Abstract
Viral infections can be a serious complication of therapy in children with acute lymphoblastic leukemia (ALL). In this study, we focused on the incidence and the profile of viral infection in children with ALL treated in 17 pediatric oncology centers in Poland in the two-year periods of 2018–2019 and 2020–2021. We also compared the frequency of viral infections in 2018–2019 to that in 2020–2021. In 2020–2021, a total of 192 children with ALL had a viral infection during intensive chemotherapy. A total number of 312 episodes of viral infections were diagnosed. The most common infections detected in the samples were: COVID-19 (23%), rhinovirus (18%), and respiratory syncytial virus (14%). COVID-19 and BK virus infections were the reason for the death 1% of all patients. In 2018–2019, a total of 53 ALL patients who had a viral infection were reported and 72 viral events were observed, mainly adenovirus (48.6%), rotavirus (31.9%), and herpes zoster (8.3%). No deaths were reported during this period. The cumulative incidence of viral infections in 2018–2019 was 10.4%, while for 2020–2021, it was 36.7%. In conclusion, a high incidence of COVID-19 infection was observed among pediatric patients with ALL in Poland. The mortality rate in our material was low. The viral profile in ALL children undergoing chemotherapy can be useful for clinicians to improve prophylactic and therapeutic strategies.
1. Introduction
Viral infections can be a serious complication of therapy in children with acute lymphoblastic leukemia (ALL). There are many factors such as the disease itself, intensive chemotherapy and steroid therapy, and prolonged neutropenia that make ALL pediatric patients vulnerable to them [1]. Additionally, coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) β-coronavirus has increased the risk of mortality in children with malignancies [2]. Patients who tested positive were either isolated at home or were directed to specialized units of infectious diseases if they were symptomatic or required medical treatment [2,3]. The European Centre for Disease Prevention and Control (ECDC) developed guidelines for COVID-19 isolation that were issued September 2, 2020 [4]. New therapeutic approaches such as monoclonal antibodies in childhood ALL can result in an improved prognosis, but on the other hand, can also increase susceptibility to viral infections [1]. Therefore, the prevention and improvement of supportive care, as well as the assessment of the incidence of infectious complications, are of great importance for pediatric patients with ALL [1,5,6].
The aim of the study was to analyze the incidence and the profile of viral infection in 1021 children with newly diagnosed acute lymphoblastic leukemia treated in 17 pediatric oncology centers in Poland in the two-year periods of 2018–2019 and 2020–2021. Additionally, we also compared the frequency of viral infections in 2018–2019 to that in 2020–2021.
2. Materials and Methods
Children with ALL received conventional chemotherapy in accordance with the therapeutic protocol in force in Poland. The study was approved by the Ethics Committee of Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun, Poland.
2.1. Definition
Testing for viruses was conducted in ALL patients with clinical symptoms of infection. Viral infections were described when the virus was confirmed by the polymerase chain reaction (PCR) method in material derived from blood (cytomegalovirus, CMV; Epstein–Barr virus, EBV; adenovirus, ADV; coronaviruses OC43 and HKU1; and parvovirus B19), urine (BKV or ADV), stool (ADV, rotavirus, norovirus, sapovirus, or astrovirus) or cerebrospinal fluid (CMV, EBV, or HHV6). Respiratory syncytial virus (RSV), parainfluenza virus (PIF), influenza (FLU), metapneumovirus (MPV), rhinovirus (HRV), bocavirus (HboV) were detected using PCR from respiratory swabs or bronchoalveolar lavage. In all pediatric oncology centers in Poland, the detection of respiratory viruses is conducted using multiplex PCR, while singleplex PCR is used for other viral infections such as CMV, EBV, BKV, and ADV. Nasopharynx swabs were used as the material for virus testing, and PCR tests were performed to diagnose SARS-CoV-2 replication [3,7,8,9].
Viral infections were identified as sporadic and latent. The following sporadic viral infections were analyzed: influenza, PIF, MPV, RSV, ADV, coronaviruses, HRV, rotavirus, norovirus, sapovirus, astroviruses, poxvirus, and bocavirus. Many viruses such as EBV, BKV, VZV, HHV6, HSV, and CMV tend to cause latent infections. Herpesviruses infections were reported when clinical symptoms occurred (HSV or VZV) or viremia was diagnosed by PCR (CMV, EBV, or HHV6) [7,10].
