Abstract
Thiosulfate leaching is a promising alternative to cyanidation, and the main hindrances for its wide commercial application are the high thiosulfate consumption and the difficult recovery of dissolved gold. In this review, the four solutions to reduce the consumption of thiosulfate, including the control of reaction conditions, the use of additives, the generation of thiosulfate in situ, and the replacement of traditional cupric-ammonia catalysis, are introduced and evaluated after the presentation of background knowledge about thiosulfate consumption. The replacement of cupric-ammonia catalysis with other metals, such as nickel- and cobalt-based catalysts, is proposed. The reason is that it not only reduces thiosulfate consumption observably via decreasing the redox potential of leach solution significantly but also is beneficial to gold recovery mainly owing to eliminating the interference of cuprous thiosulfate [Cu(S2O3)3]5−. Based on the comparative analysis for five common recovery techniques of rare-noble metals from pregnant leach solution, ion-exchange resin adsorption is considered to be the most appropriate to recover aurothiosulfate [Au(S2O3)2]3− because the resin can be employed in the form of resin-in-leach/pulp and, furthermore, is able to be eluted and regenerated simultaneously at ambient temperature. At last, how to reduce the process cost of the resin adsorption technique is discussed. In order to simplify the complex two-stage elution process for loaded resins, the traditional catalysis is suggested to be replaced.
1. Introduction
Cyanidation has been the predominant gold leaching technique since it was first put forward by John Stewart MacArthur in the 1880s [] because of its simple process and low cost. However, unluckily, cyanide is highly toxic, and there have been some serious environmental accidents occurring around the world caused by the leakage of cyanide from metallurgical plants [,,]. Due to of environmental concerns, cyanide leaching has been banned in many regions at present. Furthermore, the leaching period of cyanidation is usually as long as 24 h [] and gold cannot be effectively leached from refractory gold ores. Thus, alternative lixiviants for gold have received more and more attention in recent years. Among those lixiviants, chloride, thiourea, and thiosulfate have received the most attention. The development of chloride leaching is impeded mainly by its hazardous working environment, poor reaction selectivity, and high requirements for equipment corrosion protection [,,]. The future of thiourea leaching is not attractive because the consumption and price of thiourea are both high and, furthermore, it is a suspected carcinogen [,,,]. Thiosulfate leaching is widely considered to be the most promising alternative method owing to its reduced environmental risk, high reaction selectivity, low corrosivity of leach solution, cheap reagents, etc. [,,,,].
Thiosulfate leaching was first proposed as a part of Von Patera process [], where the leaching was implemented after chloridizing roasting. In the initial stage of thiosulfate leaching, high temperature and pressure were required until an improvement was carried out by using ammoniacal thiosulfate solution to leach copper-bearing sulfide ores at ambient temperature []. From then on, the studies about cupric-ammonia catalyzed thiosulfate leaching at room temperature began to prevail. So far, there have been a large number of literature reported in this field on the effects of ore types and reaction conditions [,,,]. However, the successful commercial application of thiosulfate leaching is almost nonexistent, except that it is being used by Barrick Gold Corporation (Elko, NV, USA) in its Goldstrike deposit with the pretreatment of acidic or alkaline pressure oxidation [,,]. The carbonaceous gold ore in Goldstrike deposit cannot be efficiently leached by cyanidation due to the “preg-robbing” phenomenon, however, this phenomenon does not occur during thiosulfate leaching owing to the very weak affinity of carbonaceous substance for gold thiosulfate complex []. This also leads to the problem that the dissolved gold in thiosulfate solution cannot be effectively recovered by active carbon adsorption which is the dominant technique used for pregnant cyanide solution. The dissolved gold in the Goldstrike deposit is recovered by a resin-in-leach process, and the loaded resin is eluted with a complex two-stage process. According to the literature [,], the thiosulfate consumptions in different studies are usually over 25 kg/t-ore during leaching and the dissolved gold cannot be effectively recovered by the simple techniques of active carbon adsorption and cementation that is used widely in cyanidation, both of which make the commercial competitiveness of thiosulfate leaching weak. Thus, the industrial application of this alternative method is still rare up to now.
In this review, the background knowledge about thiosulfate consumption will be presented at first, and then four solving measures to reduce the consumption are evaluated in detail. Afterwards, five common recovery techniques for rare-noble metals from leach solution are comparatively analyzed. In the end, how to improve the economy of the resin adsorption technique is discussed. The main intentions of this review are to propose an optimal approach for the reduction of thiosulfate consumption and a commercially-viable technique to recover dissolved gold from pregnant thiosulfate solution.
