The Role of Cluster of Differentiation 39 (CD39) and Purinergic Signaling Pathway in Viral Infections
Abstract
1. Introduction
2. CD39 Role in the Lymphocytic Subsets’ Functions during Viral Infections
3. CD39 Expression and RNA Virus Infections
3.1. The Proportion and Role of CD39+ Cells during HIV Infection
3.2. CD39/73 in Viral Hepatitis
3.2.1. The Level of CD39+ Cells during Hepatitis C Virus (HCV) Infection
3.2.2. The Level of CD39+ Cells during Hepatitis E Virus (HEV) Infection
3.2.3. CD39+ Cells and Hepatitis D Virus (HDV) Infection
3.3. CD39+ Cells and Influenza Infection
3.4. CD39 and Dengue Virus Infection
3.5. CD39+ Cells and Zika Virus Infection
3.6. CD39+ Cells and Lymphocytic Choriomeningitis Virus (LCMV) Infection
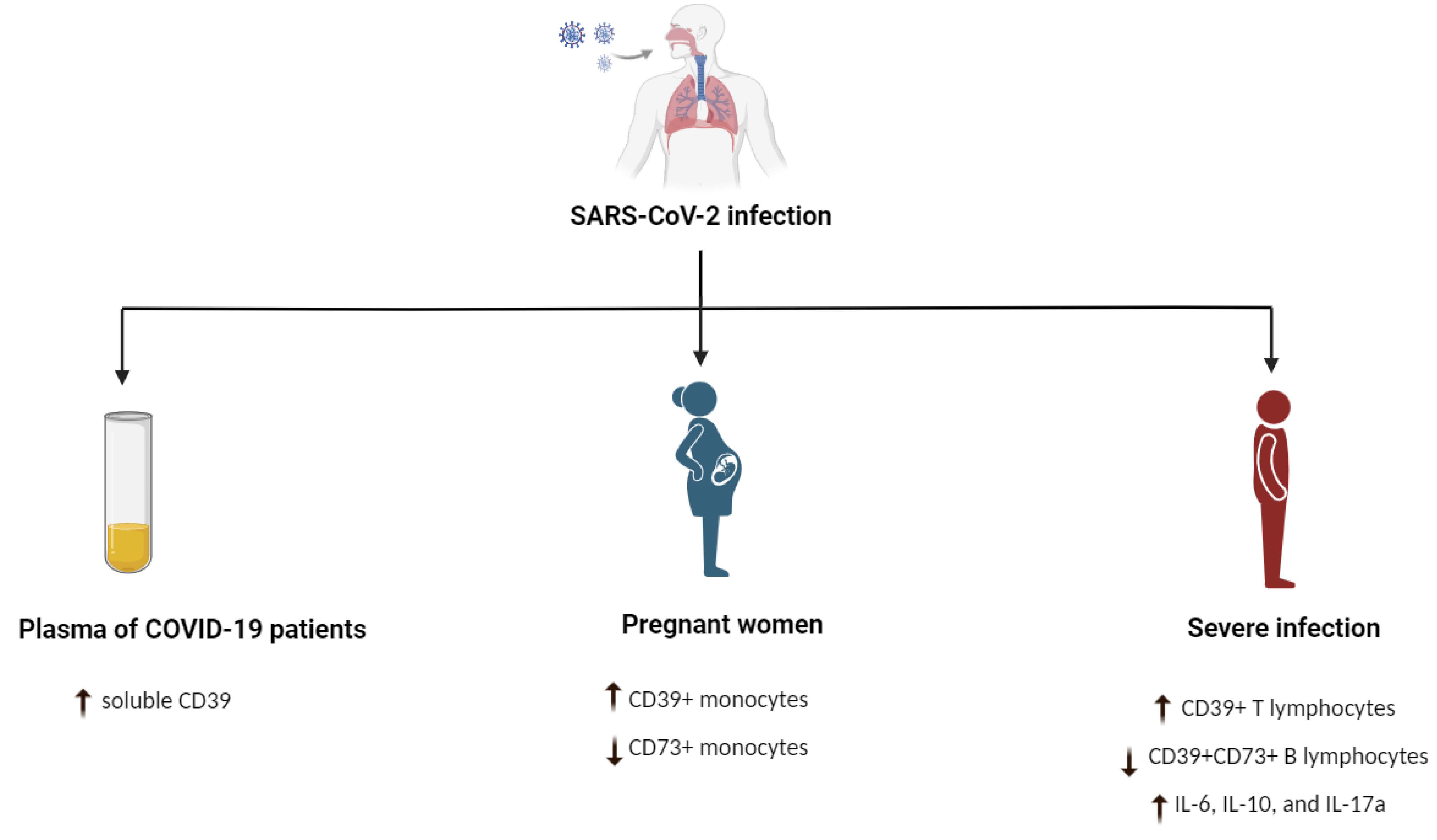
3.7. CD39+ Cells and Severe Acute Respiratory Syndrome (SARS) CoV-2 Infection
4. CD39 + Cells and Infections Caused by DNA Viruses
4.1. CD39+ Cells and Cytomegalovirus (CMV) Infection
4.2. CD39+ Cells and Epstein–Barr Virus (EBV) Infection
4.3. CD39+ Cells and Human Papillomavirus (HPV)
4.4. CD39+ Cells and Hepatitis B Virus (HBV) Infection
5. CD39-Positive Cells and HIV/Tuberculosis (TB) Coinfection
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Robson, S.C.; Sévigny, J.; Zimmermann, H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006, 2, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Maliszewski, C.R.; Delespesse, G.J.; Schoenborn, M.A.; Armitage, R.J.; Fanslow, W.C.; Nakajima, T.; Baker, E.; Sutherland, G.R.; Poindexter, K.; Birks, C.; et al. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J. Immunol. 1994, 153, 3574–3583. [Google Scholar] [CrossRef] [PubMed]
- Kansas, G.S.; Wood, G.S.; Tedder, T.F. Expression, distribution, and biochemistry of human CD39. Role in activation-associated homotypic adhesion of lymphocytes. J. Immunol. 1991, 146, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Pulte, D.; Furman, R.R.; Broekman, M.J.; Drosopoulos, J.H.; Ballard, H.S.; Olson, K.E.; Kizer, J.R.; Marcus, A.J. CD39 expression on T lymphocytes correlates with severity of disease in patients with chronic lymphocytic leukemia. Clin. Lymphoma Myeloma Leuk. 2011, 11, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G.; Wieringa, B.; Robson, S.C.; Jalkanen, S. Metabolism of circulating ADP in the bloodstream is mediated via integrated actions of soluble adenylate kinase-1 and NTPDase1/CD39 activities. Faseb J. 2012, 26, 3875–3883. [Google Scholar] [CrossRef]
- Wang, T.-F.; Guidotti, G. CD39 Is an Ecto-(Ca2+, Mg2+)-apyrase (*). J. Biol. Chem. 1996, 271, 9898–9901. [Google Scholar] [CrossRef]
- Burnstock, G. Purine and purinergic receptors. Brain Neurosci. Adv. 2018, 2, 2398212818817494. [Google Scholar] [CrossRef]
- Sueyoshi, K.; Ledderose, C.; Shen, Y.; Lee, A.H.; Shapiro, N.I.; Junger, W.G. Lipopolysaccharide suppresses T cells by generating extracellular ATP that impairs their mitochondrial function via P2Y11 receptors. J. Biol. Chem. 2019, 294, 6283–6293. [Google Scholar] [CrossRef]
- Young, A.; Ngiow, S.F.; Barkauskas, D.S.; Sult, E.; Hay, C.; Blake, S.J.; Huang, Q.; Liu, J.; Takeda, K.; Teng, M.W. Co-inhibition of CD73 and A2AR adenosine signaling improves anti-tumor immune responses. Cancer Cell 2016, 30, 391–403. [Google Scholar] [CrossRef]
- Harvey, J.B.; Phan, L.H.; Villarreal, O.E.; Bowser, J.L. CD73’s potential as an immunotherapy target in gastrointestinal cancers. Front. Immunol. 2020, 11, 508. [Google Scholar] [CrossRef]
- Powderly, J.D.; de Souza, P.L.; Gutierrez, R.; Horvath, L.; Seitz, L.; Ashok, D.; Park, A.; Walters, M.J.; Karakunnel, J.J.; Berry, W. AB928, a novel dual adenosine receptor antagonist, combined with chemotherapy or AB122 (anti-PD-1) in patients (pts) with advanced tumors: Preliminary results from ongoing phase I studies. Am. Soc. Clin. Oncol. 2019, 37, 2604. [Google Scholar] [CrossRef]
- Willingham, S.B.; Ho, P.Y.; Hotson, A.; Hill, C.; Piccione, E.C.; Hsieh, J.; Liu, L.; Buggy, J.J.; McCaffery, I.; Miller, R.A. A2AR Antagonism with CPI-444 Induces Antitumor Responses and Augments Efficacy to Anti–PD-(L) 1 and Anti–CTLA-4 in Preclinical ModelsA2AR Antagonism with CPI-444 Stimulates Antitumor Immunity. Cancer Immunol. Res. 2018, 6, 1136–1149. [Google Scholar] [CrossRef]
- Fredholm, B.B.; AP, I.J.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef]
- Müller, C.E.; Jacobson, K.A. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim. Biophys. Acta 2011, 1808, 1290–1308. [Google Scholar] [CrossRef]
- Barletta, K.E.; Ley, K.; Mehrad, B. Regulation of neutrophil function by adenosine. Arter. Thromb Vasc Biol. 2012, 32, 856–864. [Google Scholar] [CrossRef]