2.2. Supportive Care
Supportive therapy was administered in accordance with institutional standards and treatment protocol recommendations. Children undergoing chemotherapy received oral co-trimoxazole and oral fluconazole. Oral acyclovir was used in patients who were in contact with persons sustaining varicella or a zoster infection and HSV. Additionally, the recombinant human granulocyte colony-stimulating factor (Rh-G-CSF) was managed during sepsis in the neutropenic phase or in high-risk pediatric patients. Preparations of immunoglobulin were used in the case of a decreased immunoglobulin concentration during infection in neutropenic patients. Children in the period of neutropenia were on a low-bacterial diet and kept under strengthened hygiene processes. There was no specific BKV prophylaxis; patients were monitored weekly [7,11].
Patients with a SARS-CoV-2 infection were separated; their isolation times depended on applicable laws in Poland that have changed during the time of the pandemic [3]. Additionally, Poland adopted the European Centre for Disease Prevention and Control (ECDC) guidelines on the ending of COVID-19 isolation [4]. Additionally, education and vaccination of all family members and healthcare professionals working with immunocompromised patients were very important [9].
2.3. Statistical Analysis
An analysis was conducted in R statistical software (version 4.2.2); α = 0.05. Respondents were divided into two groups: patients with one infection and patients with more than one infection. Dependencies between groups and other qualitative variables were analyzed using a chi-squared test or Fisher’s exact test. Quantitative variables were compared between two groups using a Mann–Whitney U test. Frequencies for all infections as well as the percentage of patients that died due to the infections are shown in the tables below. Some analyses were done using the number of infections observed in the sample (n = 312) and some using the number of patients (n = 192). Additionally, Kaplan–Meier survival curves for the cumulative incidence of viral infections were prepared and included a comparison between the periods of 2018–2019 and 2020–2021 based on a chi-squared log-rank test.
3. Results
For 2020–2021, the analysis included 510 children with initial ALL aged 1 to 18 years (median 4.62 years) at the time of diagnosis. A total number of 192/510 (37.5%) children experienced a viral infection during therapy for ALL. Among the analyzed group, 312 episodes of viral infections were diagnosed and ranged from 1 to 5 per person. The most common infections detected in the samples were as follows: COVID-19 infection (23%), rhinovirus infection (18%), and respiratory syncytial virus (RSV) infection (14%) (Table 1).

Table 1.
Characteristics of patients with ALL who experienced 1 and >1 episode of a viral infection (2020–2021).
Most of the patients (63.5%) had one episode of a viral infection. Characteristics of patients who had reported 1 and >1 episode are presented in Table 1. Patients with one or more than one event of viral infection did not differ in the proportion of females in both groups. Both groups did not differ in time from age of patient to date of ALL diagnosis. Two patients died due to these infections (1% of total group). There was no significant correlation between death and the number of infections (p > 0.999). Both patients died due to a COVID-19 infection, but one of those patients also had a BKV (BK virus) infection. The time from diagnosis to death for a patient who died due to COVID-19 only was 53 days; for the second patient, the time from diagnosis of BKV to death was 14 days, while that from diagnosis of COVID-19 to death was 51 days.
In 2018–2019, a total number of 53/511 (10.4%) of the ALL patients were reported to have a viral infection during chemotherapy. When analyzing this group, 72 viral infection events were observed, mainly ADV (48.6%), rotavirus (31.9%), and herpes zoster (8.3%). No deaths were reported during this period. The comparison of 2018–2019 and 2020–2021 revealed significant differences in the frequency of different types of viruses (Table 2).

Table 2.
The distribution of viral infection episodes and the comparison of frequency of types of viruses between 2018–2019 and 2020–2021.
The cumulative incidence of viral infections in 2018–2019 (53 patients, 72 infections) was 10.4% CI95 [7.7%; 13.0%], while for 2020–2021 (192 patients, 312 infections), it was 36.7% CI95 [32.4%; 40.7%]; p (log-rank) < 0.001. The cumulative incidence of viral infections is illustrated in Figure 1.
