Abstract
Tick-borne rickettsioses represent a severe public health problem that has increased in recent decades by several activities that place human populations in contact with a wide range of vectors. In particular, Rickettsia parkeri, an eschar-associated spotted fever agent, represents an emerging pathogen that has been gradually identified throughout America. In the present work, we compiled an occurrence database of these bacteria, as well as its vectors, in order to identify the potential distribution of these bacteria and to detect the risk areas where this emerging pathogen may be circulating. The results show the at-risk areas to be broad regions in Central America, on the coast of Venezuela, Colombia, Ecuador, Peru, and Chile, part of Brazil and Argentina, and the greater part of Bolivia, Paraguay, and Uruguay. Particularly, in Mexico, conditions exist for widespread dissemination. Our results must be considered for the establishment of active acarological surveillance in previously unsampled areas, as well as the establishment of prevention measures for vulnerable populations and risk groups participating in outdoor activities that can place them in contact with this pathogen.
1. Introduction
The genus Rickettsia harbors around 15 strict intracellular bacterial pathogens that cause several emerging and reemerging diseases of great impact on public health, among which the members of the spotted fevers group (SFG) stand out. The members of the SFG are mainly transmitted by hard ticks of the genera Amblyomma, Dermacentor, Ixodes, and Rhipicephalus in America [1]. At least nine Rickettsia species of the SFG have recorded in Latin America and the Caribbean, however only five of them have been recorded recognized as human pathogens (R. africae, R. massiliae, R. parkeri, R. philipi, R. rickettsii). The species with the highest number of confirmed records per country was R. rickettsii, the causative agent of the Rocky Mountain spotted fever, followed by R. parkeri, the etiological agent of R. parkeri rickettsiosis [1,2,3].
Rickettsia parkeri was isolated from the Gulf coast tick Amblyomma maculatum in the early 1900s in the United States [4]. It was considered a non-pathogenic Rickettsia for six decades until 2004, when it was recovered from a human patient with an eschar-associated fever after a tick bite [5]. Subsequently, its epidemiological relevance increased because new human cases began to be detected in the United States, as well as in some countries of the southern cone of America (Uruguay, Argentina, and Brazil), and it is considered an emerging rickettsiosis in America [1,6,7,8,9].
Although the members of the complex Amblyomma maculatum (A. maculatum, A. triste, and A. tigrinum) are considered the main vectors of R. parkeri in America [2], active acarological surveillance identified 18 species of hard ticks infected with different genetic lineages of this Rickettsia species in eight countries. This includes the human pathogen R. parkeri strain Atlantic Rainforest in A. ovale and A. aureolatum [10,11], and others whose pathogenicities are still unknown, such as R. parkeri strain NOD39 in A. nodosum and R. parkeri strain Black Gap in Dermacentor parumapertus [1,12,13,14]. Rickettsia parkeri is recorded from the United States to Argentina, its distribution shows a discontinuous pattern, with regions absent from records probably due to the lack of surveillance, even though several vector species are included in the local faunistic inventories. These gaps in the knowledge of the real distribution of the species have important epidemiological implications because we do not know the areas where active transmission of this microorganism can occur presently. In addition, the increase in human activities (e.g., hunting, eco-tourism, expansion of sites over wilderness areas) could strongly impact the distribution and determinants of the disease caused by this species. One of the aspects that attracts interest is that the patterns of SF cases in humans have been recorded from the United States to Argentina and Uruguay, and thus far, no human case has been reported in intermediate regions where there are records of ticks infected with R. parkeri (e.g., Mexico, Belize, Colombia, and Peru) [14,15,16,17].