2. Background Knowledge about Thiosulfate Consumption
High thiosulfate consumption is one of bottlenecks for the wide industrialization of thiosulfate leaching. Yen et al. [] thought thiosulfate leaching is not competitive compared with cyanidation unless the thiosulfate consumption is controlled below 10 kg/t-ore. However, as mentioned above, the consumption is usually higher than 25 kg/t-ore, and sometimes it even exceeds 100 kg/t-ore. For example, as reported in the literature [], the thiosulfate consumption reached up to 165 kg/t-ore for a copper bearing gold ore. The main reasons for excessive thiosulfate consumption are as follows: thiosulfate itself is metastable and easily oxidized by Cu(II) and, furthermore, certain associated minerals can accelerate its oxidation [,].
2.1. Electrochemical-Catalytic Mechanism of Thiosulfate Leaching
The total reaction of thiosulfate leaching of pure gold is indicated in Equation (1), and the values of standard Gibbs free energy for Equations (1)–(13) were all calculated based on the thermodynamic data in the literature []. The essence of this reaction is an electrochemical process consisting of the oxidation of gold at the anode and reduction of oxygen at the cathode [,]. In fact, the reaction is very sluggish without the presence of Cu(II) and ammonia, and the speed of gold leaching increases 18 to 20 times with their catalysts []. The electrochemical-catalytic mechanism of thiosulfate leaching of gold foil is described in Figure 1. In anodic areas, NH3 moves to the gold surface and complexes with Au+ to generate the complex [Au(NH3)2]+. Then NH3 is substituted by S2O32− and, thus, the more stable complex [Au(S2O3)2]3− is formed. Thus, NH3 can not only stabilize Cu2+ in alkaline media by forming [Cu(NH3)4]2+, but also catalyzes the complex reaction between Au+ and S2O32−. These anodic reactions are shown as Equations (2)–(4). In cathodic areas, [Cu(NH3)4]2+ is reduced to [Cu(S2O3)3]5−, and newly-generated [Cu(S2O3)3]5− is quickly oxidized back into [Cu(NH3)4]2+ by dissolved oxygen in the solution. Thus, [Cu(NH3)4]2+ catalyzes the reaction of oxygen reduction. These cathodic reactions are listed as Equations (5) and (6).
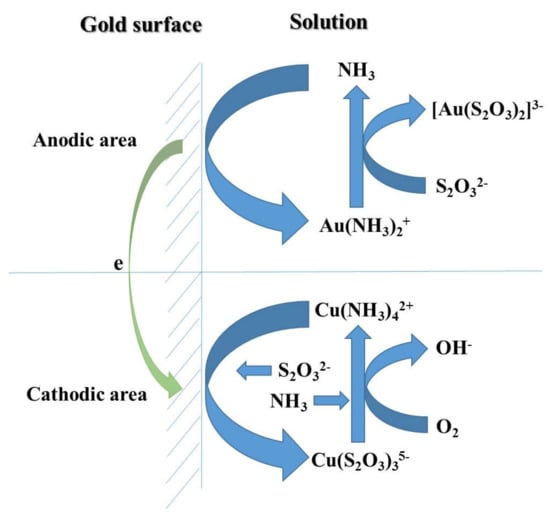
Figure 1.
Sketch map of the electrochemical-catalytic mechanism of thiosulfate leaching.
It is worth noting that [Cu(NH3)4]2+ as a catalyzer boosts gold dissolution dramatically, but it also markedly accelerates thiosulfate decomposition due to its relatively strong oxidizing ability []. As shown in Equation (7), S2O32− can be easily oxidized to S4O62− by [Cu(NH3)4]2+ whilst the [Cu(NH3)4]2+ itself is reduced to [Cu(S2O3)3]5−. As mentioned above, the [Cu(S2O3)3]5− will be also oxidized back into [Cu(NH3)4]2+ rapidly by oxygen. Thus, [Cu(NH3)4]2+ does not only catalyze the reduction of oxygen, but also catalyzes the oxidation of thiosulfate.