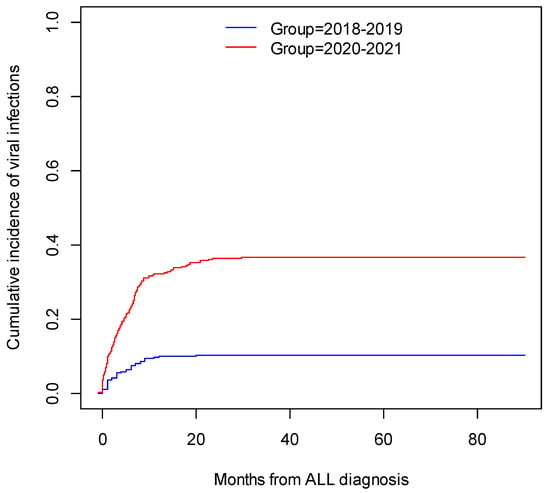
Figure 1.
The cumulative incidence of viral infections in patients with ALL.
4. Discussion
Currently, the overall survival of children with ALL has improved, with Jeha’s recent trial demonstrating 96% [12]. This approach was made possible by several factors such as new diagnostic and therapeutic methods, the evaluation of minimal residual disease, and supportive care. However, the toxicities of chemotherapy, infections leading to the interruption of chemotherapy, and prolonged hospitalization are still the causes of death in children with ALL [13,14,15,16].
O’Connor et al. reported 75 patients with ALL and infection-related mortality (IRM) in the United Kingdom Childhood Acute Lymphoblastic Leukaemia Randomised Trial 2003 (UKALL 2003). In this study, the 5-year cumulative incidence of IRM was 2.4% (95% confidence interval (CI): 1.9–3.0%), accounting for 75 (30%) of 249 deaths. Seven (12%) children died due to the viral infection (adenovirus 3/43%, RSV 2/28%, and VZV 2/29%) [17].
In the Inaba trial, the cumulative risk of infection-related mortality was low (1.0%) compared to other studies (1.7–2.4%), although different therapeutic protocols made it difficult to directly compare [5,6,18].
Katsimpardi et al. described 58 pediatric cases (9.5% of the total) of specific viral infections. Of those, varicella-zoster virus (VZV) infections were the most common diagnosis (26 cases, 45%) followed by HSV infection (25 cases, 43%). Coxsackie virus, Epstein–Barr virus, and mumps virus were confirmed in two cases each (3% each), and one case of parvovirus B19 infection (2%) was diagnosed. No patient died due to a viral infection [19].
In our previous study published in 2019, we also reported on an analysis of viral episodes in pediatric patients with ALL during 2012–2017. A total of 5/251 (2%) children died due to a viral infection (CMV—2 cases, AH1N1—2 cases, and RSV—1 case) [11]. In this previous study, the cumulative incidence was analyzed over two-year periods and we compared these results to those from 2020–2021. The highest frequency of viral infections was observed in the analyzed period of 2020–2021. The cumulative incidence of viral infections in 2020–2021 was over threefold higher than in 2018–2019. This was associated with the emergence of COVID-19 infections, which moreover resulted in the death of two (1%) children. Additionally, in 2020–2021, patients were often admitted to the oncology centers in advanced disease states complicated by infections (viral, bacterial, or fungal) due to fewer visits to primary health providers and the reluctance of families to expose their children to the virus, which delayed the final diagnosis. Moreover, monoclonal antibodies were introduced for the treatment of ALL in Poland at that time, which also increased the frequency of viral infections.
In the other study, the authors presented epidemiology and deaths in children with malignancies and COVID-19 at a reference hospital in Recife, Brazil. A total of 48 children were included, mostly with leukemia (64.5%). The patients had a delay in chemotherapy in the presence of a COVID-19 infection with a median of 15 days in leukemia and four (12.9%) patients that died, mainly during the induction phase of therapy. The authors emphasized that early mortality was less than 2% in cancer patients in the last five years. However, COVID-19 infections increased the death rate more than tenfold [20].
Rojas et al. described 15 pediatric patients with cancer and COVID-19 infections that included 8 children with ALL. Chemotherapy was interrupted in one child but no serious adverse events were observed. The authors reported that the COVID-19 infection rate was 1.3% among this pediatric patients over the first 2 months of the pandemic [21].
In the presented study, we observed that COVID-19 infections were predominant in the last two years. In Poland, guidelines related to vaccination children during chemotherapy and after stem cell transplantation have been in place since May 2021. Pediatric patients above the age of 12 years could be vaccinated 3 to 7 days after chemotherapy to minimize severe adverse events from COVID-19 and delays in cancer treatment [22].