New technological tools exist that are based on algorithms that associate occurrences of any species with the environmental conditions that estimate and project potential geographic distributions of a variety of infectious diseases [18]. The Ecological Niche Model (ENM) is frequently used to analyze and predict spatial patterns and the distribution of vector-borne diseases. Multiple abiotic and biotic factors have been associated with the ecological niche as precipitation, temperature, altitude, latitude, physical barriers, and host distribution and abundance [19,20]. ENMs used in emerging diseases or invasive species, such as in the case of the vector Aedes albopictus [21], have also been used to validate ecological niche models of the same species in other periods [22]. In that context, and due to the importance of R. parkeri as an emerging rickettsiosis agent in America, the goal of this study was to predict the potential geographic distribution of R. parkeri, using the occurrence data of ticks infected and ENM theory in America to implement surveillance and vector control measures in the different areas.
2. Results
A total of 1479 candidate models were built for this species, while 1295 models were statistically significant. Finally, 10 candidate models met the statistically significant model omission rate and AICc criteria. The best candidate model was a model with set environmental predictors (set 2), regularization multiple (N = 4), feature classes (product), partial ROC (N = 1.06), omission rate 5% (N = 0.03), AICc (N = 2623.39), Delta AICc (N = 0.00), AICc weight (N = 0.81), and number parameters (N = 5) (Table 1).

Table 1.
Model performance under optimal parameters using regularization multiplier (RM).
The size of the M region (a set of areas that are accessible to the species) for R. parkeri was 22,819,910 km2, distributed between United States–Mexico through Central America into South America (Colombia, Venezuela, Ecuador, Peru, Brazil, Bolivia, Paraguay, Uruguay, Argentina, and Chile). Ecological Niche Modelling (ENM) for R. parkeri was suitable between 0.08–0.82. The ENM was predicted the potential geographic distribution for the United States and Mexico to Chile and Argentina, except for Central Mexico, Venezuela, Guyana, Suriname, French Guiana, part of Colombia, and Brazil. ENM of R. parkeri was also presented in Ontario, Canada. For the United States, the species was predicted in 22 states and 20 states for Mexico. Finally, the ENM was projected in Central America, the coast of Venezuela, Colombia, Ecuador, Peru, and Chile, part of Brazil and Argentina, and the greater part of Bolivia, Paraguay, and Uruguay (Figure 1).
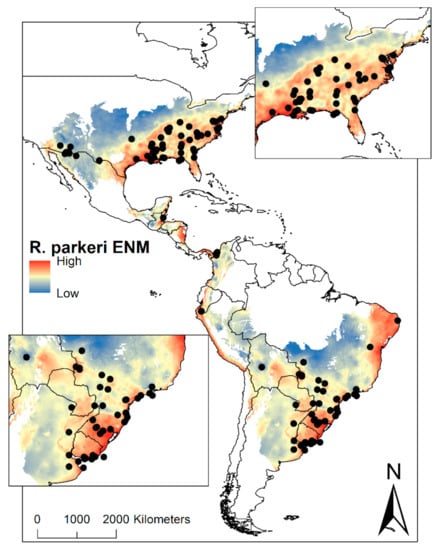
Figure 1.
Ecological niche modelling (ENM) of R. parkeri in America. On the maps, the suitability of R. parkeri from highest to lowest can be visualized. The black dots represent the occurrences of ticks infected with R. parkeri. In the upper right box, a zoom in the United States region can be visualized, while in the lower-left box, the zoom of South America can be visualized.
In the future, all the projections of the climate change scenarios (representative concentration pathways (RCPs) and shared socioeconomic pathways (SSPs)) presented similar geographic distributions. The different projections in the RCP scenarios (2.6, 4.5, 6.0, and 8.5) are similar, mainly affecting regions in Texas, Louisiana, Arkansas, Mississippi, Alabama, Florida, Georgia, South Carolina, North Carolina, and Virginia in the USA, Sonora, Nuevo Leon, Tamaulipas, Oaxaca, Veracruz, Chiapas in Mexico, Guatemala, Costa Rica, Colombia, Peru, Argentina, Uruguay, and Brazil (Figure 2). However, the projections in the SSP scenarios were much more conservative for the year 2041–2060 in the similar regions of the RCPs, mainly in United States, North of Mexico, Brazil, Argentina, and Uruguay. The current ecological niche overlap of R. parkeri with the different RCP scenarios presents lower values than with the SSP scenarios (Table 2). The comparisons between the different RCPs were similar (0.954–0.956), compared to the equality of the SSPs values between them (D = 1.000) (Table 2).