4Au + 8S2O32− + O2 + 2H2O → 4Au(S2O3)23− + 4OH− ΔG0 = −97.9 kJ/mol
Au → Au+ + e ΔG0 = 163.2 kJ/mol
Au+ + 2NH3 → Au(NH3)2+ ΔG0 = −74.1 kJ/mol
Au(NH3)2+ + 2S2O32− → 2NH3 + Au(S2O3)23− ΔG0 = −74.9 kJ/mol
Cu(NH3)42+ + 3S2O32− + e → Cu(S2O3)35− + 4NH3 ΔG0 = −21.9 kJ/mol
4Cu(S2O3)35− + 16NH3 + O2 + 2H2O → 4Cu(NH3)42+ + 4OH− + 12S2O32− ΔG0 = −67.1 kJ/mol
2Cu(NH3)42+ + 8S2O32− → 2Cu(S2O3)35− + S4O62− + 8NH3 ΔG0 = −19.8 kJ/mol
2.2. Route of Thiosulfate Decomposition
Thiosulfate is metastable in ammoniacal thiosulfate solution and tends to decompose into some intermediate products, such as S4O62−, S3O62−, and SO32−, due to its oxidizability [,] and the final oxidation product is SO42−. The simplified decomposition route of S2O32− is shown in Figure 2, and the corresponding reactions are listed as Equations (8)–(11). Equation (8) actually represents the total chemical reaction of Equations (6) and (7). According to the literature [], S2O32− is oxidized into S4O62− by Cu(II) involving the formation of the mixed ligand complex Cu(NH3)4S2O3, and the oxidation process is depicted in Figure 3. S2O32− can change the coordination sphere of [Cu(NH3)4]2+ via complexing with a central cupric ion at its axial coordination site, then [Cu(NH3)4]2+ easily obtains an electron from S2O32− and is reduced whilst S2O32− is oxidized. Thiosulfate can also self-decompose or be reduced into S0, S2−, and SO32−, as indicated by Equations (12) and (13) []. Elemental sulfur and sulfide deriving from these reactions deposit on gold surface and passivate its dissolution. In addition, polythionates generated from thiosulfate decomposition can recapture aurothiosulfate [Au(S2O3)2]3− from ion exchange resins and thus hinder gold recovery by resin adsorption [,]. Thus, thiosulfate decomposition not only results in reagent consumption, but also inhibits the leaching and recovery of gold.
4S2O32− + O2 + 2H2O → 2S4O62− + 4OH− ΔG0 = −106.7 kJ/mol
4S4O62− + 6OH− → 5S2O32− + 2S3O62− + 3H2O ΔG0 = −183.4 kJ/mol
2S3O62− + 6OH− → S2O32− + 4SO32− + 3H2O ΔG0 = −330.2 kJ/mol
2SO32− + O2 → 2SO42− ΔG0 = −516.4 kJ/mol
S2O32− → SO32− + S ΔG0 = 45.7 kJ/mol
S2O32− + 2e → SO32− + S2− ΔG0 = 137.6 kJ/mol
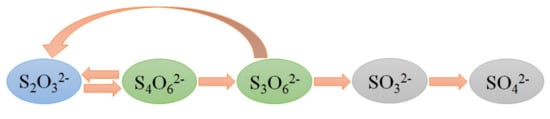
Figure 2.
Simplified route of thiosulfate decomposition.
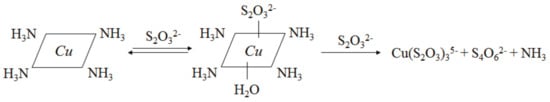
Figure 3.
Oxidation of thiosulfate by Cu(II) involving the formation of Cu(NH3)4S2O3.
2.3. Effect of Associated Minerals
It is demonstrated that associated minerals, such as pyrite and hematite, can catalyze the decomposition of thiosulfate [,]. By the comparison of four common sulfide minerals, Feng and Van Deventer [] concluded that the catalytic effect of pyrite on thiosulfate decomposition is stronger than that of arsenopyrite, pyrrhotite, and chalcopyrite. Xu and Schoonen [] attributed this effect of pyrite to its band structure. As Figure 4 shows, Chen [] divided the oxidation of thiosulfate catalyzed by pyrite into several steps. At first, thiosulfate is absorbed onto pyrite surface due to its strong affinity for S2O32−. After the adsorption, electrons move from thiosulfate to the surface, and then further transfer from the anodic area to the cathodic area via the conduction band of minerals. Finally, oxygen obtains electrons easily from the cathodic area of the mineral surface. In the presence of Cu(II)-ammine complex, it will preferentially gain an electron because its diffusibility in the solution is stronger than that of oxygen []. It is easy to know from this figure that pyrite catalyzes thiosulfate oxidation by providing a “bridge” for electron transfer. In addition, Chen [] also found that the presence of pyrite, pyrrhotite, chalcopyrite, arsenopyrite, chalcocite, bornite, and galena has significant detrimental effects on both of thiosulfate decomposition and gold leaching.
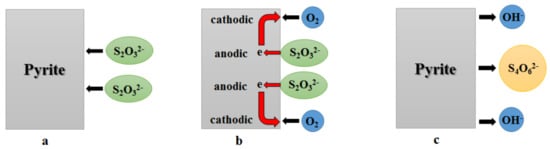
Figure 4.