- Cohen, H.B.; Ward, A.; Hamidzadeh, K.; Ravid, K.; Mosser, D.M. IFN-γ prevents adenosine receptor (A2bR) upregulation to sustain the macrophage activation response. J. Immunol. 2015, 195, 3828–3837. [Google Scholar] [CrossRef]
- Cohen, H.B.; Briggs, K.T.; Marino, J.P.; Ravid, K.; Robson, S.C.; Mosser, D.M. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood 2013, 122, 1935–1945. [Google Scholar] [CrossRef]
- Haskó, G.; Pacher, P. Regulation of macrophage function by adenosine. Arter. Thromb Vasc Biol. 2012, 32, 865–869. [Google Scholar] [CrossRef]
- Kronlage, M.; Song, J.; Sorokin, L.; Isfort, K.; Schwerdtle, T.; Leipziger, J.; Robaye, B.; Conley, P.B.; Kim, H.-C.; Sargin, S.J. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci. Signal. 2010, 3, ra55. [Google Scholar] [CrossRef]
- Dombrowski, K.E.; Ke, Y.; Thompson, L.F.; Kapp, J.A. Antigen recognition by CTL is dependent upon ectoATPase activity. J. Immunol. 1995, 154, 6227–6237. [Google Scholar] [CrossRef]
- Gupta, P.K.; Godec, J.; Wolski, D.; Adland, E.; Yates, K.; Pauken, K.E.; Cosgrove, C.; Ledderose, C.; Junger, W.G.; Robson, S.C.; et al. CD39 Expression Identifies Terminally Exhausted CD8+ T Cells. PLOS Pathog. 2015, 11, e1005177. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.; Moulana, Z.; Sepidarkish, M.; Bagherzadeh, M.; Rezanejad, M.; Mirzakhani, M.; Jafari, M.; Mohammadnia-Afrouzi, M. Pronounce expression of Tim-3 and CD39 but not PD1 defines CD8 T cells in critical Covid-19 patients. Microb. Pathog. 2021, 153, 104779. [Google Scholar] [CrossRef] [PubMed]
- Canale, F.P.; Ramello, M.C.; Núñez, N.; Araujo Furlan, C.L.; Bossio, S.N.; Gorosito Serrán, M.; Tosello Boari, J.; Del Castillo, A.; Ledesma, M.; Sedlik, C.; et al. CD39 Expression Defines Cell Exhaustion in Tumor-Infiltrating CD8(+) T Cells. Cancer Res. 2018, 78, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xia, Y.; Lin, Z.; Qu, Y.; Qi, Y.; Chen, Y.; Zhou, Q.; Zeng, H.; Wang, J.; Chang, Y.; et al. Tumor-infiltrating CD39(+)CD8(+) T cells determine poor prognosis and immune evasion in clear cell renal cell carcinoma patients. Cancer Immunol. Immunother. 2020, 69, 1565–1576. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [CrossRef]
- Jung, M.K.; Shin, E.C. Regulatory T Cells in Hepatitis B and C Virus Infections. Immune Netw. 2016, 16, 330–336. [Google Scholar] [CrossRef]
- Dietze, K.K.; Schimmer, S.; Kretzmer, F.; Wang, J.; Lin, Y.; Huang, X.; Wu, W.; Wang, B.; Lu, M.; Dittmer, U. Characterization of the Treg response in the hepatitis B virus hydrodynamic injection mouse model. PLoS ONE 2016, 11, e0151717. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, L.; Zheng, Y.; Ni, B.; Wu, Y. Expression of CD39 on FoxP3+ T regulatory cells correlates with progression of HBV infection. BMC Immunol. 2012, 13, 17. [Google Scholar] [CrossRef]
- Dwyer, K.M.; Hanidziar, D.; Putheti, P.; Hill, P.A.; Pommey, S.; McRae, J.L.; Winterhalter, A.; Doherty, G.; Deaglio, S.; Koulmanda, M.; et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am. J. Transpl. 2010, 10, 2410–2420. [Google Scholar] [CrossRef]
- Schulze zur Wiesch, J.; Thomssen, A.; Hartjen, P.; Toth, I.; Lehmann, C.; Meyer-Olson, D.; Colberg, K.; Frerk, S.; Babikir, D.; Schmiedel, S.; et al. Comprehensive Analysis of Frequency and Phenotype of T Regulatory Cells in HIV Infection: CD39 Expression of FoxP3+ T Regulatory Cells Correlates with Progressive Disease. J. Virol. 2010, 85, 1287–1297. [Google Scholar] [CrossRef]
- Ouyang, W.; Kolls, J.K.; Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008, 28, 454–467. [Google Scholar] [CrossRef]
- Otsuka, A.; Hanakawa, S.; Miyachi, Y.; Kabashima, K. CD39: A new surface marker of mouse regulatory γδ T cells. J. Allergy Clin. Immunol. 2013, 132, 1448–1451. [Google Scholar] [CrossRef]
- Kolbe, K.; Wittner, M.; Hartjen, P.; Hüfner, A.D.; Degen, O.; Ackermann, C.; Cords, L.; Stellbrink, H.J.; Haag, F.; Schulze Zur Wiesch, J. Inversed Ratio of CD39/CD73 Expression on γδ T Cells in HIV Versus Healthy Controls Correlates With Immune Activation and Disease Progression. Front. Immunol. 2022, 13, 867167. [Google Scholar] [CrossRef]
- Mizumoto, N.; Kumamoto, T.; Robson, S.C.; Sévigny, J.; Matsue, H.; Enjyoji, K.; Takashima, A. CD39 is the dominant Langerhans cell–associated ecto-NTPDase: Modulatory roles in inflammation and immune responsiveness. Nat. Med. 2002, 8, 358–365. [Google Scholar] [CrossRef]
- Kaczmarek, E.; Koziak, K.; Sévigny, J.; Siegel, J.B.; Anrather, J.; Beaudoin, A.R.; Bach, F.H.; Robson, S.C. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J. Biol. Chem. 1996, 271, 33116–33122. [Google Scholar] [CrossRef]