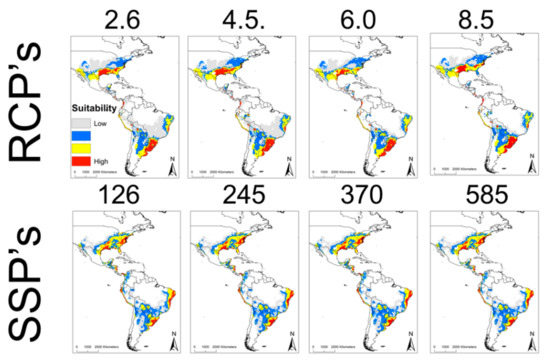
Figure 2.
Ecological niche modeling in climate change scenarios of R. parkeri in America. The upper panel represents the RCP’s scenarios, while the lower panel represents the SSP’s scenarios. The suitability of the different models is observed from lowest to highest (red). In both scenarios (RCP’s and SSP’s), foci of R. parkeri distribution can be observed in the United States and a large part of Brazil, Uruguay, and Argentina.

Table 2.
Niche overlap values using Schoener’s D index between all different times for R. parkeri in America. Niche overlap is represented from 0 (no overlap) to 1 (full overlap).
The occurrence data of R. parkeri were divided into four important strains: Black Gap (N = 4), Parkeri sensu stricto (N = 85), Atlantic Rainforest (N = 22), and NOD39 (N = 6). The Black Gap strain occurs on the United States–Mexico border, compared to the Parkeri sensu stricto strain found in the United States, Mexico, Ecuador, Peru, Brazil, Bolivia, Argentina, Paraguay, and Uruguay. The Atlantic Rainforest strain is located mainly in Mexico, Central America, Colombia, and the intersection of Bolivia, Argentina, Paraguay, and Uruguay. Finally, the NOD39 strain is located in Brazil (Figure 3).
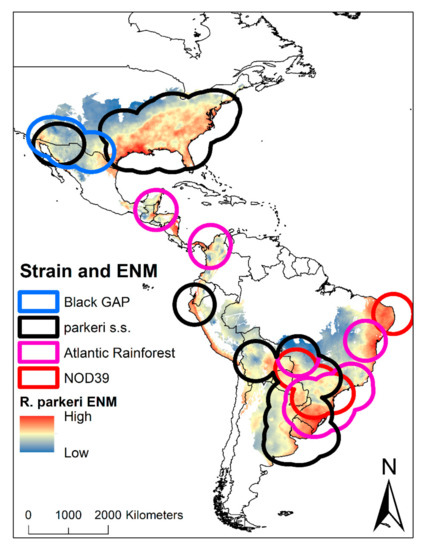
Figure 3.
Ecological niche modelling of R. parkeri in America with overlapping areas by strains. In the map, the suitability of R. parkeri can be observed, as well as the potential buffer areas with the representation of the four strains for America.
3. Discussion
In this study, we show the first database compilation on R. parkeri with an ecological analysis of its potential distribution in America. Rickettsia parkeri is an emerging pathogen that causes spotted fever rickettsiosis, which has been recorded in several species of ticks of the genus Amblyomma (A. americanum, A. aureolatum, A. dubidatum, A. longirostre, A. maculatum/A. triste, A. nodosum, A. ovale, A. parkeri, and A. tigrinum). However, it has also been detected in other hard ticks, such as Ixodes scapularis, Rhipicephalus sanguineus s.l., D. parumapertus, and D. variabilis [5,6,7,8,9,10,11,12,23]. Currently, only data from positive infection occurrences have been compared to data from other species of ticks that could be incidental. It is important to consider that several of the tick species incriminated as vectors (e.g., A. maculatum and A. nodosum), of some of these strains (Parkeri s.s. and NOD39) develop their life cycles in multiple hosts, among which are birds, which in their migratory routes could transfer and disseminate infected ticks from one zoogeographic region to another [2,3,13].
