Evaluating the Efficacy of Enzyme-Induced Carbonate Precipitation (EICP) for Aeolian Sand Fixation
Abstract
1. Introduction
2. Materials and Methods
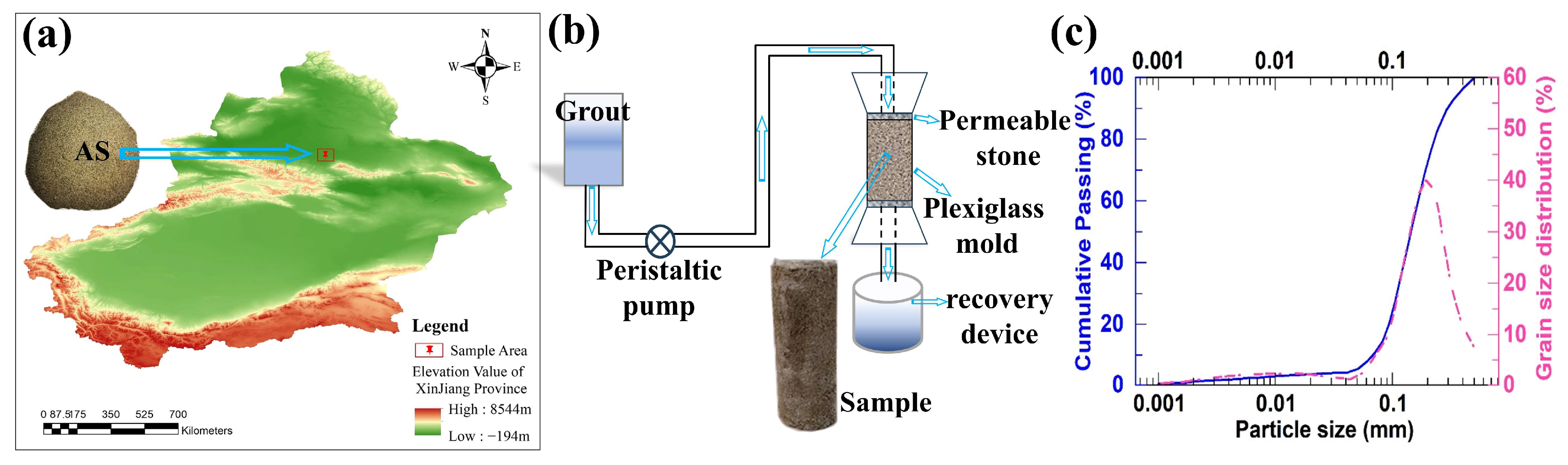
2.1. Aeolian Sand
2.2. Basic Physical and Chemical Indicators and UCS
2.3. Soybean Crude Urease
2.4. Determination of Urease Activity
2.5. Solid-to-Liquid Ratio
2.6. Calcium Source
2.7. Temperature, ELR, and pH
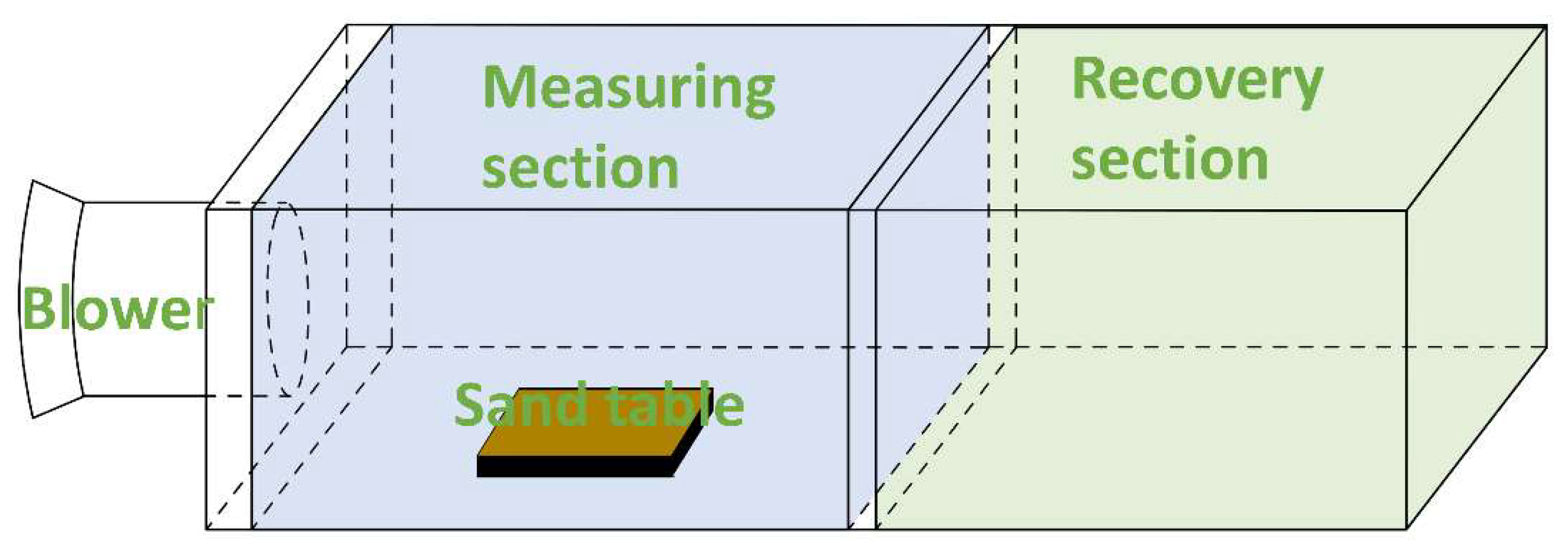
2.8. Wind Erosion Resistance Rate
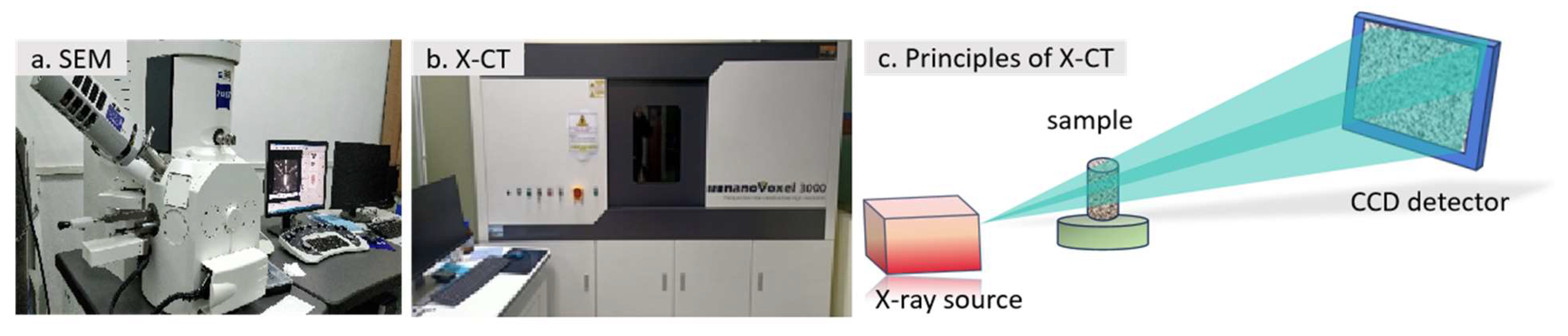
2.9. SEM and X-CT