- Dierks, P.; Wroblewski, R.; Eberhard, J.M.; Martrus, G.; Degen, O.; Hertling, S.; Schmiedel, S.; Lunemann, S.; Hüfner, A.; Lohse, A.W.; et al. Brief Report: Increased Frequency of CD39+ CD56bright Natural Killer Cells in HIV-1 Infection Correlates With Immune Activation and Disease Progression. J. Acquir. Immune. Defic. Syndr. 2017, 74, 467–472. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.H.; Tu, B.; Zhou, M.J.; Hu, W.; Fu, Y.L.; Li, X.Y.; Yang, T.; Song, J.W.; Fan, X.; et al. Reversal of the CD8(+) T-Cell Exhaustion Induced by Chronic HIV-1 Infection Through Combined Blockade of the Adenosine and PD-1 Pathways. Front. Immunol. 2021, 12, 687296. [Google Scholar] [CrossRef]
- Jenabian, M.-A.; Seddiki, N.; Yatim, A.; Carriere, M.; Hulin, A.; Younas, M.; Ghadimi, E.; Kök, A.; Routy, J.-P.; Tremblay, A. Regulatory T cells negatively affect IL-2 production of effector T cells through CD39/adenosine pathway in HIV infection. PLoS Pathog. 2013, 9, e1003319. [Google Scholar] [CrossRef]
- Fenoglio, D.; Dentone, C.; Signori, A.; Di Biagio, A.; Parodi, A.; Kalli, F.; Nasi, G.; Curto, M.; Cenderello, G.; De Leo, P.; et al. CD8(+)CD28(−)CD127(lo)CD39(+) regulatory T-cell expansion: A new possible pathogenic mechanism for HIV infection? J. Allergy Clin. Immunol. 2018, 141, 2220–2233. [Google Scholar] [CrossRef]
- Seddiki, N.; Cook, L.; Hsu, D.C.; Phetsouphanh, C.; Brown, K.; Xu, Y.; Kerr, S.J.; Cooper, D.A.; Munier, C.M.; Pett, S.; et al. Human antigen-specific CD4⁺ CD25⁺ CD134⁺ CD39⁺ T cells are enriched for regulatory T cells and comprise a substantial proportion of recall responses. Eur. J. Immunol. 2014, 44, 1644–1661. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Ackermann, C.; Tóth, I.; Dierks, P.; Eberhard, J.M.; Wroblewski, R.; Scherg, F.; Geyer, M.; Schmidt, R.E.; Beisel, C.; et al. Down-regulation of CD73 on B cells of patients with viremic HIV correlates with B cell activation and disease progression. J. Leukoc Biol. 2017, 101, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Cachem, F.; Dias, A.S.; Monteiro, C.; Fernandes, G.; Delphim, L.; Tavares, F.; Maciel, A.M.A.; Amendola-Pires, M.M.; Brandão-Mello, C.E.; Andrade, R.M.; et al. Different core-specific T cell subsets are expanded in chronic hepatitis C with advanced liver disease. Cytokine 2019, 124, 154456. [Google Scholar] [CrossRef] [PubMed]
- Keoshkerian, E.; Hunter, M.; Cameron, B.; Nguyen, N.; Sugden, P.; Bull, R.; Zekry, A.; Maher, L.; Seddiki, N.; Zaunders, J. Hepatitis C-specific effector and regulatory CD 4 T-cell responses are associated with the outcomes of primary infection. J. Viral Hepat. 2016, 23, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Wildner, N.H.; Walker, A.; Brauneck, F.; Ditt, V.; Peine, S.; Huber, S.; Haag, F.; Beisel, C.; Timm, J.; Schulze Zur Wiesch, J. Transcriptional Pattern Analysis of Virus-Specific CD8+ T Cells in Hepatitis C Infection: Increased Expression of TOX and Eomesodermin During and After Persistent Antigen Recognition. Front. Immunol. 2022, 13, 886646. [Google Scholar] [CrossRef]
- Rathod, S.B.; Das, R.; Thanapati, S.; Arankalle, V.A.; Tripathy, A.S. Suppressive activity and altered conventional phenotype markers/mediators of regulatory T cells in patients with self-limiting hepatitis E. J. Viral Hepat. 2014, 21, 141–151. [Google Scholar] [CrossRef]
- Taylor, J.M.; Han, Z. Purinergic receptor functionality is necessary for infection of human hepatocytes by hepatitis delta virus and hepatitis B virus. PLoS ONE 2010, 5, e15784. [Google Scholar] [CrossRef]
- Lu, C.; Zanker, D.; Lock, P.; Jiang, X.; Deng, J.; Duan, M.; Liu, C.; Faou, P.; Hickey, M.J.; Chen, W. Memory regulatory T cells home to the lung and control influenza A virus infection. Immunol. Cell Biol. 2019, 97, 774–786. [Google Scholar] [CrossRef]
- Hall, O.J.; Limjunyawong, N.; Vermillion, M.S.; Robinson, D.P.; Wohlgemuth, N.; Pekosz, A.; Mitzner, W.; Klein, S.L. Progesterone-Based Therapy Protects Against Influenza by Promoting Lung Repair and Recovery in Females. PLoS Pathog. 2016, 12, e1005840. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Liong, K.H.; Gunalan, M.G.; Li, N.; Lim, D.S.L.; Fisher, D.A.; MacAry, P.A.; Leo, Y.S.; Wong, S.-C.; Puan, K.J. Type I IFNs and IL-18 regulate the antiviral response of primary human γδ T cells against dendritic cells infected with Dengue virus. J. Immunol. 2015, 194, 3890–3900. [Google Scholar] [CrossRef]
