lowest prevalence was reported by a multi-continent study (0.57%) [24].
The heterogeneity among the studies was assessed to be high (I2 = 97%; p < 0.001) (Figure 2a). Hence, the random effects model was applied to determine the pooled prevalence.
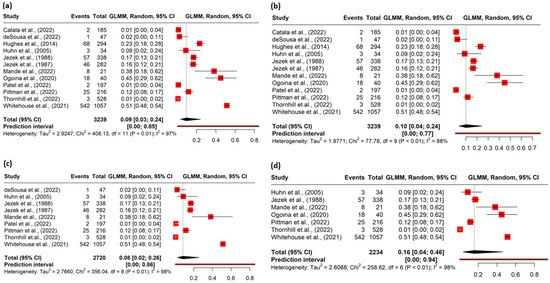
Figure 2.
(a) Forest plot of the pooled magnitude of ophthalmic manifestations among mpox cases. (b) Forest plot of sensitivity analysis conducted after excluding outliers. (c) Forest plot of sensitivity analysis conducted after excluding poor quality studies. (d) Forest plot of sensitivity analysis conducted after excluding studies with potential overlap of cases.
3.3. Pooled Prevalence
The meta-analysis included 3239 confirmed mpox cases, among which 755 patients reported ophthalmic manifestations. The pooled prevalence of ophthalmic lesions in the mpox patients was 9% (95% CI, 3–24) (Figure 2a). In the identification of potential outliers, the tests were able to identify two studies with a large impact on the cluster make-up: study 11 and study 12. (Figures S1–S4). The sensitivity analysis after removing the outliers yielded a prevalence of 10% (95% CI 4–24), I2 = 88% (Figure 2b) (Table 2).

Table 2.
Pooled prevalence of the ophthalmic manifestations among mpox patients.
3.4. Risk of Bias
Nine studies were rated as fair or good quality based on the risk of bias assessment (Tables S4a and S4b). The sensitivity analysis conducted with studies of good or fair quality studies (nine) yielded a pooled prevalence of 8% (95% CI 2.0–26) (Figure 2c) (Table 2), which was close to the overall prevalence (9%).
The sensitivity analysis conducted after removing the studies with potential overlap of the cases revealed a pooled prevalence of 16% (95% CI 4–46) (Figure 2d) (Table 2).
The Doi plot (Figure 3a) shows a symmetrical distribution of the studies included in the meta-analysis, which was further corroborated by using the Egger’s statistics (0.18, p = 0.874). Figure 3b shows the meta-regression in the form of a bubble plot, which indicates that the prevalence of the ocular manifestations was directly proportional to the sample size of the included studies.
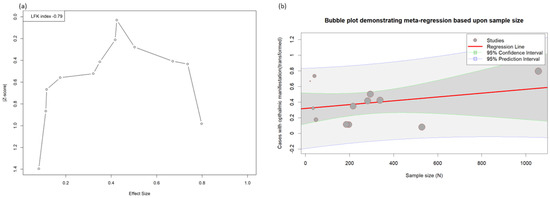
Figure 3.
(a) Doi plot of the ophthalmic manifestations in the mpox. (b) Meta-regression showing the effect size with sample size.
3.5. Subgroup Analysis
Based on the geography where the study was conducted, studies from Europe reported a very low prevalence of ocular manifestations of 0.98% (95% CI 0.14–2.31), while studies from Africa reported a higher prevalence of 27.22% (95% CI 12.69–43.26). Similarly, the prevalence of ocular manifestations differed significantly according to the endemicity of mpox, with the mpox-endemic countries having a higher prevalence (27.22% (95% CI 13.69–43.26) than the non-endemic countries (1.05% (95% CI 0.09–2.68)). The prevalence of ocular lesions has been lower during the ongoing multi-country outbreak (0.61% (95% CI 0.13–1.31)) than in the cases reported before the 2022 outbreak (24.78% (95% CI 12.41–39.62)). The subgroup analysis based on the period of occurrence eliminated the heterogeneity among the studies which reported cases from the ongoing 2022 outbreak (I2 = 0%; p-0.55). Subgrouping by endemicity reduced the heterogeneity among studies from non-endemic countries (I2 = 53.93%; p-0.07), though it remained marginally on the higher side. However, subgrouping based on geography (continent) did not reduce the heterogeneity (Table 3).