Our current data (Supplementary Table S1; Available in https://doi.org/10.6084/m9.figshare.14452680; accessed on 29 April 2021) present a new perspective of R. parkeri in the Americas, showing a wide scope of environmental factors associated with the pathogen’s distribution, as well as the most extensive revision of R. parkeri in America (N = 66 published articles). Previous reports have been isolated to nine countries: Argentina (6), Belize (1), Brazil (21), Bolivia (1), Colombia (2), Mexico (2), Peru (1), Uruguay (5) and United States (27) (Figure 1), yet our map shows geographic continuity, making it plausible for the pathogen to have a much wider distribution than expected by sanitary authorities, such as in several unsampled countries of South America (Chile, Paraguay, Ecuador, and Venezuela) and most countries of Central America. Ecological niche modelling of R. parkeri was performed for each separate strain: Black Gap, Parkeri sensu stricto, Atlantic Rainforest, and NOD39 (Figure 3). Two of these strains (Atlantic Rainforest and Parkeri s.s.) have a distribution in both the north and south of Latin America; while Black GAP is more restricted to the United States and Mexico border, and NOD39 more restricted to Brazil. It is important to highlight that the Atlantic Rainforest strain has been reported in Argentina and Brazil since 2010, while in the last six years it has been reported in Bolivia and Belize. As of 2018, migrant caravans from Honduras and El Salvador to the Mexico-United States (Available in https://rosanjose.iom.int/SITE/en) border could serve to disperse this strain to new regions in North America. Recently, in Central Mexico, there is no evidence of R. parkeri, but we must consider that there is the presence of these vectors in much of the country.
The ecological niche of R. parkeri coincides with the ecological niche of several members of the A. cajennense complex, a species not yet found with infection [24]. It also coincides with the ecological niche of infected species, such as A. americanum [25], and A. maculatum [26]. The center of Mexico, where no infection with R. parkeri exists, is also highlighted, but with areas suitable for distribution of I. scapularis [14,16,27]. Thus far, this is the first article in which different scenarios are evaluated (AR5 and AR6). Previously, ENMs were made for ticks using the RCP scenarios (2050 and 2070). However, none have used the new SSP scenarios [28]. Both scenarios range from conservative projections to very extreme projections. The potential for the increased geographic spread of R. parkeri is worrisome, regardless of scenario (RCP or SSP) both in the United States and South America. Therefore, it is important to improve epidemiological control, and surveillance measures must be implemented against R. parkeri.
4. Materials and Methods
4.1. Database and Accessible Area M
We generated a data set according to the ten tick species infected by R. parkeri: A. aureolatum (2), A. dubitatum (3), A. longirostre (1), A. maculatum (58), A. nodosum (4), A. parkeri (1), A. ovale (20), A. trigrinum (2), A. triste (22), D. parumapertus (4), Amblyomma spp (7), and Dermacentor spp. (4). The unique occurrences (N = 128) were obtained from 66 manuscripts that reported the presence of R. parkeri in Argentina (7), Belize (1), Bolivia (1), Brazil (30), Colombia (4), Mexico (1), Peru (3), Uruguay (12), and the United States (69) (Table S1; Available in https://doi.org/10.6084/m9.figshare.14452680; accessed on 29 April 2021). We eliminated duplicate occurrences and reduced effects of spatial autocorrelation by thinning records with a distance of 5 km between occurrences using the spThin R package [29]. We set aside on the data subset and split the remaining occurrences randomly into two subsets: calibration (70%) and testing (30%) using the “random k-fold” method. The latter method partitions the occurrence localities randomly into a user-specified number of (k) bins as described in detail in the protocol of Muscarella et al. [30]. The accessible area M is an important component in the biotic, abiotic and movement diagram [31]. Accessible area M represents the areas to which a species has had access over a relevant time-period because of its movement and colonizing capacities and the structure of barriers and distances [30]. We constructed an accessible area M used 200 km radius buffer around each point to extend the limits of the calibration region. This buffer was subsequently overlain on the ecoregion shapefile of World Wildlife Fund [19,32].