- Guerra-Gomes, I.C.; Gois, B.M.; Peixoto, R.F.; Palmeira, P.H.S.; Dias, C.N.S.; Csordas, B.G.; Araújo, J.M.G.; Veras, R.C.; de Medeiros, I.A.; de Azevedo, F.; et al. Phenotypical characterization of regulatory T cells in acute Zika infection. Cytokine 2021, 146, 155651. [Google Scholar] [CrossRef]
- Pietrobon, A.J.; Andrejew, R.; Custódio, R.W.A.; Oliveira, L.M.; Scholl, J.N.; Teixeira, F.M.E.; de Brito, C.A.; Glaser, T.; Kazmierski, J.; Goffinet, C.; et al. Dysfunctional purinergic signaling correlates with disease severity in COVID-19 patients. Front. Immunol. 2022, 13, 1012027. [Google Scholar] [CrossRef]
- Cérbulo-Vázquez, A.; García-Espinosa, M.; Briones-Garduño, J.C.; Arriaga-Pizano, L.; Ferat-Osorio, E.; Zavala-Barrios, B.; Cabrera-Rivera, G.L.; Miranda-Cruz, P.; García de la Rosa, M.T.; Prieto-Chávez, J.L.; et al. The percentage of CD39+ monocytes is higher in pregnant COVID-19+ patients than in nonpregnant COVID-19+ patients. PLoS ONE 2022, 17, e0264566. [Google Scholar] [CrossRef]
- Dorneles, G.P.; Teixeira, P.C.; da Silva, I.M.; Schipper, L.L.; Santana Filho, P.C.; Rodrigues Junior, L.C.; Bonorino, C.; Peres, A.; Fonseca, S.G.; Monteiro, M.C.; et al. Alterations in CD39/CD73 axis of T cells associated with COVID-19 severity. J. Cell Physiol. 2022, 237, 3394–3407. [Google Scholar] [CrossRef]
- Díaz-García, E.; García-Tovar, S.; Alfaro, E.; Zamarrón, E.; Mangas, A.; Galera, R.; Ruíz-Hernández, J.J.; Solé-Violán, J.; Rodríguez-Gallego, C.; Van-Den-Rym, A.; et al. Role of CD39 in COVID-19 Severity: Dysregulation of Purinergic Signaling and Thromboinflammation. Front. Immunol. 2022, 13, 847894. [Google Scholar] [CrossRef]
- Luciw, P.A. Human immunodeficiency viruses and their replication. In Fields Virology, 3rd ed.; Fields, B.N., Knipe, D.M., Eds.; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1996; pp. 1881–1952. [Google Scholar]
- Shaw, G.M.; Hunter, E. HIV transmission. Cold Spring Harb. Perspect. Med. 2012, 2, a006965. [Google Scholar] [CrossRef]
- Okoye, A.A.; Picker, L.J. CD4(+) T-cell depletion in HIV infection: Mechanisms of immunological failure. Immunol Rev. 2013, 254, 54–64. [Google Scholar] [CrossRef]
- Leal, D.B.; Streher, C.A.; Bertoncheli, C.d.M.; Carli, L.F.; Leal, C.A.; da Silva, J.E.; Morsch, V.M.; Schetinger, M.R. HIV infection is associated with increased NTPDase activity that correlates with CD39-positive lymphocytes. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2005, 1746, 129–134. [Google Scholar] [CrossRef]
- Leal, D.B.; Schetinger, M.R.; Leal, C.A.; Bertoncheli Cde, M.; Morsch, V.M. NTPDase activity in human lymphocytes is not affected by therapeutic doses of anti-HIV drugs. Biomed. Pharm. 2011, 65, 594–596. [Google Scholar] [CrossRef]
- Barat, C.; Martin, G.; Beaudoin, A.R.; Sévigny, J.; Tremblay, M.J. The nucleoside triphosphate diphosphohydrolase-1/CD39 is incorporated into human immunodeficiency type 1 particles, where it remains biologically active. J. Mol. Biol. 2007, 371, 269–282. [Google Scholar] [CrossRef]
- Moreno-Fernandez, M.E.; Rueda, C.M.; Rusie, L.K.; Chougnet, C.A. Regulatory T cells control HIV replication in activated T cells through a cAMP-dependent mechanism. Blood 2011, 117, 5372–5380. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.; Carriere, M.; Jenabian, M.-A.; Limou, S.; Younas, M.; Kök, A.; Huë, S.; Seddiki, N.; Hulin, A.; Delaneau, O. CD39/adenosine pathway is involved in AIDS progression. PLoS Pathog. 2011, 7, e1002110. [Google Scholar] [CrossRef] [PubMed]
- Yero, A.; Shi, T.; Farnos, O.; Routy, J.P.; Tremblay, C.; Durand, M.; Tsoukas, C.; Costiniuk, C.T.; Jenabian, M.A. Dynamics and epigenetic signature of regulatory T-cells following antiretroviral therapy initiation in acute HIV infection. EBioMedicine 2021, 71, 103570. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-W.; Huang, H.-H.; Zhang, C.; Yang, H.-G.; Zhang, J.-Y.; Xu, R.-N.; Jin, L.; Shi, M.; Wang, F.-S.; Jiao, Y.-M. Expression of CD39 Is Correlated With HIV DNA Levels in Naïve Tregs in Chronically Infected ART Naïve Patients. Front. Immunol. 2019, 10, 02465. [Google Scholar] [CrossRef]
- Hoffmann, M.; Pantazis, N.; Martin, G.E.; Hickling, S.; Hurst, J.; Meyerowitz, J.; Willberg, C.B.; Robinson, N.; Brown, H.; Fisher, M.; et al. Exhaustion of Activated CD8 T Cells Predicts Disease Progression in Primary HIV-1 Infection. PLoS Pathog. 2016, 12, e1005661. [Google Scholar] [CrossRef]
- Schachter, J.; Delgado, K.V.; Barreto-de-Souza, V.; Bou-Habib, D.C.; Persechini, P.M.; Meyer-Fernandes, J.R. Inhibition of ecto-ATPase activities impairs HIV-1 infection of macrophages. Immunobiology 2015, 220, 589–596. [Google Scholar] [CrossRef]