Table 3.
Sub-group analysis of the ophthalmic manifestations of the mpox patients.
The site-wise prevalence of ophthalmic manifestations and complications is enumerated in Table 4.

Table 4.
Site-wise lesions and complications in eyes among the patients with mpox.
The most affected site in the eye among mpox cases was the conjunctiva, with 11 of 12 studies reporting conjunctival lesions. Among them, data were available from six studies, which revealed a pooled prevalence of conjunctivitis of 13.89% (95% CI 6.92–22.67). Eye rash and conjunctival lesion (unspecified) were found to have a pooled prevalence of 14.37% (95% CI 6.91–23.71) and 1.62% (95% CI 0.80–2.69), respectively. Pustules or pseudo-pustules on the eyelids (1.08%), conjunctival ulcers (2.13%), redness, pain, and discharge in the eye (9.26%) were the other manifestations. The most common ophthalmic complication reported was photophobia in two studies [15,20], with the highest pooled prevalence of 30.87% (95% CI 28.13–33.67). Keratitis [21,22], corneal ulceration [16,21,22] (3.33%), corneal opacities (38.1%) [23], vision loss [16] (7.69%), and vision changes (2.31%) were the other complications reported in the included studies (Table 4). None of the patients from non-African countries reported vision loss.
4. Discussion
In mpox patients, the pooled prevalence of ophthalmic manifestations has been determined at 9%, globally. Previous analyses based on a smaller number of studies found that the prevalence of ocular lesions ranges from 0.09 to 5% [7,29]. Although this is lower compared to manifestations involving other systems, such as rash, fever, and lymphadenopathy, one in ten patients with mpox could have ophthalmic lesions. Studies conducted among African mpox patients had a significantly higher prevalence of ophthalmic lesions than their European counterparts. A similar geographical variation in the prevalence of rash (the most common symptom of mpox) and oral lesions was also noted from previous reviews [7,11]. This is also closely related to the endemicity of mpox, as all African studies included were from mpox-endemic countries, and all studies outside Africa were from non-endemic countries. In terms of the time period, the ongoing outbreak of mpox showed a relatively lower prevalence of ophthalmic lesions than previous studies. Thus, there is a significant difference in the prevalence of ophthalmic lesions according to geography, endemicity, and the time period of cases.
The conjunctival lesion was the most common manifestation, photophobia was the most common complication, and vision loss was the most severe complication. The presence of conjunctivitis has been shown to have an association with other symptoms of mpox, including systemic and oral lesions [13]. Conjunctivitis has been reported as a factor of hospitalization [28] and a predictor of bed riddance among children [13]. Thus, ocular lesions may have a prognostic value in the outcomes of mpox. Corneal ulcerations, in combination with secondary bacterial infections, have been associated with severe ocular complications, including blindness [29]. Topical trifluridine, when administered together with tecovirimat for mpox patients with varying ophthalmic lesions, has improved the condition without complications [30]. Vision loss as a complication was reported only among endemic African countries, which could be due to the lack of adequate and appropriate treatment. Less severe ocular complications in non-African countries might also be due to the isolation of the less severe variant of MPXV (West African clade) as a causal agent in these countries [31]. None of the studies included in our analysis discussed the impact of ocular lesions on outcomes such as duration of hospital stay and mortality.
The source of the acquisition of mpox had a differential impact on the occurrence of ocular manifestations, with primary cases (contracted from animals) having an ocular lesion prevalence of 10%, while the secondary cases (contracted from humans) had a lower prevalence of 5%, including complications [16]. Isolation of MPXV in the conjunctiva of prairie dogs’ conjunctiva has been reported [32]. Considering the above reports, there is a high suspicion of animal-to-human transmission of mpox through the ocular route, which might cause a higher prevalence of ocular lesions and complications among such cases. The isolation of MPXV from the ocular vesicles and conjunctiva of mpox patients has been reported [14,33]. However, MPXV transmission through human ocular secretions was not reported in any of the reviewed studies.