It is known that viral infections that occur during ALL chemotherapy can cause serious consequences. This is why it is important to reduce the risk of morbidity and mortality via early initiation of antiviral therapy and by educating parents regarding hygiene measures. Education and vaccination (for seasonal influenza, varicella, COVID-19, etc.) of families and medical staff who deal with immunocompromised patients are particularly essential.
5. Conclusions
In conclusion, a high incidence of COVID-19 infections was observed among pediatric patients with ALL in Poland. The mortality rate in our cohort was low (1%). The viral profile in ALL children undergoing chemotherapy can be useful for clinicians to improve prophylactic and therapeutic strategies.
Author Contributions
Conceptualization, J.Z.; data curation, K.D., K.C., M.D., K.J., P.Z.-W., A.S.-B., Ł.H., M.M., W.C., W.B. (Walentyna Balwierz), I.Ż., M.S.-B., B.K., G.W., K.K., R.T., T.S., O.Z.-S., J.W., M.P., M.K.-R., A.K., T.O., T.U., F.P., W.M., J.U.-R., K.M. (Katarzyna Machnik), S.P., W.B. (Wanda Badowska), T.B., K.M. (Katarzyna Mycko), H.M.-G., A.U.-D., G.K., A.M.-M., W.S., K.S.-K., J.M., R.C., N.I.-J. and E.B.; writing—original draft preparation, J.Z.; visualization, J.Z.; supervision, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moschovi, M.; Adamaki, M.; Vlahopoulos, S.A. Progress in Treatment of Viral Infections in Children with Acute Lymphoblastic Leukemia. Oncol. Rev. 2016, 10, 300. [Google Scholar] [CrossRef]
- Węcławek-Tompol, J.; Zakrzewska, Z.; Gryniewicz-Kwiatkowska, O.; Pierlejewski, F.; Bień, E.; Zaucha-Prażmo, A.; Zając-Spychała, O.; Szmydki-Baran, A.; Mizia-Malarz, A.; Bal, W.; et al. COVID-19 in pediatric cancer patients is associated with treatment interruptions but not with short-term mortality: A Polish national study. J. Hematol. Oncol. 2021, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Regulation of the Minister of Health. 2020. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20200001506/O/D20201506.pdf (accessed on 2 September 2020).
- Guidance for Discharge and Ending Isolation of People with COVID-19—Second Update. 2020. Available online: https://www.ecdc.europa.eu/sites/default/fles/documents/Guidance-for-discharge-and-ending-of-isolation-of-people-with-COVID-19.pdf (accessed on 16 May 2021).
- Inaba, H.; Pei, D.; Wolf, J.; Howard, S.C.; Hayden, R.T.; Go, M.; Varechtchouk, O.; Hahn, T.; Buaboonnam, J.; Metzger, M.L.; et al. Infection-related complications during treatment for childhood acute lymphoblastic leukemia. Ann. Oncol. 2017, 28, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Styczynski, J.; Czyzewski, K.; Zalas-Wiecek, P.; Gryniewicz-Kwiatkowska, O. Profile of infections in Polish pediatric hematology, oncology and stem cell transplantation centers in 2014-2015. Post. N. Med. 2016, 8, 555–560. [Google Scholar] [CrossRef]
- Styczyński, J. Infectious complications in children and adults with hematological malignancies. Acta Haematol. Pol. 2019, 50, 167–173. [Google Scholar] [CrossRef][Green Version]
- Styczynski, J.; Czyzewski, K.; Wysocki, M.; Gryniewicz-Kwiatkowska, O.; Kolodziejczyk-Gietka, A.; Salamonowicz, M.; Hutnik, L.; Zajac-Spychala, O.; Zaucha-Prazmo, A.; Chelmecka-Wiktorczyk, L.; et al. Increased risk of infections and infection-related mortality in children undergoing haematopoietic stem cell transplantation compared to conventional anticancer therapy: A multicentre nationwide study. Clin. Microbiol. Infect. 2016, 22, 179.e1–179.e10. [Google Scholar] [CrossRef]