- Cao, W.J.; Zhang, X.C.; Wan, L.Y.; Li, Q.Y.; Mu, X.Y.; Guo, A.L.; Zhou, M.J.; Shen, L.L.; Zhang, C.; Fan, X.; et al. Immune Dysfunctions of CD56(neg) NK Cells Are Associated With HIV-1 Disease Progression. Front. Immunol. 2021, 12, 811091. [Google Scholar] [CrossRef]
- Qian, S.; Xiong, C.; Wang, M.; Zhang, Z.; Fu, Y.; Hu, Q.; Ding, H.; Han, X.; Shang, H.; Jiang, Y. CD38(+)CD39(+) NK cells associate with HIV disease progression and negatively regulate T cell proliferation. Front. Immunol. 2022, 13, 946871. [Google Scholar] [CrossRef]
- Catanese, M.T.; Uryu, K.; Kopp, M.; Edwards, T.J.; Andrus, L.; Rice, W.J.; Silvestry, M.; Kuhn, R.J.; Rice, C.M. Ultrastructural analysis of hepatitis C virus particles. Proc. Natl. Acad. Sci. USA 2013, 110, 9505–9510. [Google Scholar] [CrossRef]
- Alter, H.J.; Seeff, L.B. Recovery, persistence, and sequelae in hepatitis C virus infection: A perspective on long-term outcome. Semin. Liver Dis. 2000, 20, 17–35. [Google Scholar] [CrossRef]
- Abdelwahab, S.F. Cellular immune response to hepatitis-C-virus in subjects without viremia or seroconversion: Is it important? Infect. Agents Cancer 2016, 11, 23. [Google Scholar] [CrossRef]
- Li, Z.; Ping, Y.; Yu, Z.; Wang, M.; Yue, D.; Zhang, Z.; Li, J.; Zhang, B.; Shi, X.; Zhang, Y. Dynamic changes in CD45RA(-)Foxp3(high) regulatory T-cells in chronic hepatitis C patients during antiviral therapy. Int. J. Infect. Dis. 2016, 45, 5–12. [Google Scholar] [CrossRef]
- Sayed, I.M.; Vercouter, A.S.; Abdelwahab, S.F.; Vercauteren, K.; Meuleman, P. Is hepatitis E virus an emerging problem in industrialized countries? Hepatology 2015, 62, 1883–1892. [Google Scholar] [CrossRef]
- Sayed, I.M.; Vercauteren, K.; Abdelwahab, S.F.; Meuleman, P. The emergence of hepatitis E virus in Europe. Future Virol. 2015, 10, 763–778. [Google Scholar] [CrossRef]
- Sayed, I.M.; Vercouter, A.S.; Meuleman, P. Hepatitis E virus in acute liver failure: An unusual suspect? Hepatology 2016, 64, 1837–1839. [Google Scholar] [CrossRef]
- Kamar, N.; Selves, J.; Mansuy, J.M.; Ouezzani, L.; Péron, J.M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef]
- Pischke, S.; Hartl, J.; Pas, S.D.; Lohse, A.W.; Jacobs, B.C.; Van der Eijk, A.A. Hepatitis E virus: Infection beyond the liver? J. Hepatol. 2017, 66, 1082–1095. [Google Scholar] [CrossRef]
- Tripathy, A.S.; Das, R.; Rathod, S.B.; Gurav, Y.K.; Arankalle, V.A. Peripheral T regulatory cells and cytokines in hepatitis E infection. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 179–184. [Google Scholar] [CrossRef]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.-F.; Enjyoji, K.; Linden, J.; Oukka, M. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef]
- Rathod, S.B.; Tripathy, A.S. TGF-β(1) and contact mediated suppression by CD4+CD25+CD127− T regulatory cells of patients with self-limiting hepatitis E. Hum. Immunol. 2016, 77, 1254–1263. [Google Scholar] [CrossRef]
- Koytak, E.S.; Yurdaydin, C.; Glenn, J.S. Hepatitis D. Curr. Treat. Options Gastroenterol. 2007, 10, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Seeger, C.; Zoulim, F.; Mason, W. Hepadnaviruses. Fields Virol. 2007, 2, 2977–3029. [Google Scholar]
- Negro, F. Hepatitis D virus coinfection and superinfection. Cold Spring Harb. Perspect. Med. 2014, 4, a021550. [Google Scholar] [CrossRef] [PubMed]
- Rizzetto, M.; Canese, M.G.; Arico, S.; Crivelli, O.; Trepo, C.; Bonino, F.; Verme, G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 1977, 18, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Corriden, R.; Insel, P.A. Basal release of ATP: An autocrine-paracrine mechanism for cell regulation. Sci. Signal. 2010, 3, re1. [Google Scholar] [CrossRef]
- Faas, M.; Sáez, T.; De Vos, P. Extracellular ATP and adenosine: The Yin and Yang in immune responses? Mol. Asp. Med. 2017, 55, 9–19. [Google Scholar] [CrossRef]
- Arbeitskreis, B. Untergruppe «Bewertung Blutassoziierter Krankheitserreger». Influenza Virus. Transfus. Med. Hemother. 2009, 36, 32–39. [Google Scholar]
- Zambon, M.C. The pathogenesis of influenza in humans. Rev. Med. Virol. 2001, 11, 227–241. [Google Scholar] [CrossRef]
- Saito, T.; Tanikawa, T.; Uchida, Y.; Takemae, N.; Kanehira, K.; Tsunekuni, R. Intracontinental and intercontinental dissemination of Asian H5 highly pathogenic avian influenza virus (clade 2.3.4.4) in the winter of 2014–2015. Rev. Med. Virol. 2015, 25, 388–405. [Google Scholar] [CrossRef]
- Aeffner, F.; Woods, P.S.; Davis, I.C. Ecto-5’-nucleotidase CD73 modulates the innate immune response to influenza infection but is not required for development of influenza-induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L1313–L1322. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef]