3. Results and Discussions
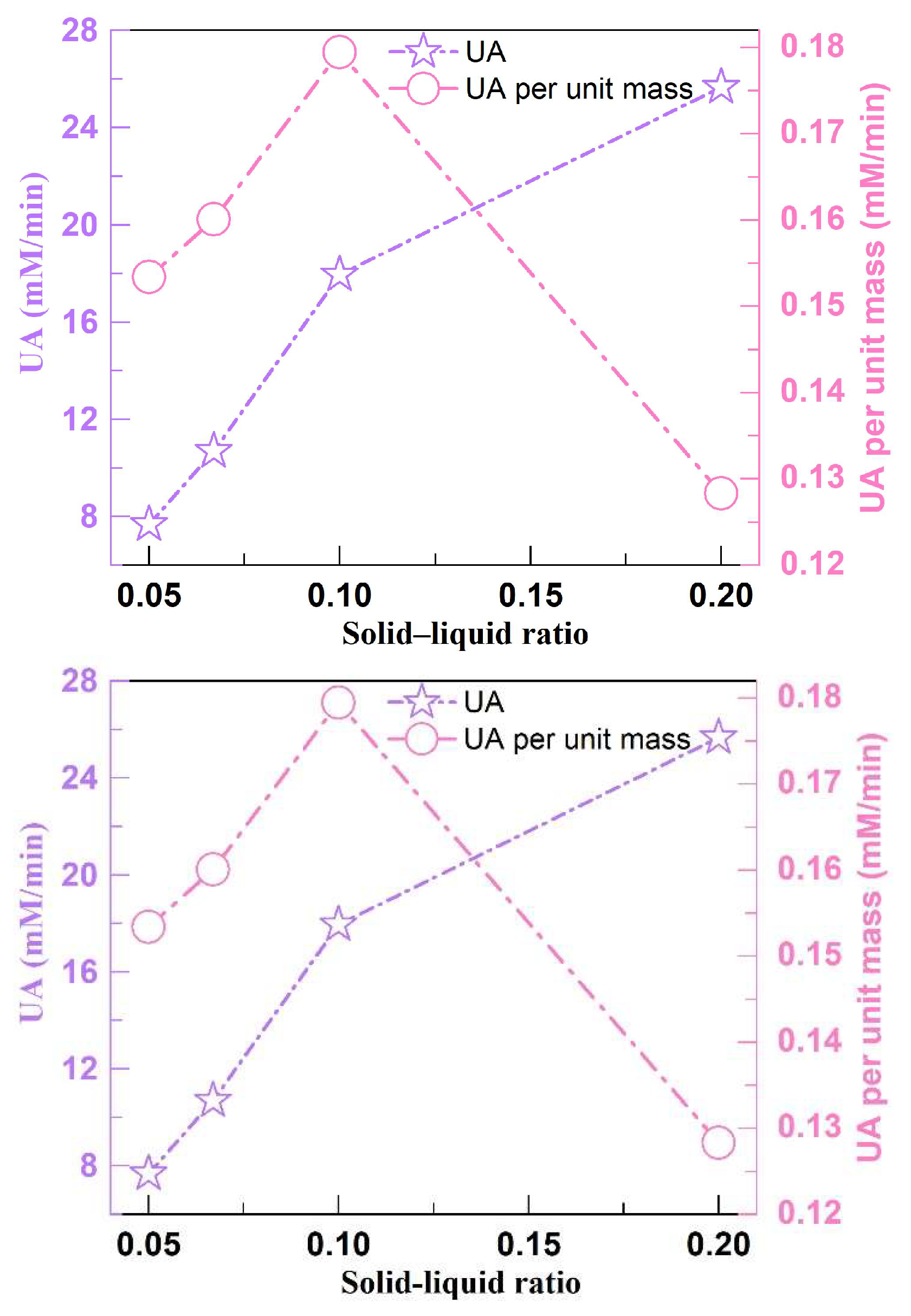
3.1. The Activity of Crude Soybean Urease with Different Solid–Liquid Ratios
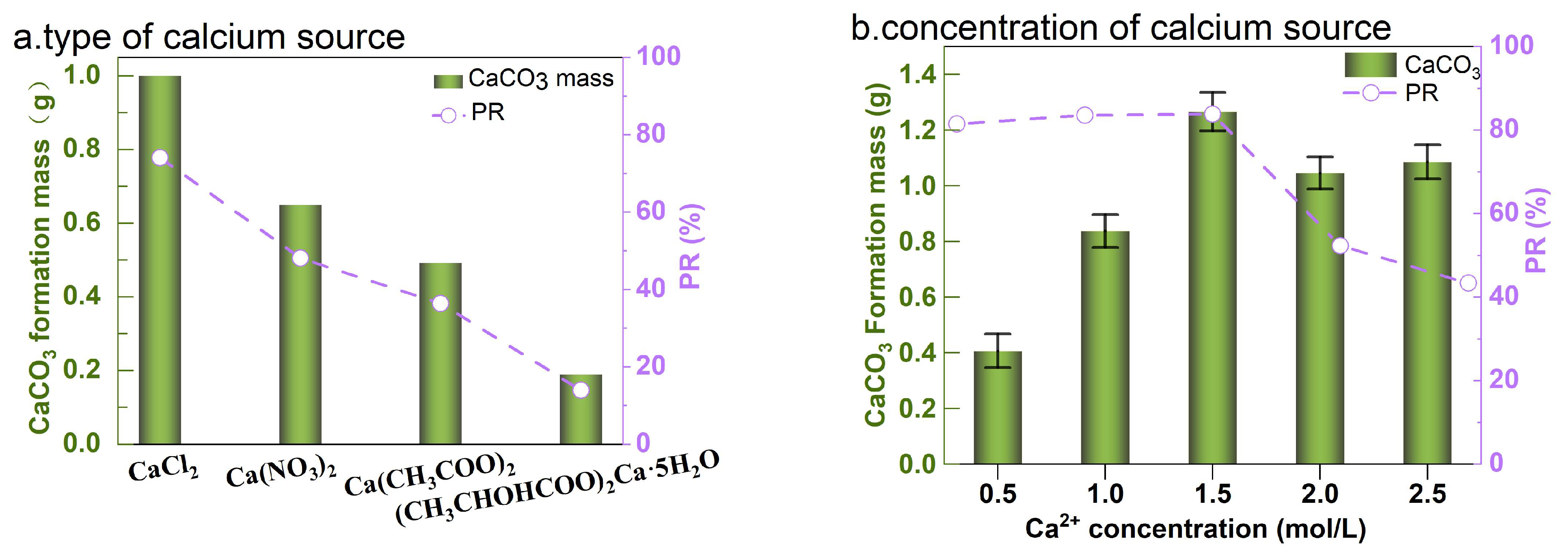
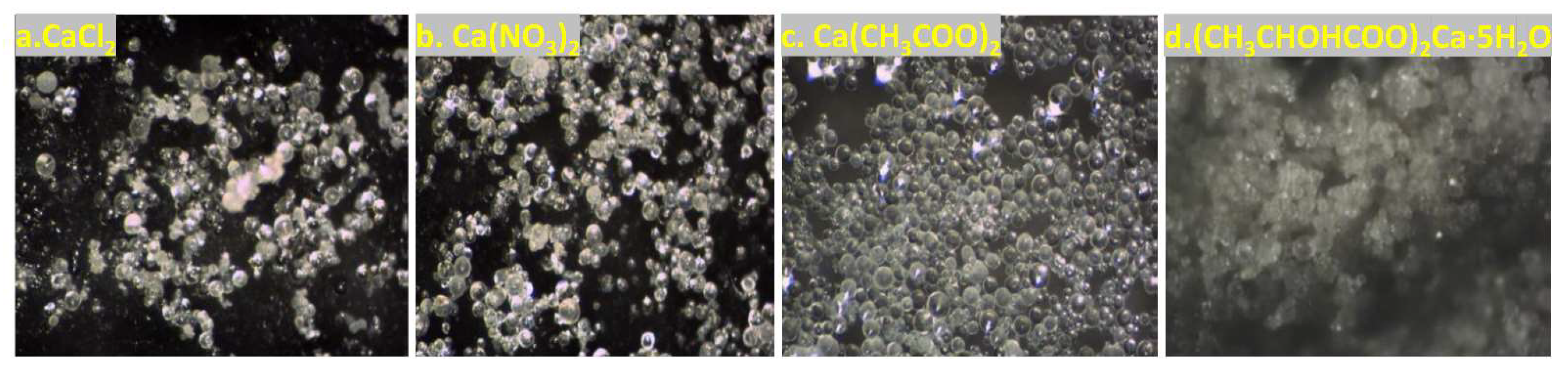
3.2. Effect of Calcium Source and Calcium Ion Concentration
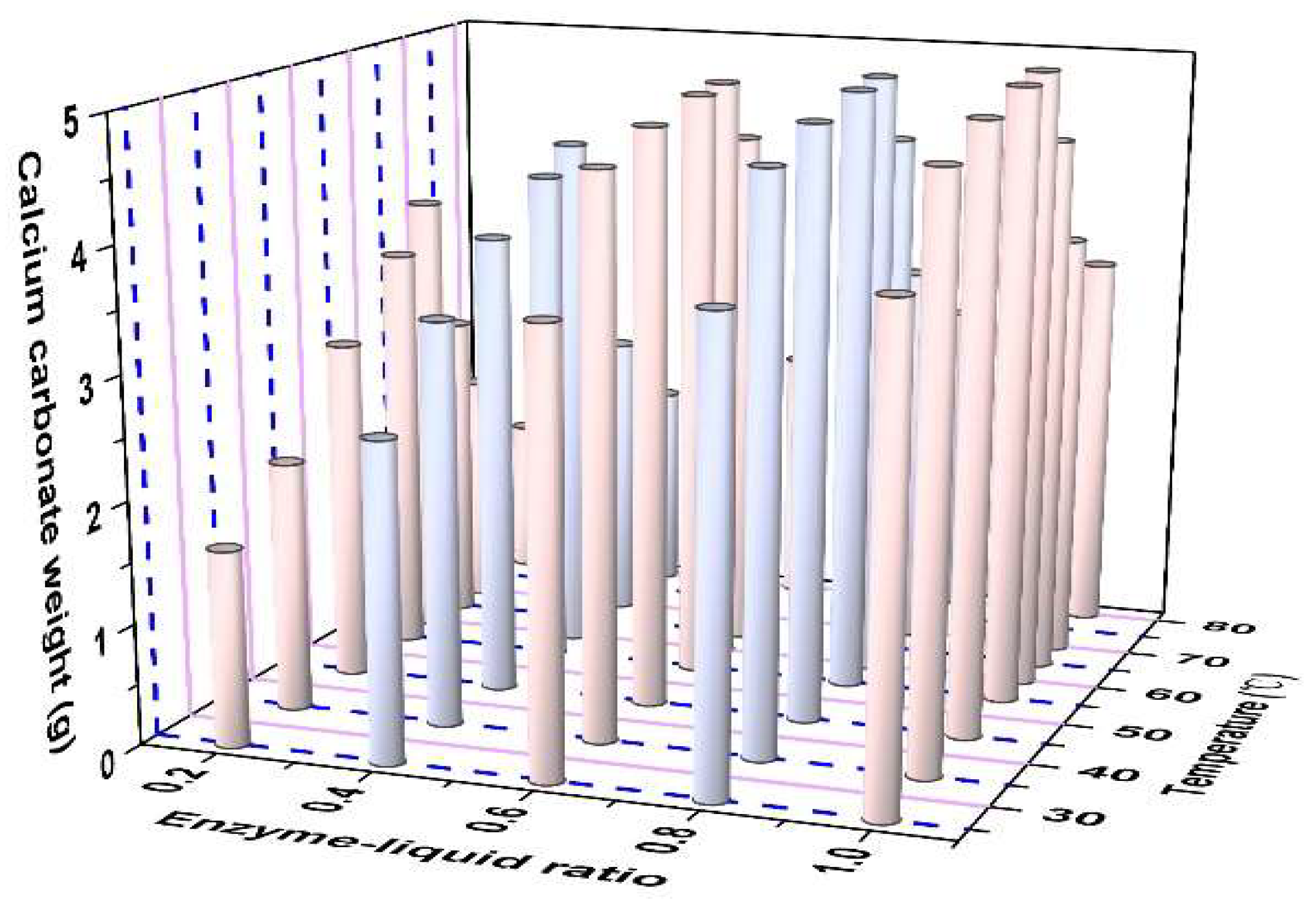
3.3. Effects of Temperature and ELR on UA
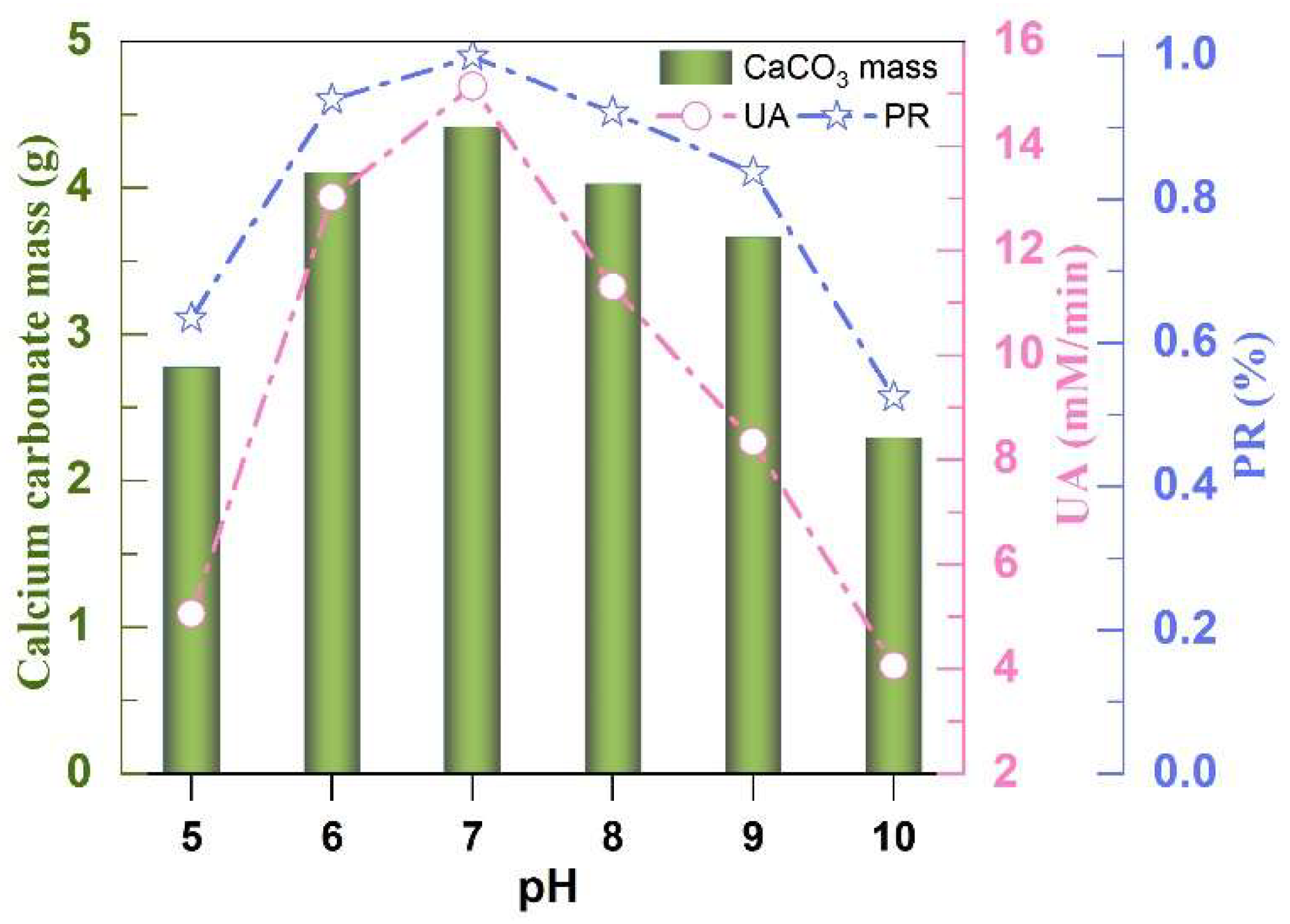
3.4. Effect of pH Value on UA
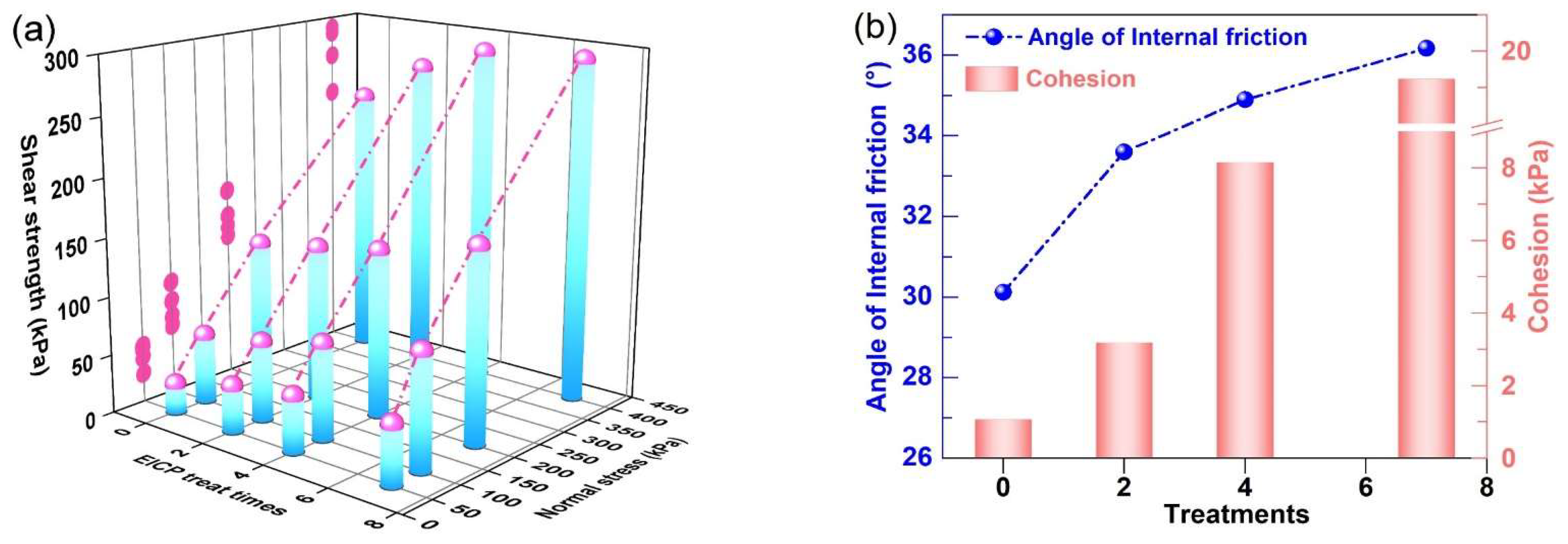
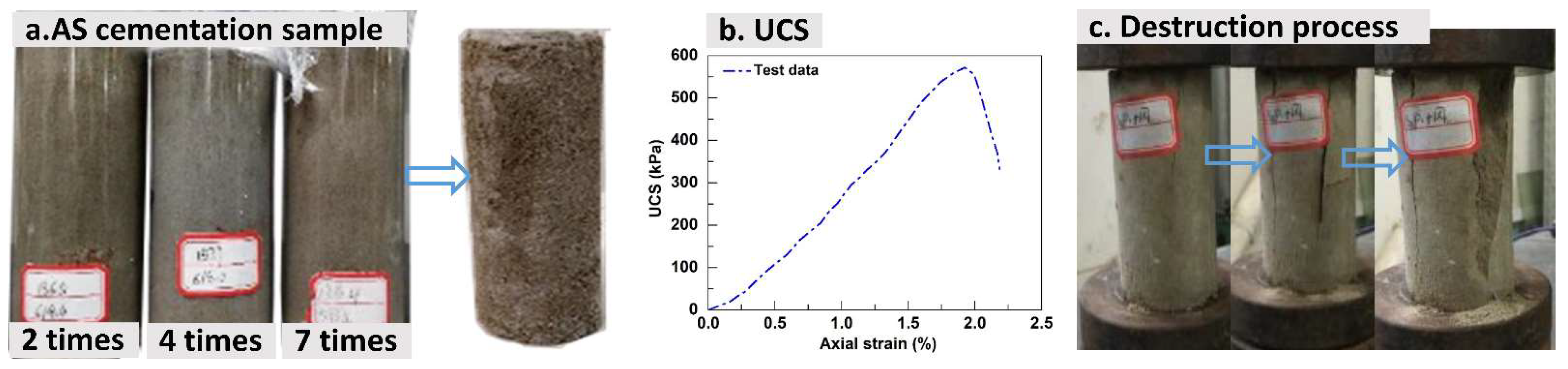