4.2. Bioclim Variables
Fifteen (out 19) Bioclim variables (1970–2000) were used to construct ENMs. The 15 layers were downloaded from the WorldClim database version 2.0 [33], excluding those that combined temperature (Bio8 and Bio9) and precipitation (Bio18 and Bio19) owing to known artifacts, consistent with Escobar et al. [34]. We used three sets of environmental predictors (Table 3) [22]: set 1: 15 variables from WorldClim; set 2: variables used for the construction of ENMs of species general included species of medical importance [19,35]; set 3: a jackknife process in MaxEnt used to select distinct sets of variables that contributed mostly to models (>90%) [19,22]. The ENMs were projected for the current period (1970–2000) and two scenarios: RCPs (representative concentration pathways) for year 2050 and SSPs (Shared socioeconomic pathways) for year 2041–2060. ENMs were projected using the four RCPs from the Fifth Assessment Report (AR5), representing the lowest to highest estimated greenhouse gas emissions: 2.6 (>430 ppm CO2), 4.5 (580–720 ppm CO2), 6.0 (720–1000 ppm CO2), and 8.5 (>1000 ppm CO2). Finally, ENMs were modelled using the four SSPs from the Six Assessment Report (AR6). These SSPs evaluated the four different ways, i.e., SSP126 (445.6 ppm CO2), SSP245 (602.8 ppm CO2), SSP370 (867.2 ppm CO2), and SSP585 (1135.2 ppm CO2), the world might evolve in the absence of climate policy and how different levels of climate change mitigation could be achieved when the mitigation targets of RCPs are combined with SSPs. For the climate model, we obtained the variables at the spatial resolution of 2.5 min (approximately 5 × 5 km per pixel), which is an adequate coarse resolution at which climate influences species distributions [36].

Table 3.
Set of Bioclim variables used to the construction of the ecological niche modeling for Rickettsia parkeri.
4.3. ENM
The ENM was constructed using the kuenm package in R software based in MaxEnt [24,37]. We created 1479 candidate models by combining three sets of bioclimatic variables, 17 values of regularization multiplier (0.1–1 with intervals of 0.1, 2–6 with intervals of 1, and 8 and 10), and all 29 possible combinations of five feature classes (linear = l, quadratic = q, product = p, threshold = t, and hinge = h) [24]. Regularization multiplier forces have many coefficients that are zero; therefore, a greater deviation from zero corresponds to a greater penalty in the model fit. The feature classes are the response categories that the data can belong to using n binary characteristics [37,38]. The candidate model performance and best model were selected according to the criteria proposed by Cobos et al. [24], as follows: AICc, Delta AICc, and Weight AICc. The final model was based on the criteria established by Cobos et al. [24]. After model calibration, we created final models with the selected parameter values, using all occurrences after the corresponding thinning process, with 10 bootstrap replicates with logistic outputs. To further visualize the effects of uncontrolled model projections on the future climate, we also developed predictions allowing predictions in novel climates (i.e., extrapolation with activated clamping) for a transferred-use type of extrapolation with clamping to the RCPs and SSPs [38]. We evaluated the ecological niche overlap using the Schoener D index in the ENMTools software with a range from 0 (no overlap) to 1 (total overlap) [39]. Finally, a 200 km radius buffer was created around each point to extend the limits of the occurrence points of four strains of R. parkeri.