A study from the DRC reported a differential prevalence of ocular manifestations between vaccinated (13%) and unvaccinated (17%) people against smallpox [21]. Smallpox vaccination offers protection against mpox, which may have reduced the incidence of ocular manifestations in the vaccinated.
The pooled estimate of the ocular manifestations of mpox patients has been assessed systematically for the first time in the index meta-analysis. Risk of bias assessment has been performed for the included studies using standard tools, and the robustness of the result was improved by conducting a sensitivity analysis after excluding the poor-quality studies. The heterogeneity of the included studies in the pooled prevalence was high, which is a limitation. We addressed the heterogeneity by conducting subgroup analysis accordingly, identifying geography and time of occurrence of cases as the potential factors for heterogeneity. Nevertheless, heterogeneity was high among African studies regardless of subgroup assessment. This could be due to the variations in sex, the genetic strain of the virus (clade), age groups of the patients reported in those studies, and/or other unidentified confounders. Therefore, pooled analysis findings must be interpreted with caution. Additionally, different diagnostic techniques employed in the different countries could also affect the proportion of ophthalmic manifestations in the studies included. Although we attempted to factor in the overlap of the cases among the studies by conducting a sensitivity analysis, an accurate estimate of the ocular manifestations might be elusive in the current analysis.
In conclusion, the pooled prevalence of ocular manifestations among the mpox patients is 9%. There is a high variation in the prevalence of ocular manifestations according to the geography of the patients, with a higher prevalence of ocular manifestations found in endemic African countries. Healthcare workers involved in managing mpox in these African countries must be educated on the ocular manifestations of mpox for early detection and management. This, in turn, may prevent ocular complications, including vision loss. Robust strategies to address the overlap of cases from multiple studies need to be identified and applied in future reviews. Studies should report on the modality utilized for the diagnosis of the ophthalmic morbidity, which in turn will improve the comparability and pooling of the results from the studies. Furthermore, studies on the conjunctiva being a potential route of mpox transmission, therapeutics, and the prognostic role of ocular manifestations on the outcomes of mpox in endemic and non-endemic countries must be undertaken.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pathogens12030452/s1: Table S1: PRISMA Checklist (2020); Table S2: Inclusion and exclusion criteria; Table S3: The adjusted search terms as per searched electronic databases (as of 12 December 2022); Table S4: (a) Quality assessment of included case series with the use of National Heart, Lung, and Blood Institute (NHLBI) quality assessment tool; (b) Quality assessment of included cross-sectional and cohort studies with the use of National Heart, Lung, and Blood Institute (NHLBI) quality assessment tool; Supplementary Figures S1–S4 (PPT file): Identification of the potential outliers among the included studies.
Author Contributions
Conceptualization, B.K.P., J.J.B. and A.D.; Data curation, A.P.G., P.C.G. and T.K.S.; Formal analysis, B.K.P., P.S., D.A.L.-F. and J.J.B.; Methodology, A.P.G., P.C.G., M.S., T.K.S., M.A.S., P.S., R.S., D.A.L.-F., A.J.R.-M. and A.D.; Software, M.S. and M.A.S.; Supervision, B.K.P., A.J.R.-M., J.J.B. and A.D.; Validation, A.P.G., P.C.G., M.S., T.K.S., M.A.S. and R.S.; Writing—original draft, A.P.G., P.C.G. and B.K.P.; Writing—review and editing, M.S., T.K.S., M.A.S., P.S., R.S., D.A.L.-F., A.J.R.-M., J.J.B. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. All authors had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Institutional Review Board Statement
Ethical review was not applicable since it was a systematic review and meta-analysis of the data from published literature.
Informed Consent Statement
Not applicable.
Data availability statement
Documents containing all extracted data have been made available in the manuscript and the accompanying Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A Human Infection Caused by Monkeypox Virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar] [PubMed]
- Huang, Y.; Mu, L.; Wang, W. Monkeypox: Epidemiology, Pathogenesis, Treatment and Prevention. Signal Transduct. Target. Ther. 2022, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- World Health Orginazation Multi-Country Outbreak of Monkeypox, External Situation Report #12—14 December 2022. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report-12--14-december-2022 (accessed on 26 December 2022).