3.5. Analysis of Mechanical Properties of Aeolian Sand
3.6. UCS
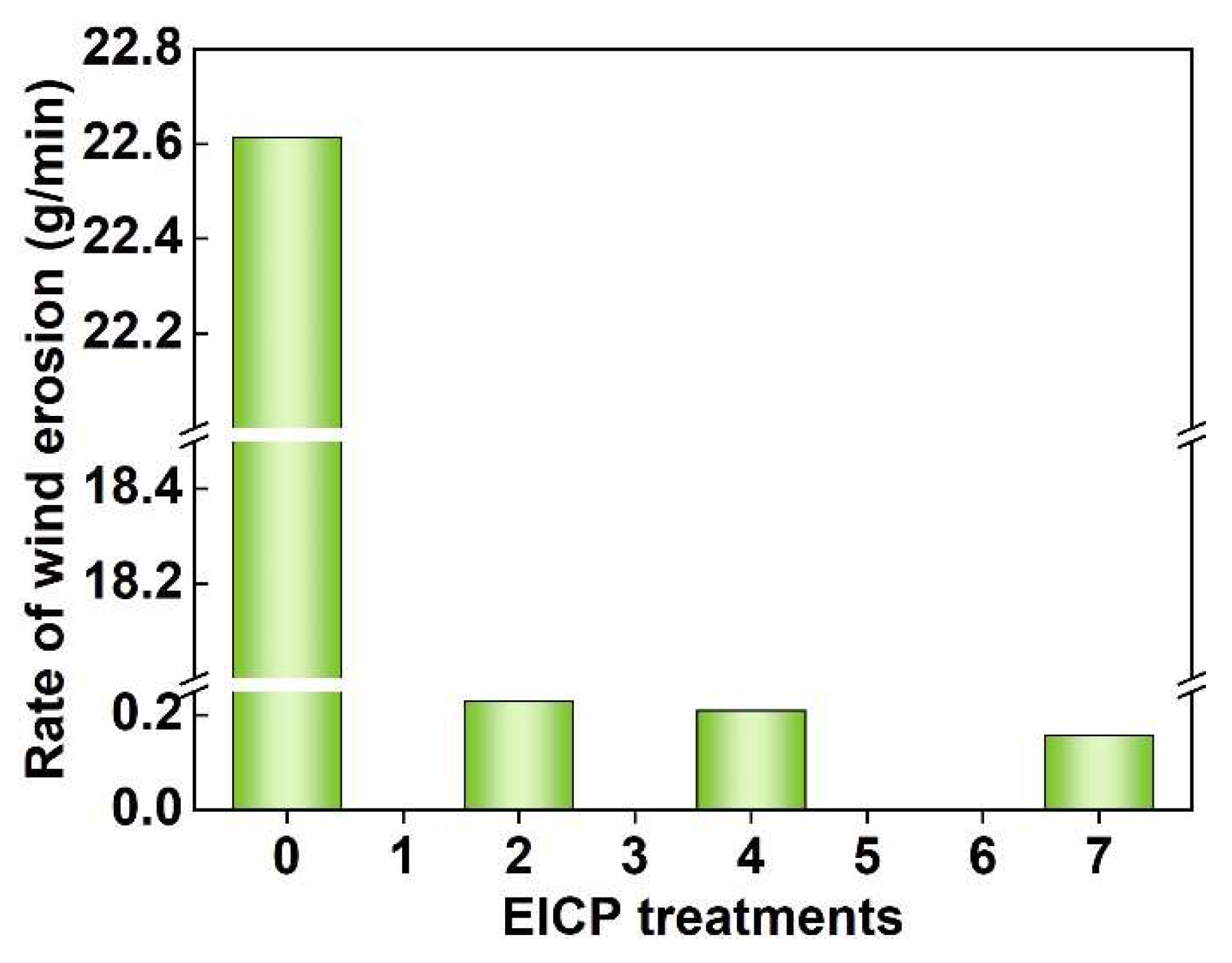
3.7. Wind Erosion Resistance
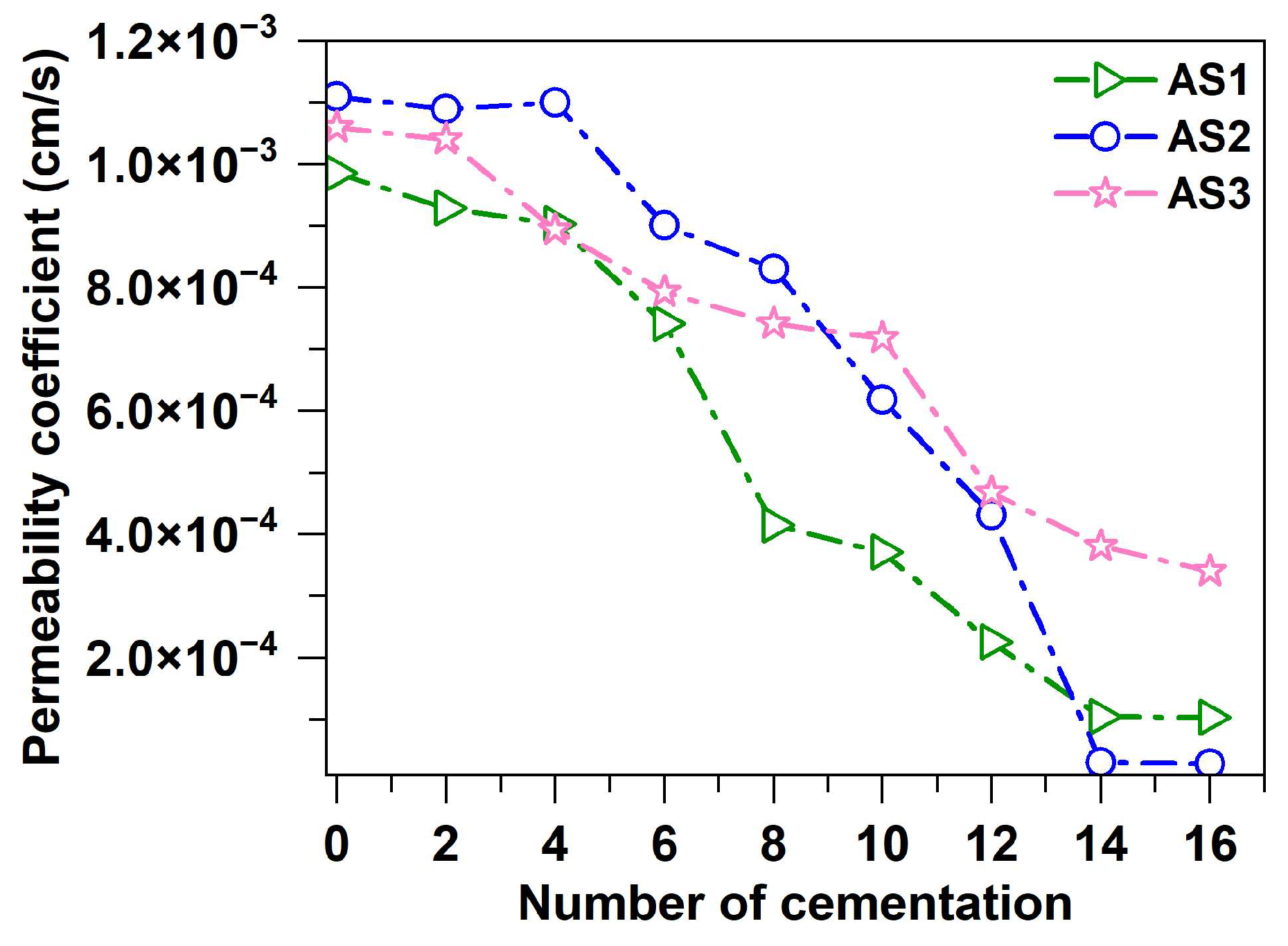
3.8. Permeability Coefficient
3.9. Microscopic Characteristics
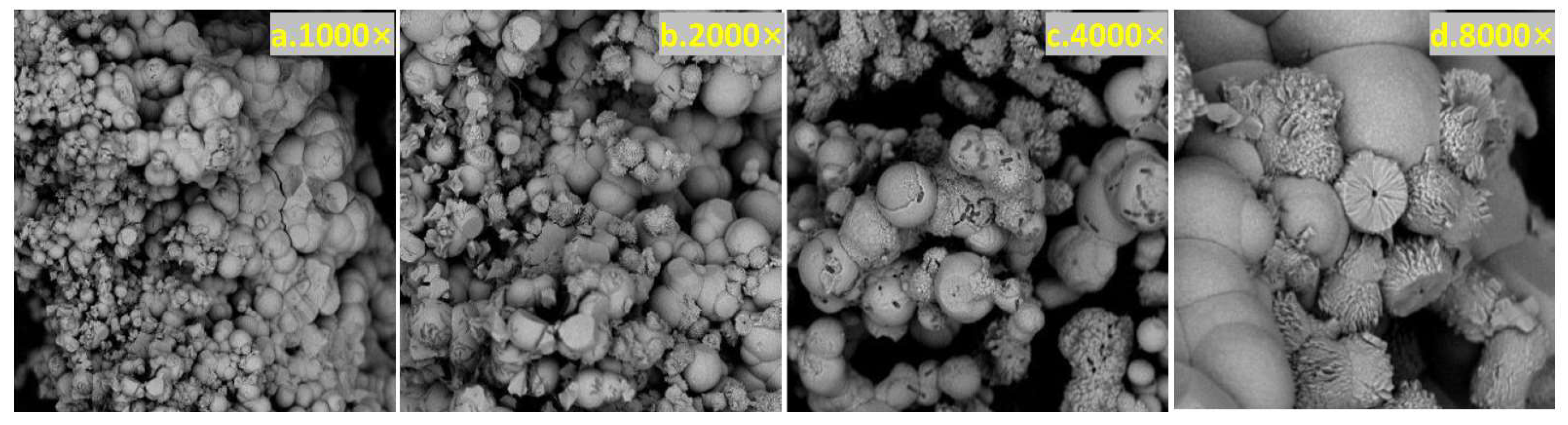
3.9.1. SEM
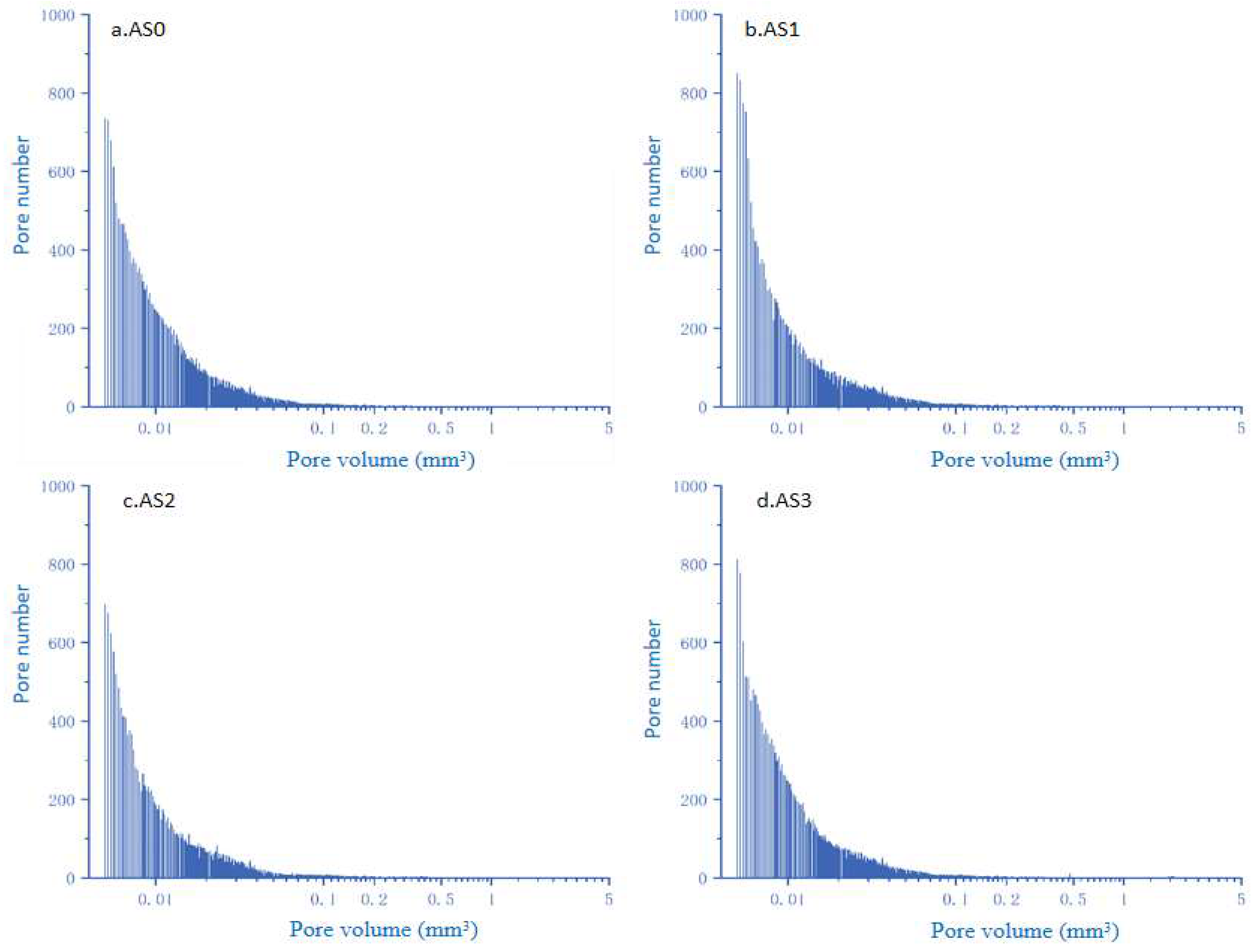
3.9.2. X-CT
4. Future Scope and Recommendation
5. Conclusions
- The optimal EICP parameters include a 1:10 solid-to-liquid ratio, CaCl2 as the preferred calcium source (1.0~1.5 mol/L concentration range), a