- Woods, P.S.; Doolittle, L.M.; Hickman-Davis, J.M.; Davis, I.C. ATP catabolism by tissue nonspecific alkaline phosphatase contributes to development of ARDS in influenza-infected mice. Am. J. Physiol -Lung Cell. Mol. Physiol. 2018, 314, L83–L92. [Google Scholar] [CrossRef]
- Leyva-Grado, V.H.; Ermler, M.E.; Schotsaert, M.; Gonzalez, M.G.; Gillespie, V.; Lim, J.K.; García-Sastre, A. Contribution of the Purinergic Receptor P2X7 to Development of Lung Immunopathology during Influenza Virus Infection. mBio 2017, 8, e00229-17. [Google Scholar] [CrossRef]
- Weinberg, A.; Muresan, P.; Richardson, K.M.; Fenton, T.; Dominguez, T.; Bloom, A.; Watts, D.H.; Abzug, M.J.; Nachman, S.A.; Levin, M.J. Determinants of vaccine immunogenicity in HIV-infected pregnant women: Analysis of B and T cell responses to pandemic H1N1 monovalent vaccine. PLoS ONE 2015, 10, e0122431. [Google Scholar] [CrossRef]
- Weinberg, A.; Muresan, P.; Richardson, K.; Fenton, T.; Dominguez, T.; Bloom, A.; Watts, D.H.; Abzug, M.J.; Nachman, S.A.; Levin, M.J. Heterogeneity of T Cell Responses to Pandemic pH1N1 Monovalent Vaccine in HIV-Infected Pregnant Women. AIDS Res. Hum. Retrovir. 2015, 31, 1170–1177. [Google Scholar] [CrossRef]
- Mathew, A.; Rothman, A.L. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol. Rev. 2008, 225, 300–313. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef]
- Malhão, T.A.; Resende, C.M.; Gamerman, D.; Medronho Rde, A. [A Bayesian model to investigate excess mortality during the dengue epidemic in Greater Metropolitan Rio de Janeiro, Brazil, in 2007–2008]. Cad. Saude Publica 2013, 29, 2057–2070. [Google Scholar] [CrossRef]
- Corrêa, G.; Lindenberg, C.d.A.; Fernandes-Santos, C.; Gandini, M.; Paiva, F.P.; Coutinho-Silva, R.; Kubelka, C.F. The purinergic receptor P2X7 role in control of Dengue virus-2 infection and cytokine/chemokine production in infected human monocytes. Immunobiology 2016, 221, 794–802. [Google Scholar] [CrossRef]
- Patkar, C.; Giaya, K.; Libraty, D.H. Dengue virus type 2 modulates endothelial barrier function through CD73. Am. J. Trop. Med. Hyg. 2013, 88, 89. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Chen, G.; Zhu, N.; Wu, D.; Wu, Y.; James, T.D. Recent progresses and remaining challenges for the detection of Zika virus. Med. Res. Rev. 2021, 41, 2039–2108. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Novella, I.S.; Teng, M.N.; Oldstone, M.B.; de La Torre, J.C. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J. Virol. 2000, 74, 3470–3477. [Google Scholar] [CrossRef] [PubMed]
- Oldstone, M.B.A.; Ahmed, R.; Byrne, J.; Buchmeier, M.J.; Riviere, Y.; Southern, P. Virus and immune responses: Lymphocytic choriomeningitis virus as a prototype model of viral pathogenesis. Br. Med. Bull. 1985, 41, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Bonthius, D.J. Lymphocytic choriomeningitis virus: An underrecognized cause of neurologic disease in the fetus, child, and adult. Semin. Pediatr. Neurol. 2012, 19, 89–95. [Google Scholar] [CrossRef]
- Schøller, A.S.; Nazerai, L.; Christensen, J.P.; Thomsen, A.R. Functionally Competent, PD-1(+) CD8(+) Trm Cells Populate the Brain Following Local Antigen Encounter. Front. Immunol. 2020, 11, 595707. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef]
- Hatmal, M.m.M.; Alshaer, W.; Al-Hatamleh, M.A.; Hatmal, M.; Smadi, O.; Taha, M.O.; Oweida, A.J.; Boer, J.C.; Mohamud, R.; Plebanski, M. Comprehensive structural and molecular comparison of spike proteins of SARS-CoV-2, SARS-CoV and MERS-CoV, and their interactions with ACE2. Cells 2020, 9, 2638. [Google Scholar] [CrossRef]
- Masters, P.S. Coronavirus genomic RNA packaging. Virology 2019, 537, 198–207. [Google Scholar] [CrossRef]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe covid-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292.e286. [Google Scholar] [CrossRef]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.X.; Gong, L.; et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef]
- Ulrich, H.; Pillat, M.M. CD147 as a target for COVID-19 treatment: Suggested effects of azithromycin and stem cell engagement. Stem Cell Rev. Rep. 2020, 16, 434–440. [Google Scholar] [CrossRef]
- da Silva, G.B.; Manica, D.; da Silva, A.P.; Kosvoski, G.C.; Hanauer, M.; Assmann, C.E.; Simões, J.L.B.; Pillat, M.M.; de Lara, J.D.; Marafon, F.; et al. High levels of extracellular ATP lead to different inflammatory responses in COVID-19 patients according to the severity. J. Mol. Med. 2022, 100, 645–663. [Google Scholar] [CrossRef]