5. Conclusions
The findings of the present study have important implications in public health because they reveal the presence of a zoonotic agent that circulates in several tick species in different localities across the Americas. Given that the members of this group have cross-reactivity in the serological tests, it is possible the patients may be infected by this species that presents with a milder rickettsiosis than those infected by R. rickettsii, the etiological agent of Rocky Mountain Spotted Fever [1,2]. For this reason, it is essential to perform an intentional search for patients infected with this eschar-associated pathogen, as well as reinforce acarological surveillance in regions with the potential presence of the pathogen to establish the degree of exposure for the human population.
Supplementary Materials
The following are available online at: https://doi.org/10.6084/m9.figshare.14452680, Table S1: Occurrence records of R. parkeri in America.
Author Contributions
Conceptualization, D.A.M.-L. and S.S.-M.; methodology, D.A.M.-L.; software, D.A.M.-L., A.C.M.d.O.-A., and S.S.-M.; validation, D.A.M.L. and S.S.-M.; formal analysis, D.A.M.-L. and S.S.-M.; investigation, D.A.M.-L., A.C.M.d.O.-A., D.R.-S., and S.S.-M.; resources, D.A.M.-L., A.C.M.d.O.-A., and S.S.-M.; data curation, D.A.M.L. and S.S.-M.; writing—original draft preparation, D.A.M.-L., A.C.M.d.O.-A., D.R.-S., and S.S.-M.; writing—review and editing, D.A.M.-L., A.C.M.d.O.-A., and S.S.-M.; visualization, D.A.M.-L. and S.S.-M.; supervision, D.A.M.-L., and S.S.-M.; project administration, S.S.-M.; funding acquisition, S.S.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are publicly available in the manuscript text. Occurrence records of R. parkeri in America were deposited via the Figshare repository in https://doi.org/10.6084/m9.figshare.14452680.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sánchez-Montes, S.; Colunga-Salas, P.; Lozano-Sardaneta, Y.N.; Zazueta-Islas, H.M.; Ballados-González, G.G.; Salceda-Sánchez, B.; Huerta-Jiménez, H.; Torres-Castro, M.; Panti-May, J.A.; Peniche-Lara, G.; et al. The genus Rickettsia in Mexico: Current knowledge and perspectives. Ticks Tick-Borne Dis. 2021, 12, 101633. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the World: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef] [PubMed]
- Labruna, M.B.; Salim-Mattar, V.; Nava, S.; Bermúdez, S.; Venzal, J.; Dolz, G.; Abarca, K.; Romero, L.; de Souza, R.; Oteo, J.; et al. Rickettsiosis in Latin America, Caribbean, Spain and Portugal. Rev. MVZ Cordoba 2011, 16, 2435–2457. [Google Scholar] [CrossRef]
- Parker, R.R.; Kohls, G.M.; Cox, G.W.; Davis, G.E. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep. 1939, 54, 1482. [Google Scholar] [CrossRef]
- Paddock, C.D.; Sumner, J.W.; Comer, J.A.; Zaki, S.R.; Goldsmith, C.S.; Goddard, J.; McLellan, S.L.F.; Tamminga, C.L.; Ohl, C.A. Rickettsia parkeri: A newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 2004, 38, 805–811. [Google Scholar] [CrossRef]
- Conti-Díaz, I.A.; Moraes-Filho, J.; Pacheco, R.C.; Labruna, M.B. Serological evidence of Rickettsia parkeri as the etiological agent of rickettsiosis in Uruguay. Revista do Instituto de Medicina Tropical de São Paulo 2009, 51, 337–339. [Google Scholar] [CrossRef]