catalytic temperature window of 35~65 °C, and pH 7. Elevated calcium concentrations moderately enhanced CaCO3 yield and precipitation efficiency.
- Porous media critically influenced CaCO3 crystallization: homogeneous spherical particles formed in solution, while rhombohedral calcite dominated in porous environments. These crystals strengthened AS via dual mechanisms of surface coating and interparticle pore filling. As a result, UCS increased to 580 kPa, and the wind erosion rate decreased to 0.151 g/min, significantly surpassing the minimum requirements for desert highway subbase (UCS ≥ 550 kPa, wind erosion rate ≤ 0.2 g/min). SCU-induced mineralization significantly improved wind erosion resistance, achieving stabilization thresholds with minimal treatments.
- Infiltration-based EICP effectively consolidated loose AS, with cementation efficacy positively correlating with treatment cycles. Progressive alterations in particle morphology, spacing, and cementation intensity elevated internal friction angles and cohesion. Post-treatment specimens demonstrated enhanced integrity, compressive strength, and brittle failure characteristics, with cohesion being particularly treatment-dependent.
- ELR optimization (0.8) maximized reductions in permeability coefficients, pore counts, volumes, and porosity. Permeability exhibited strong dependence on pore quantity. Microscopic analysis confirms that calcium carbonate crystals fill and bridge the pores, reducing the permeability coefficient by two orders of magnitude and effectively blocking water infiltration and sand migration pathways.