- Romão, P.R.; Teixeira, P.C.; Schipper, L.; da Silva, I.; Santana Filho, P.; Júnior, L.C.R.; Peres, A.; Gonçalves da Fonseca, S.; Chagas Monteiro, M.; Lira, F.S.; et al. Viral load is associated with mitochondrial dysfunction and altered monocyte phenotype in acute severe SARS-CoV-2 infection. Int. Immunopharmacol. 2022, 108, 108697. [Google Scholar] [CrossRef]
- Kas-Deelen, A.; Bakker, W.; Olinga, P.; Visser, J.; De Maar, E.; Van Son, W.; The, T.; Harmsen, M.J. Cytomegalovirus infection increases the expression and activity of ecto-ATPase (CD39) and ecto-5′ nucleotidase (CD73) on endothelial cells. FEBS Lett. 2001, 491, 21–25. [Google Scholar] [CrossRef]
- Schwele, S.; Fischer, A.M.; Brestrich, G.; Wlodarski, M.W.; Wagner, L.; Schmueck, M.; Roemhild, A.; Thomas, S.; Hammer, M.H.; Babel, N.; et al. Cytomegalovirus-specific regulatory and effector T cells share TCR clonality--possible relation to repetitive CMV infections. Am. J. Transpl. 2012, 12, 669–681. [Google Scholar] [CrossRef]
- De Rossi, A.; Calabro, M.L.; D’Andrea, E.; Panozzo, M.; Saggiori, D.; Mammano, F.; Roncella, S.; Ferrarini, M.; Chieco-Bianchi, L. Morphological and phenotypical changes in EBV positive lymphoblastoid cells infected by HIV-1. Leukemia 1992, 6 (Suppl. S3), 38s–40s. [Google Scholar]
- Falk, M.H.; Trauth, B.C.; Debatin, K.M.; Klas, C.; Gregory, C.D.; Rickinson, A.B.; Calender, A.; Lenoir, G.M.; Ellwart, J.W.; Krammer, P.H.; et al. Expression of the APO-1 antigen in Burkitt lymphoma cell lines correlates with a shift towards a lymphoblastoid phenotype. Blood 1992, 79, 3300–3306. [Google Scholar] [CrossRef] [PubMed]
- Auclair, H.; Ouk-Martin, C.; Roland, L.; Santa, P.; Al Mohamad, H.; Faumont, N.; Feuillard, J.; Jayat-Vignoles, C. EBV Latency III-Transformed B Cells Are Inducers of Conventional and Unconventional Regulatory T Cells in a PD-L1-Dependent Manner. J. Immunol. 2019, 203, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Dadaglio, G.; Oberkampf, M.; Di Carlo, S.; Peduto, L.; Laubreton, D.; Desrues, B.; Sun, C.M.; Montagutelli, X.; Leclerc, C. B cells promote tumor progression in a mouse model of HPV-mediated cervical cancer. Int. J. Cancer 2016, 139, 1358–1371. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hoya, A.; Zerecero-Carreón, O.; Valle-Mendiola, A.; Moreno-Lafont, M.; López-Santiago, R.; Weiss-Steider, B.; Soto-Cruz, I. Cervical Cancer Cells Express Markers Associated with Immunosurveillance. J. Immunol. Res. 2019, 2019, 1242979. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.N.; Zhang, N.; Liu, H.H.; Xia, P.; Zhang, C.; Song, J.W.; Fan, X.; Shi, M.; Jin, L.; Zhang, J.Y.; et al. Skewed CD39/CD73/adenosine pathway contributes to B-cell hyperactivation and disease progression in patients with chronic hepatitis B. Gastroenterol. Rep. 2021, 9, 49–58. [Google Scholar] [CrossRef]
- Schottstedt, V.; Blümel, J.; Burger, R.; Drosten, C.; Gröner, A.; Gürtler, L.; Heiden, M.; Hildebrandt, M.; Jansen, B.; Montag-Lessing, T.; et al. Human Cytomegalovirus (HCMV)—Revised. Transfus. Med. Hemother. 2010, 37, 365–375. [Google Scholar] [CrossRef]
- Hosie, L.; Pachnio, A.; Zuo, J.; Pearce, H.; Riddell, S.; Moss, P. Cytomegalovirus-Specific T Cells Restricted by HLA-Cw*0702 Increase Markedly with Age and Dominate the CD8(+) T-Cell Repertoire in Older People. Front. Immunol. 2017, 8, 1776. [Google Scholar] [CrossRef]
- Soldan, S.S.; Lieberman, P.M. Epstein–Barr virus and multiple sclerosis. Nat. Rev. Microbiol. 2022. [Google Scholar] [CrossRef]
- Thorley-Lawson, D.A. EBV Persistence--Introducing the Virus. Curr. Top. Microbiol. Immunol. 2015, 390, 151–209. [Google Scholar] [CrossRef]
- Young, L.S.; Yap, L.F.; Murray, P.G. Epstein-Barr virus: More than 50 years old and still providing surprises. Nat. Rev. Cancer 2016, 16, 789–802. [Google Scholar] [CrossRef]
- Nie, K.; Zheng, G.G.; Zhang, X.J.; Lin, Y.M.; Wang, L.; Li, G.; Song, Y.H.; Wu, K.F. CD 39-associated high ATPase activity contribute to the loss of P 2 X 7-mediated calcium response in LCL cells. Leuk. Res. 2005, 29, 1325–1333. [Google Scholar] [CrossRef]
- Simoni, Y.; Becht, E.; Li, S.; Loh, C.Y.; Yeong, J.P.S.; Lim, T.K.H.; Takano, A.; Tan, D.S.W.; Newell, E.W. Partial absence of PD-1 expression by tumor-infiltrating EBV-specific CD8(+) T cells in EBV-driven lymphoepithelioma-like carcinoma. Clin. Transl. Immunol. 2020, 9, e1175. [Google Scholar] [CrossRef]
- Brianti, P.; De Flammineis, E.; Mercuri, S.R. Review of HPV-related diseases and cancers. New Microbiol. 2017, 40, 80–85. [Google Scholar]
- zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef]