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Damon, I.K. Status of Human Monkeypox: Clinical Disease, Epidemiology and Research. Vaccine 2011, 29, D54–D59. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.S.; Lin, C.Y.; Urbina, A.N.; Wang, W.H.; Assavalapsakul, W.; Tseng, S.P.; Lu, P.L.; Chen, Y.H.; Yu, M.L.; Wang, S.F. The First Case of Monkeypox Virus Infection Detected in Taiwan: Awareness and Preparation. Int. J. Infect. Dis. 2022, 122, 991–995. [Google Scholar] [CrossRef]
- Benites-Zapata, V.A.; Ulloque-Badaracco, J.R.; Alarcon-Braga, E.A.; Hernandez-Bustamante, E.A.; Mosquera-Rojas, M.D.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Clinical Features, Hospitalisation and Deaths Associated with Monkeypox: A Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 36. [Google Scholar] [CrossRef]
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 Outbreak and the Pathobiology of the Monkeypox Virus. J. Autoimmun. 2022, 131, 102855. [Google Scholar] [CrossRef]
- Pourriyahi, H.; Aryanian, Z.; Afshar, Z.M.; Goodarzi, A. A Systematic Review and Clinical Atlas on Mucocutaneous Presentations of Monkeypox: With a Comprehensive Approach to All Aspects of the New and Previous Monkeypox Outbreaks. J. Med. Virol. 2022, 95, e28230. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox—A Potential Threat? A Systematic Review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Gandhi, P.A.; Patro, S.K.; Sandeep, M.; Satapathy, S.; Shamim, M.A.; Kumar, V.; Aggarwal, A.K.; Padhi, B.K.; Sah, R. Oral Manifestation of the Monkeypox Virus: A Systematic Review and Meta-Analysis. eClinicalMedicine 2023, 56, 101817. [Google Scholar] [CrossRef]
- Abdelaal, A.; Serhan, H.A.; Mahmoud, M.A.; Rodriguez-Morales, A.J.; Sah, R. Ophthalmic Manifestations of Monkeypox Virus. Eye 2022, 37, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.; McCollum, A.; Pukuta, E.; Karhemere, S.; Nguete, B.; Lushima, R.S.; Kabamba, J.; Balilo, M.; Tamfum, J.-J.M.; Wemakoy, O. Ocular Complications Associated with Acute Monkeypox Virus Infection, DRC. Int. J. Infect. Dis. 2014, 21, 276–277. [Google Scholar] [CrossRef]
- Ly-Yang, F.; Miranda-Sánchez, A.; Burgos-Blasco, B.; Fernández-Vigo, J.I.; Gegúndez-Fernández, J.A.; Díaz-Valle, D. Conjunctivitis in an Individual With Monkeypox. JAMA Ophthalmol. 2022, 140, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; Iroezindu, M.; James, H.I.; Oladokun, R.; Yinka-Ogunleye, A.; Wakama, P.; Otike-Odibi, B.; Usman, L.M.; Obazee, E.; Aruna, O.; et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin. Infect. Dis. 2020, 71, e210–e214. [Google Scholar] [CrossRef] [PubMed]
- Jezek, Z.; Grab, B.; Szczeniowski, M.; Paluku, K.M.; Mutombo, M. Clinico-Epidemiological Features of Monkeypox Patients with an Animal or Human Source of Infection. Bull. World Health Organ. 1988, 66, 459–464. [Google Scholar] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A Basic Introduction to Fixed-Effect and Random-Effects Models for Meta-Analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M. Research Note: In a Meta-Analysis, the I(2) Index Does Not Tell Us How Much the Effect Size Varies across Studies. J. Physiother. 2020, 66, 135–139. [Google Scholar] [CrossRef]
- Comprehensive Meta-Analysis Software (CMA). Available online: https://www.meta-analysis.com/ (accessed on 1 March 2023).