- Under optimized conditions, EICP technology enables efficient cementation of AS, eliminating the need for long-distance material transportation required by traditional gravel bases. This approach allows for the direct utilization of in situ AS in road construction, reducing engineering costs and mitigating chemical pollution risks. The solidified layer offers both high load-bearing capacity and ecological adaptability.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Ren, Y.; Hong, Z.; Ping, W.; Yang, Y. Research on Surface Hydrophobic Modification Methods and Mechanisms of Recycled Aeolian Sand Aggregates. J. Build. Mater. 2025, 28, 270–1275. [Google Scholar]
- Wang, Y.; Sun, X.; Miao, L.; Wang, H.; Wu, L.; Shi, W.; Kawasaki, S. State-of-the-art review of soil erosion control by MICP and EICP techniques: Problems, applications, and prospects. Sci. Total Environ. 2024, 912, 169016. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wu, L.; Sun, X. Enzyme-catalysed mineralisation experiment study to solidify desert sands. Sci. Rep. 2020, 10, 10611. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Lei, J.; Xu, X.; Wang, S.; Fan, J.; Fan, S. Damage by wind-blown sand and its control measures along the Taklimakan Desert Highway in China. J. Arid Land 2020, 13, 98–106. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, R.; Wu, X.; Zhao, D.; Xue, H.; Zhang, Y.; Dai, N.; Song, D.; Zhang, M.; Ding, H. Preparation and Properties of Bio-Based Attapulgite Copolymer (BAC) Sand-Fixing Material. Polymers 2023, 15, 265. [Google Scholar] [CrossRef]
- Qi, Y.; Gao, Y.; He, J.; Zhou, Y.; Yan, B. Effects researches of soluble soybean polysaccharides on solidifying aeolian sand by soybean-urease induced carbonate precipitation. Chin. J. Geotech. Eng. 2024, 46, 823–832. [Google Scholar]
- Zhang, J.; Yin, Y.; Shi, W.; Bian, H.; Shi, L.; Wu, L.; Han, Z.; Zheng, J.; He, X. Strength and uniformity of EICP-treated sand under multi-factor coupling effects. Biogeotechnics 2023, 1, 100007. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, C.-S.; Jiang, N.-J.; Pan, X.; Liu, B.; Wang, Y.; Shi, B. Microbial-induced carbonate precipitation (MICP) technology: A review on the fundamentals and engineering applications. Environ. Earth Sci. 2023, 82, 229. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Guo, Z.; Xu, Q. Durability of MICP-reinforced calcareous sand in marine environments: Laboratory and field experimental study. Biogeotechnics 2023, 1, 100018. [Google Scholar] [CrossRef]
- Salifu, E.; Maclachlan, E.; Iyer, K.; Knapp, C.; Tarantino, A. Application of microbially induced calcite precipitation in erosion mitigation and stabilisation of sandy soil foreshore slopes: A preliminary investigation. Eng. Geol. 2015, 201, 96–105. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Luo, Y.; Li, Y.; Yang, Q.; Shen, Z. An experimental study of mitigating coastal dune erosion by using bio-carbonization of reactive magnesia cement. Mar. Georesour. Geotechnol. 2022, 42, 161–175. [Google Scholar] [CrossRef]
- Qian, C.; Rong, H.; Yu, X.; Wang, X. Experiments on and predictions about properties of sand bonded by microbe cement. Sci. China Technol. Sci. 2016, 59, 1186–1193. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, Y.; Sun, J.; Liu, S.; Liu, J. Research and Application Status of Sand Fixation Materials in China. Mater. Rev. 2012, 26, 332–334. [Google Scholar]
- Lai, J.; Zhang, K.; Wang, W.; Wang, Y.; Xu, X.; Qu, J.; Xiao, J. Research Progress and Prospects of Chemical Sand Fixation Materials. Chin. J. Desert Res. 2017, 37, 644–658. [Google Scholar]
- Dilrukshi, R.A.N.; Kawasaki, S. Effective Use of Plant-Derived Urease in the Field of Geoenvironmental/Geotechnical Engineering. J. Civ. Environ. Eng. 2016, 6, 2. [Google Scholar]
- Zhang, Q.; Ye, W.M.; Liu, Z.R.; Wang, Q.; Chen, Y.G. Advances in soil cementation by biologically induced calcium carbonate precipitation. Rock Soil Mech. 2022, 43, 345–357. [Google Scholar]
- Zhang, Q.; Ye, W.; Liu, Z.; Wang, Q.; Chen, Y. Influence of injection methods on calcareous sand cementation by EICP technique. Constr. Build. Mater. 2023, 363, 129724. [Google Scholar] [CrossRef]
- Xie, D.; Zhang, R.; Wang, J. The influence of environmental factors and precipitation precursors on enzyme-induced carbonate precipitation (EICP) process and its application on modification of recycled concrete aggregates. J. Clean. Prod. 2023, 395, 136444. [Google Scholar] [CrossRef]
- Kidanemariam, T.G.; Gebru, K.A.; Gebretinsae, H.K. A mini review of enzyme-induced calcite precipitation (EICP) technique for eco-friendly bio-cement production. Environ. Sci. Pollut. Res. Int. 2024, 31, 16206–16215. [Google Scholar] [CrossRef]
- Lemboye, K.; Almajed, A.; Hamid, W.; Arab, M. Permeability investigation on sand treated using enzyme-induced carbonate precipitation and biopolymers. Innov. Infrastruct. Solut. 2021, 6, 167. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Yuan, J.; Wang, H.; Wu, L. Application of enzymatic calcification for dust control and rainfall erosion resistance improvement. Sci. Total Environ. 2020, 759, 143468. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-Y.; Tirkolaei, H.K.; Hua, M.; De Rosa, I.M.; Carlson, L.; Kavazanjian, E.; He, X. Durable and ductile double-network material for dust control. Geoderma 2020, 361, 114090. [Google Scholar] [CrossRef]
- Cui, M.; Xiong, H.; Zheng, J.; Cui, M.; Lv, S.; Lai, H. Experimental Study on Multiscale Engineering Properties of EICP Combined with Xanthan Gum Solidified Sand. J. Mater. Civ. Eng. 2024, 36, 04024108. [Google Scholar] [CrossRef]
- Cui, M.; Xiong, H.; Lv, S.; Zheng, J.; Cui, M.; Zeng, C. Multivariate Experimental Study on EICP Combined with Polypropylene Fiber Solidified Desert Sand. KSCE J. Civ. Eng. 2024, 28, 2221–2230. [Google Scholar] [CrossRef]
- Dakhane, A.; Das, S.; Hansen, H.; O’Donnell, S.; Hanoon, F.; Rushton, A.; Perla, C.; Neithalath, N. Crack Healing in Cementitious Mortars Using Enzyme-Induced Carbonate Precipitation: Quantification Based on Fracture Response. J. Mater. Civ. Eng. 2018, 30, 04018035. [Google Scholar] [CrossRef]
- Choi, S.-G.; Park, S.-S.; Wu, S.; Chu, J. Methods for Calcium Carbonate Content Measurement of Biocemented Soils. J. Mater. Civ. Eng. 2017, 29, 06017015. [Google Scholar] [CrossRef]
- Gomez, M.; Martinez, B.; Dejong, J.; Hunt, C.; DeVlaming, L.; Major, D.; Dworatzek, S. Field-scale bio-cementation tests to improve sands. Proc. ICE-Ground Improv. 2014, 168, 206–216. [Google Scholar] [CrossRef]
- Arab, M.; Rohy, H.; Zeiada, W.; Almajed, A.; Omar, M. One-Phase EICP Biotreatment of Sand Exposed to Various Environmental Conditions. J. Mater. Civ. Eng. 2021, 33, 4020489. [Google Scholar] [CrossRef]
- GB/T 50123-2019; Standard for Geotechnical Testing Method. Construction Ministry of PRC: Beijing, China, 2019.