- Spolidorio, M.G.; Labruna, M.B.; Mantovani, E.; Brandão, P.E.; Richtzenhain, L.J.; Yoshinari, N.H. Novel spotted fever group rickettsiosis, Brazil. Emerg. Infect. Dis. 2010, 16, 521–523. [Google Scholar] [CrossRef]
- Romer, Y. Rickettsia parkeri Rickettsiosis, Argentina. Emerg. Infect. Dis. 2011, 17, 1169–1173. [Google Scholar] [CrossRef]
- Eisen, R.J.; Kugeler, K.J.; Eisen, L.; Beard, C.B.; Paddock, C.D. Tick-borne zoonoses in the United States: Persistent and emerging threats to human health. ILAR J. 2017, 58, 319–335. [Google Scholar] [CrossRef]
- Barbieri, A.R.; Filho, J.M.; Nieri-Bastos, F.A.; Souza, J.C.; Szabó, M.P.; Labruna, M.B. Epidemiology of Rickettsia sp. strain Atlantic rainforest in a spotted fever-endemic area of southern Brazil. Ticks Tick-Borne Dis. 2014, 5, 848–853. [Google Scholar] [CrossRef]
- Nieri-Bastos, F.A.; Horta, M.C.; Barros-Battesti, D.M.; Moraes-Filho, J.; Ramirez, D.G.; Martins, T.F.; Labruna, M.B. Isolation of the pathogen Rickettsia sp. strain Atlantic Rainforest from its presumed tick vector, Amblyomma ovale (Acari: Ixodidae), from two areas of Brazil. J. Med. Éntomol. 2016, 53, 977–981. [Google Scholar] [CrossRef]
- Paddock, C.D.; Allerdice, M.E.J.; Karpathy, S.E.; Nicholson, W.L.; Levin, M.L.; Smith, T.C.; Becker, T.; Delph, R.J.; Knight, R.; Ritter, J.M.; et al. Unique strain of Rickettsia parkeri associated with the hard tick Dermacentor parumapertus Neumann in the Western United States. Appl. Environ. Microbiol. 2017, 83, e03463-16. [Google Scholar] [CrossRef]
- Nieri-Bastos, F.A.; Marcili, A.; De Sousa, R.; Paddock, C.D.; Labruna, M.B. Phylogenetic evidence for the existence of multiple strains of Rickettsia parkeri in the New World. Appl. Environ. Microbiol. 2018, 84, e02872-17. [Google Scholar] [CrossRef]
- Sánchez-Montes, S.; López-Pérez, A.M.; Guzmán-Cornejo, C.; Colunga-Salas, P.; Becker, I.; Delgado-de la Mora, J.; Licona-Enríquez, J.D.; Delgado-de la Mora, D.; Karpathy, S.E.; Paddock, C.D.; et al. Rickettsia parkeri in Dermacentor parumapertus ticks. Mexico. Emerg. Infect. Dis. 2018, 24, 1108–1111. [Google Scholar] [CrossRef]
- Tomassone, L.; Conte, V.; Parrilla, G.; De Meneghi, D. Rickettsia infection in dogs and Rickettsia parkeri in Amblyomma tigrinum ticks, Cochabamba Department, Bolivia. Vector-Borne Zoonotic Dis. 2010, 10, 953–958. [Google Scholar] [CrossRef]
- Lopes, M.G.; Junior, J.M.; Foster, R.J.; Harmsen, B.J.; Sanchez, E.; Martins, T.F.; Quigley, H.; Marcili, A.; Labruna, M.B. Ticks and rickettsiae from wildlife in Belize, Central America. Parasites Vectors 2016, 9, 1–7. [Google Scholar] [CrossRef]
- La Mora, J.D.-D.; Sánchez-Montes, S.; Licona-Enríquez, J.D.; La Mora, D.D.-D.; Paddock, C.D.; Beati, L.; Colunga-Salas, P.; Guzmán-Cornejo, C.; Zambrano, M.L.; Karpathy, S.E.; et al. Rickettsia parkeri and Candidatus Rickettsia andeanae in tick of the Amblyomma maculatum group, Mexico. Emerg. Infect. Dis. 2019, 25, 836–838. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2012; ISBN 978-0-691-13686-8. [Google Scholar]
- Moo-Llanes, D.; Ibarra-Cerdeña, C.N.; Rebollar-Tellez, E.A.; Ibáñez-Bernal, S.; Gonzalez, C.; Ramsey, J.M. Current and future niche of North and Central American sand flies (Diptera: Psychodidae) in climate change scenarios. PLoS Negl. Trop. Dis. 2013, 7, e2421. [Google Scholar] [CrossRef]