- Whitehouse, E.R.; Bonwitt, J.; Hughes, C.M.; Lushima, R.S.; Likafi, T.; Nguete, B.; Kabamba, J.; Monroe, B.; Doty, J.B.; Nakazawa, Y.; et al. Clinical and Epidemiological Findings from Enhanced Monkeypox Surveillance in Tshuapa Province, Democratic Republic of the Congo During 2011–2015. J. Infect. Dis. 2021, 223, 1870–1878. [Google Scholar] [CrossRef]
- Jezek, Z.; Szczeniowski, M.; Paluku, K.M.; Mutombo, M. Human Monkeypox: Clinical Features of 282 Patients. J. Infect. Dis. 1987, 156, 293–298. [Google Scholar] [CrossRef]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical Characteristics of Human Monkeypox, and Risk Factors for Severe Disease. Clin. Infect. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef]
- Mande, G.; Akonda, I.; De Weggheleire, A.; Brosius, I.; Liesenborghs, L.; Bottieau, E.; Ross, N.; Gembu, G.-C.; Colebunders, R.; Verheyen, E.; et al. Enhanced Surveillance of Monkeypox in Bas-Uélé, Democratic Republic of Congo: The Limitations of Symptom-Based Case Definitions. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2022, 122, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Da Silva Fontoura, D.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; et al. Clinical Features and Novel Presentations of Human Monkeypox in a Central London Centre during the 2022 Outbreak: Descriptive Case Series. BMJ 2022, 378, e072410. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.; Patrocínio, J.; Frade, J.; Brazão, C.; Mancha, D.; Correia, C.; Borges-Costa, J.; Filipe, P. Monkeypox Diagnosis by Cutaneous and Mucosal Findings. Infect. Dis. Rep. 2022, 14, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Pittman, P.R.; Martin, J.W.; Kingebeni, P.M.; Tamfum, J.-J.M.; Wan, Q.; Reynolds, M.G.; Quinn, X.; Norris, S.; Townsend, M.B.; Satheshkumar, P.S.; et al. Clinical Characterization of Human Monkeypox Infections in the Democratic Republic of the Congo. medRxiv 2022. [Google Scholar] [CrossRef]
- Català, A.; Clavo-Escribano, P.; Riera-Monroig, J.; Martín-Ezquerra, G.; Fernandez-Gonzalez, P.; Revelles-Peñas, L.; Simon-Gozalbo, A.; Rodríguez-Cuadrado, F.J.; Castells, V.G.; de la Torre Gomar, F.J.; et al. Monkeypox Outbreak in Spain: Clinical and Epidemiological Findings in a Prospective Cross-Sectional Study of 185 Cases. Br. J. Dermatol. 2022, 187, 765–772. [Google Scholar] [CrossRef]
- Taha, M.J.J.; Abuawwad, M.T.; Alrubasy, W.A.; Sameer, S.K.; Alsafi, T.; Al-Bustanji, Y.; Abu-Ismail, L.; Nashwan, A.J. Ocular Manifestations of Recent Viral Pandemics: A Literature Review. Front. Med. 2022, 9, 1011335. [Google Scholar] [CrossRef]
- Shamim, M.A.; Padhi, B.K.; Satapathy, P.; Veeramachaneni, S.D.; Chatterjee, C.; Tripathy, S.; Akhtar, N.; Pradhan, A.; Dwivedi, P.; Mohanty, A. The Use of Antivirals in the Treatment of Human Monkeypox Outbreaks: A Systematic Review: Antivirals in Treatment of Monkeypox: Systematic Review. Int. J. Infect. Dis. 2022, 127, 150–161. [Google Scholar] [CrossRef]
- Vogel, L. Making Sense of Monkeypox Death Rates. C. Can. Med. Assoc. J. 2022, 194, E1097. [Google Scholar] [CrossRef]
- Guarner, J.; Johnson, B.J.; Paddock, C.D.; Shieh, W.-J.; Goldsmith, C.S.; Reynolds, M.G.; Damon, I.K.; Regnery, R.L.; Zaki, S.R. Monkeypox Transmission and Pathogenesis in Prairie Dogs. Emerg. Infect. Dis. 2004, 10, 426–431. [Google Scholar] [CrossRef]
- Mazzotta, V.; Mondi, A.; Carletti, F.; Baldini, F.; Santoro, R.; Meschi, S.; Moccione, M.; Teklè, S.G.; Minosse, C.; Camici, M. Ocular Involvement in Monkeypox: Description of an Unusual Presentation during the Current Outbreak. J. Infect. 2022, 85, 573–607. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).