- Cesaro, S.; Ljungman, P.; Mikulska, M.; Hirsch, H.; Lilienfeld-Toal, M.; Cordonnier, C.; Meylan, S.; Mehra, V.; Styczynski, J.; Marchesi, F.; et al. Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference on Infections in Leukaemia (ECIL 9). Leukemia 2022, 36, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Styczynski, J. ABC of viral infections in hematology: Focus on herpesviruses. Acta Haematol. Pol. 2019, 50, 159–166. [Google Scholar] [CrossRef][Green Version]
- Zawitkowska, J.; Drabko, K.; Szmydki-Baran, A.; Zaucha-Prazmo, A.; Lejman, M.; Czyzewski, K.; Zalas-Wiecek, P.; GryniewiczeKwiatkowska, O.; Czajnska-Deptuła, A.; Kulicka, E.; et al. Infectious profile in children with ALL during chemotherapy: A report of study group for infections. J. Infect. Chemother. 2019, 25, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Jeha, S.; Pei, D.; Choi, J.; Cheng, C.; Sandlund, J.T.; Coustan-Smith, E.; Campana, D.; Inaba, H.; Rubnitz, J.E.; Ribeiro, R.C.; et al. Improved CNS Control of Childhood Acute Lymphoblastic Leukemia Without Cranial Irradiation: St Jude Total Therapy Study 16. J. Clin. Oncol. 2019, 37, 3377–3391. [Google Scholar] [CrossRef] [PubMed]
- Kuhlen, M.; Klusmann, J.H.; Hoell, J.I. Molecular Approaches to Treating Pediatric Leukemias. Front. Pediatr. 2019, 7, 368. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved Survival for Children and Adolescents with Acute Lymphoblastic Leukemia Between 1990 and 2005: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 1663–1669. [Google Scholar] [CrossRef]
- Pui, C.H.; Yang, J.J.; Hunger, S.P.; Pieters, R.; Schrappe, M.; Biondi, A.; Vora, A.; Baruchel, A.; Silverman, L.B.; Schmiegelow, K.; et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. J. Clin. Oncol. 2015, 33, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Schmiegelow, K.; Muller, K.; Mogensen, S.S.; Mogensen, P.R.; Wolthers, B.O.; Stoltze, U.K.; Tuckuviene, R.; Frandsen, T. Noninfectious chemotherapy-associated acute toxicities during childhood acute lymphoblastic leukemia therapy. F1000Research 2017, 6, 444. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Bate, J.; Wade, R.; Clack, R.; Dhir, S.; Hough, R.; Vora, A.; Goulden, N.; Samarasinghe, S. Infection-related mortality in children with acute lymphoblastic leukemia: An analysis of infectious deaths on UKALL2003. Blood 2014, 124, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Pui, C.H. Advances in the Diagnosis and Treatment of Pediatric Acute Lymphoblastic Leukemia. J. Clin. Med. 2021, 10, 1926. [Google Scholar] [CrossRef]
- Katsimpardi, K.; Papadakis, V.; Pangalis, A.; Parcharidou, A.; Panagiotou, J.P.; Soutis, M.; Papandreou, E.; Polychronopoulou, S.; Haidas, S. Infections in a pediatric patient cohort with acute lymphoblastic leukemia during the entire course of treatment. Support. Care Cancer 2006, 14, 277–284. [Google Scholar] [CrossRef]
- Lima, A.L.M.A.; Borborema, M.C.D.; Matos, A.P.R.; Oliveira, K.M.M.; Mello, M.J.G.; Lins, M.M. COVID-19 cohort on children with cancer: Delay in treatment and increased frequency of deaths. Rev. Bras. Saúde Matern. Infant. 2021, 21 (Suppl. 1), 299–304. [Google Scholar] [CrossRef]
- Rojas, T.; Pérez-Martínez, A.; Cela, E.; Baragaño, M.; Galán, V.; Mata, C.; Peretó, A.; Madero, L. COVID-19 infection in children and adolescents with cancer in Madrid. Pediatr. Blood Cancer 2020, 67, e28397. [Google Scholar] [CrossRef] [PubMed]
- Polish Ministry of Health. Common Guidelines for Group 1B Patients and Healthcare Professionals on Vaccination against COVID-19. [Wspólne Wytyczne dla Pacjentów z Grupy 1B i Personelu Medycznego w Zakresie Szczepienia Przeciwko COVID-19.]. 2021. Available online: https://www.gov.pl/web/szczepimysie/wspolne-wytyczne-dla-pacjentow-z-grupy-1b-i-personelu-medycznego-w-zakresie-szczepienia-przeciwko-covid-19 (accessed on 27 September 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).