- Moo-Llanes, D.A.; Pech-May, A.; Ibarra-Cerdeña, C.N.; Rebollar-Téllez, E.A.; Ramsey, J.M. Inferring distributional shifts of epidemiologically important North and Central American sandflies from Pleistocene to future scenarios. Med. Vet. Éntomol. 2019, 33, 31–43. [Google Scholar] [CrossRef]
- Pech-May, A.; Moo-Llanes, D.A.; Puerto-Avila, M.B.; Casas, M.; Danis-Lozano, R.; Ponce, G.; Tun-Ku, E.; Pinto-Castillo, J.F.; Villegas, A.; Ibáñez-Piñon, C.R.; et al. Population genetics and ecological niche of invasive Aedes albopictus in Mexico. Acta Trop. 2016, 157, 30–41. [Google Scholar] [CrossRef]
- Moo-Llanes, D.; López-Ordóñez, T.; Torres-Monzón, J.; Mosso-González, C.; Casas-Martínez, M.; Samy, A. Assessing the potential distributions of the invasive mosquito vector Aedes albopictus and its natural Wolbachia infections in Mexico. Insects 2021, 12, 143. [Google Scholar] [CrossRef]
- Ogrzewalska, M.; Nieri-Bastos, F.A.; Marcili, A.; Nava, S.; González-Acuña, D.; Muñoz-Leal, S.; Ruiz-Arrondo, I.; Venzal, J.M.; Mangold, A.; Labruna, M.B. A novel spotted fever group Rickettsia infecting Amblyomma parvitarsum (Acari: Ixodidae) in highlands of Argentina and Chile. Ticks Tick-Borne Dis. 2016, 7, 439–442. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- John, H.K.S.; Adams, M.L.; Masuoka, P.M.; Flyer-Adams, J.G.; Jiang, J.; Rozmajzl, P.J.; Stromdahl, E.Y.; Richards, A.L. Prevalence, Distribution, and development of an ecological niche model of Dermacentor variabilis ticks positive for Rickettsia montanensis. Vector-Borne Zoonotic Dis. 2016, 16, 253–263. [Google Scholar] [CrossRef]
- Raghavan, R.K.; Peterson, A.T.; Cobos, M.E.; Ganta, R.; Foley, D. Current and future distribution of the lone star tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS ONE 2019, 14, e0209082. [Google Scholar] [CrossRef]
- Pascoe, E.L.; Marcantonio, M.; Caminade, C.; Foley, J.E. Modeling potential habitat for Amblyomma tick species in California. Insects 2019, 10, 201. [Google Scholar] [CrossRef]
- Lippi, C.A.; Gaff, H.D.; White, A.L.; Ryan, S.J. Scoping review of distribution models for selected Amblyomma ticks and rickettsial group pathogens. PeerJ 2021, 9, e10596. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on earth. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Escobar, L.E.; Lira-Noriega, A.; Medina-Vogel, G.; Peterson, A.T. Potential for spread of the white-nose fungus (Pseudogymnoascus destructans) in the Americas: Use of Maxent and NicheA to assure strict model transference. Geospat. Health 2014, 9, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Rödder, D.; Schmidtlein, S.; Veith, M.; Lötters, S. Alien invasive slider turtle in unpredicted habitat: A matter of niche shift or of predictors studied? PLoS ONE 2009, 4, e7843. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Aguilar-Domínguez, A.; Moo-Llanes, D.A.; Sánchez-Montes, S.; Becker, I.; Feria-Arroyo, T.; de León, A.P.; Romero-Salas, D. Potential distribution of Amblyomma mixtum (Koch 1844) in climate change scenarios in America. Tick Tick-Borne Dis. 2021, in press. [Google Scholar]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).