- Stoler, M.H.; Rhodes, C.R.; Whitbeck, A.; Wolinsky, S.M.; Chow, L.T.; Broker, T.R. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum. Pathol. 1992, 23, 117–128. [Google Scholar] [CrossRef]
- de Lourdes Mora-García, M.; López-Cisneros, S.; Gutiérrez-Serrano, V.; García-Rocha, R.; Weiss-Steider, B.; Hernández-Montes, J.; Sánchez-Peña, H.I.; Ávila-Ibarra, L.R.; Don-López, C.A.; Muñóz-Godínez, R.; et al. HPV-16 Infection Is Associated with a High Content of CD39 and CD73 Ectonucleotidases in Cervical Samples from Patients with CIN-1. Mediat. Inflamm. 2019, 2019, 4651627. [Google Scholar] [CrossRef]
- Duurland, C.L.; Santegoets, S.J.; Abdulrahman, Z.; Loof, N.M.; Sturm, G.; Wesselink, T.H.; Arens, R.; Boekestijn, S.; Ehsan, I.; van Poelgeest, M.I.E.; et al. CD161 expression and regulation defines rapidly responding effector CD4+ T cells associated with improved survival in HPV16-associated tumors. J. Immunother. Cancer 2022, 10, e003995. [Google Scholar] [CrossRef]
- Schaefer, S. Hepatitis B virus taxonomy and hepatitis B virus genotypes. World J. Gastroenterol. 2007, 13, 14–21. [Google Scholar] [CrossRef]
- Liang, T.J. Hepatitis B: The virus and disease. Hepatology 2009, 49 (Suppl. S5), S13–S21. [Google Scholar] [CrossRef]
- Ravimohan, S.; Tamuhla, N.; Nfanyana, K.; Ni, H.; Steenhoff, A.P.; Gross, R.; Weissman, D.; Bisson, G.P. Elevated Pre-Antiretroviral Therapy CD39+CD8+ T Cell Frequency Is Associated with Early Mortality in Advanced Human Immunodeficiency Virus/Tuberculosis Co-infection. Clin. Infect. Dis. 2017, 64, 1453–1456. [Google Scholar] [CrossRef]
- Angerami, M.T.; Suarez, G.V.; Vecchione, M.B.; Laufer, N.; Ameri, D.; Ben, G.; Perez, H.; Sued, O.; Salomón, H.; Quiroga, M.F. Expansion of CD25-Negative Forkhead Box P3-Positive T Cells during HIV and Mycobacterium tuberculosis Infection. Front. Immunol. 2017, 8, 528. [Google Scholar] [CrossRef] [PubMed]
| Virus | Family | Virology | Main Findings Related to CD39+ Cells | References |
|---|---|---|---|---|
| HIV | Retroviridae |
|
| [21,38] |
| [39] | |||
| [40] | |||
| [41] | |||
| [42] | |||
| HCV | Flaviviridae |
|
| [43,44] |
| [21] | |||
| [44] | |||
| [45] | |||
| HEV | Hepeviridae |
|
| [46] |
| [46] | |||
| HDV | The Deltaviridae genus does not belong to a known family |
|
| [47] |
| Influenza | Orthomyxoviridae |
|
| [48,49] |
| Dengue virus | Flaviviridae |
|
| [50] |
| Zika virus | Flaviviridae |
|
| [51] |
| Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) | Coronaviridae |
|
| [52] |
| [53] | |||
| [54] | |||
| [55] |
| Virus | Family | Virology | Main Findings Related to CD39+ Cells | References |
|---|---|---|---|---|
| CMV | Herpesviridae |
| ↑ expression of CD39 on endothelial cells → regulates the platelet function and inflammation | [119] |
| ↑ expression on CD25+ Treg cells → recurrence and/or latent infection | [120] | |||
| EBV | Herpesviridae |
| Expressed on EBV-infected cancer B cells, Burkitt lymphoma cells, and LCL | [3,121,122] |
| EBV ↑ CD39+ Treg cells | [123] | |||
| HPV | Papillomaviridae |
| ↑ expression of CD39 on the B cells inside the tumor | [124] |
| ↑ expression of CD39 and CD73 on cervical cancer cells infected with HPV → immune escape | [125] | |||
| HBV | Hepadnaviridae |
| A lower percentage of CD39+ Treg cells in HBV-infected patients than in healthy controls | [29] |
| The expression CD39/CD73 in B cells is negatively associated with liver inflammation | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsaghir, A.; El-Sabaa, E.M.W.; Ahmed, A.K.; Abdelwahab, S.F.; Sayed, I.M.; El-Mokhtar, M.A. The Role of Cluster of Differentiation 39 (CD39) and Purinergic Signaling Pathway in Viral Infections. Pathogens 2023, 12, 279. https://doi.org/10.3390/pathogens12020279
Elsaghir A, El-Sabaa EMW, Ahmed AK, Abdelwahab SF, Sayed IM, El-Mokhtar MA. The Role of Cluster of Differentiation 39 (CD39) and Purinergic Signaling Pathway in Viral Infections. Pathogens. 2023; 12(2):279. https://doi.org/10.3390/pathogens12020279
Chicago/Turabian StyleElsaghir, Alaa, Ehsan M. W. El-Sabaa, Abdulrahman K. Ahmed, Sayed F. Abdelwahab, Ibrahim M. Sayed, and Mohamed A. El-Mokhtar. 2023. "The Role of Cluster of Differentiation 39 (CD39) and Purinergic Signaling Pathway in Viral Infections" Pathogens 12, no. 2: 279. https://doi.org/10.3390/pathogens12020279
APA StyleElsaghir, A., El-Sabaa, E. M. W., Ahmed, A. K., Abdelwahab, S. F., Sayed, I. M., & El-Mokhtar, M. A. (2023). The Role of Cluster of Differentiation 39 (CD39) and Purinergic Signaling Pathway in Viral Infections. Pathogens, 12(2), 279. https://doi.org/10.3390/pathogens12020279