- Whiffin, V.; van Paassen, L.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Cui, M.-J.; Chu, J.; Lai, H.-J. Optimization of one-phase-low-pH enzyme-induced carbonate precipitation method for soil improvement. Acta Geotech. 2024, 19, 1611–1625. [Google Scholar] [CrossRef]
- Cui, M.-J.; Lai, H.; Wu, S.-F.; Chu, J. Comparison of Soil Improvement Methods using Crude Soybean Enzyme, Bacterial Enzyme or Bacteria Induced Carbonate Precipitation. Géotechnique 2022, 74, 18–26. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S.; Saint, C. A Review of Enzyme Induced Carbonate Precipitation (EICP): The Role of Enzyme Kinetics. Sustain. Chem. 2021, 2, 92–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Cheng, X. Influences of calcium sources on microbially induced carbonate precipitation in porous media. Mater. Res. Innov. 2014, 18, S2–S79. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Xiao, Y.; Chu, J.; Xiao, P.; Wang, Y. Biocementation of calcareous sand using soluble calcium derived from calcareous sand. Bull. Eng. Geol. Environ. 2017, 77, 1781–1791. [Google Scholar] [CrossRef]
- Putra, H.; Yasuhara, H.; Kinoshita, N.; Hirata, A. Optimization of Enzyme-Mediated Calcite Precipitation as a Soil-Improvement Technique: The Effect of Aragonite and Gypsum on the Mechanical Properties of Treated Sand. Crystals 2017, 7, 59. [Google Scholar] [CrossRef]
- Neupane, D.; Yasuhara, H.; Kinoshita, N.; Ando, Y. Distribution of mineralized carbonate and its quantification method in enzyme mediated calcite precipitation technique. Soils Found. 2015, 55, 447–457. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Mourdoch University, Murdoch, WA, Australia, 2004. [Google Scholar]
- Abo-El-Enein, S.A.; Ali, A.H.; Talkhan, F.N.; Abdel-Gawwad, H.A. Utilization of microbial induced calcite precipitation for sand consolidation and mortar crack remediation. HBRC J. 2012, 8, 185–192. [Google Scholar] [CrossRef]
- Li, D.; Tian, K.-L.; Zhang, H.-L.; Wu, Y.-Y.; Nie, K.-Y.; Zhang, S.-C. Experimental investigation of solidifying desert aeolian sand using microbially induced calcite precipitation. Constr. Build. Mater. 2018, 172, 251–262. [Google Scholar]
- Zheng, T.; Qian, C. Influencing factors and formation mechanism of CaCO3 precipitation induced by microbial carbonic anhydrase. Process Biochem. 2020, 91, 271–281. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.-B.; Wang, H.; Zeng, R. Microbially induced calcium carbonate precipitation driven by ureolysis to enhance oil recovery. RSC Adv. 2017, 7, 37382–37391. [Google Scholar] [CrossRef]
- Prachayawarakorn, S.; Prachayawasin, P.; Soponronnarit, S. Effective Diffusivity and Kinetics of Urease Inactivation and Color Change During Processing of Soybeans with Superheated-Steam Fluidized Bed. Dry. Technol. 2004, 22, 2095–2118. [Google Scholar] [CrossRef]
- Akyel, A. Improving pH and Temperature Stability of Urease for Ureolysis-Induced Calcium Carbonate Precipitation. Ph.D. Thesis, Montana State University-Bozeman, College of Engineering, Bozeman, MT, USA, 2022. [Google Scholar]
- Lai, H.; Cui, M.-J.; Chu, J. Effect of pH on soil improvement using one-phase-low-pH MICP or EICP biocementation method. Acta Geotech. 2022, 18, 3259–3272. [Google Scholar] [CrossRef]

| Bulk Density (g/cm3) | Maximum Dry Density (g/cm3) | Permeability Coefficient (cm/s) | Natural Density (g/cm3) | Natural Moisture Content (%) | Gradation Coefficient | Specific Gravity of Soil Particles Gs | av (MPa−1) |
|---|---|---|---|---|---|---|---|
| 1.28 | 1.712 | 0.015 | 1.29 | 0.97 | 1.10 | 2.56 | 1.892 |
| Temperature (°C) | Soybean Urease (mL) | Deionized Water (mL) | ELR | Cementation Concentration (M) | Cementing Fluid (mL) | Total Volume of Solution (mL) |
|---|---|---|---|---|---|---|
| 25, 35, 45, 55, 60, 65, 70, 80 | 5 | 20 | 0.2 | 2.0 | 25 | 50 |
| 10 | 15 | 0.4 | ||||
| 15 | 10 | 0.6 | ||||
| 20 | 5 | 0.8 | ||||
| 25 | 0 | 1 |
| Contents | AS0 | AS1 | AS2 | AS3 |
|---|---|---|---|---|
| ELR | 0 | 0.6 | 0.8 | 1.0 |
| Sample | (cm/s) | (cm/s) | |
|---|---|---|---|
| AS1 | 9.23 × 10−4 | 1.59 × 10−5 | 58.11 |
| AS2 | 1.65 × 10−3 | 1.12 × 10−5 | 147.92 |
| AS3 | 1.33 × 10−3 | 4.66 × 10−5 | 28.53 |
| Test Specimen | Effective Volume (mm3) | Number of Pores | Void Content (mm3) | Porosity (%) | Porosity Decline Value ∆n (%) |
|---|---|---|---|---|---|
| AS0 | 9769.256 | 1,334,679 | 3525.29472 | 36.09 | - |
| AS1 | 7739.214 | 1,013,265 | 2052.647 | 26.53 | 9.56 |
| AS2 | 9131.873 | 875,841 | 2429.782 | 27.01 | 9.08 |
| AS3 | 8107.352 | 1,078,465 | 2255.765 | 29.83 | 6.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Zhang, J.; Luo, Y.; Wang, X.; Qi, X.; Hu, Z.; Hussain, J.; Jiang, G. Evaluating the Efficacy of Enzyme-Induced Carbonate Precipitation (EICP) for Aeolian Sand Fixation. Buildings 2025, 15, 1984. https://doi.org/10.3390/buildings15121984
Xiao L, Zhang J, Luo Y, Wang X, Qi X, Hu Z, Hussain J, Jiang G. Evaluating the Efficacy of Enzyme-Induced Carbonate Precipitation (EICP) for Aeolian Sand Fixation. Buildings. 2025; 15(12):1984. https://doi.org/10.3390/buildings15121984
Chicago/Turabian StyleXiao, Lina, Jiaming Zhang, Yi Luo, Xinlong Wang, Xiaojian Qi, Zhongyi Hu, Javid Hussain, and Guosheng Jiang. 2025. "Evaluating the Efficacy of Enzyme-Induced Carbonate Precipitation (EICP) for Aeolian Sand Fixation" Buildings 15, no. 12: 1984. https://doi.org/10.3390/buildings15121984
APA StyleXiao, L., Zhang, J., Luo, Y., Wang, X., Qi, X., Hu, Z., Hussain, J., & Jiang, G. (2025). Evaluating the Efficacy of Enzyme-Induced Carbonate Precipitation (EICP) for Aeolian Sand Fixation. Buildings, 15(12), 1984. https://doi.org/10.3390/buildings15121984