Step-oxidation of thiosulfate catalyzed by pyrite. (a) the adsorption of thiosulfate; (b) the transfer of electrons; (c) the formation of reaction products.
Xu et al. [] found that all studied sulfide minerals can accelerate thiosulfate consumption and hinder gold dissolution in different extent. The rates of thiosulfate consumption with the presence of sulfide minerals followed the descending order of pyrite > arsenopyrite > chalcopyrite > galena > sphalerite, and the rates of gold dissolution were in the sequence of sphalerite > arsenopyrite > pyrite ≈ galena > chalcopyrite. The dominant causes for these two detrimental effects of sulfide minerals were inferred separately via the analyses of frontier orbital energy and XPS (X-ray photoelectron spectroscopy) to be the catalyses of these minerals on thiosulfate decomposition and the passivation of gold surfaces []. From the above, it is concluded that certain associated minerals can catalyze thiosulfate oxidation and, thus, accelerate the consumption. The minerals also hinder gold leaching because of the accumulation of passivation species deriving from thiosulfate decomposition on gold surfaces.
3. Measures for the Reduction of Thiosulfate Consumption
3.1. The Control of Reaction Conditions
Favorable reaction conditions are important to control thiosulfate consumption and promote gold dissolution []. Although gold dissolution is evidently enhanced by a high Cu(II) concentration, the thiosulfate consumption deriving from its oxidation by Cu(II) also increases markedly. Yen et al. [] put forward that high concentration ammonia reduces thiosulfate decomposition, but also hinders gold dissolution because it can stabilize Cu(II) and decrease its oxidizing ability. Concentrated thiosulfate solutions, excessive dissolved oxygen and high temperature all cause high consumption of thiosulfate, but diluted thiosulfate solutions, low oxygen concentration (for example, anaerobic condition) and low temperature make gold dissolution very slow [,,]. Additionally, reagent concentrations, solution pH should also be kept in an appropriate range. Molleman and Dreisinger [] proposed that the pH range of 9–10 is preferred to simultaneously stabilize thiosulfate and copper ammonia complex. Moreover, the concentration ratio of Cu(II) to Cu(I) is also a critical factor for both of thiosulfate consumption and gold dissolution because the ratio determines the oxidation potential of leach solution [,]. Oxygen plays an important role in changing the ratio by oxidizing Cu(I) to Cu(II). Furthermore, the concentration ratio of thiosulfate to ammonia should be maintained in a range of 0.5–1.0 in order to ensure the regeneration of Cu(II), because S2O32− and NH3 are separately the dominant ligands of Cu(I) and Cu(II) in ammoniacal thiosulfate solution [,].
Most of above-mentioned reaction conditions are constantly changing during leaching, so it is very difficult to precisely control each of them. However, the adoption of relatively low reagent concentrations under limited oxygen supply is a relatively easy method to decrease thiosulfate consumption. Lampinen et al. [] demonstrated that satisfactory gold dissolution from a pressure oxidized sulfide gold concentration and negligible thiosulfate consumption can be achieved with low concentrations of ammonia (0.2 M), thiosulfate (0.2 M) and Cu(II) (1.6 mM) under continuous aeration (0.2 L/min air). Based on the literature [,,,], the mixed pulp potential determined by the Cu(II)/Cu(I) redox equilibrium potential is a key factor for thiosulfate leaching, and there is a proper mixed potential range for both of high gold leaching ratio and acceptable thiosulfate consumption. According to the above analysis, the concentrations of thiosulfate, ammonia, and dissolved oxygen all can greatly affect the ratio of Cu(II)/Cu(I) and, thus, significantly impact the mixed potential. Certain associated minerals also have obvious influence on the potential, because they will be evidently oxidized and largely consume Cu(II) in ammoniacal thiosulfate solution [,] and, moreover, the interfacial potential of mineral particles is also an important ingredient of the mixed potential of actual slurry []. For each type of ore, the mixed pulp potential should be able to be adjusted into the appropriate range mainly by controlling the concentrations of thiosulfate, copper, ammonia, and dissolved oxygen. Thus, the regulation of mixed pulp potential will likely be an effective means to improve thiosulfate leaching.
3.2. The Use of Additives
3.2.1. Inorganic Additives
Some inorganic additives can be dedicated to reduce thiosulfate consumption. At the initial development stage, SO32− and S2− were added into thiosulfate solution []. As shown in Equations (14)–(16), SO32− can reduce tetrathionate to thiosulfate in alkaline media, and trithionate and tetrathionate can also be reduced to thiosulfate by S2−. However, the S2− will precipitate dissolved gold and impede gold leaching. Certain additives can easily complex with the central cupric ion of Cu(II) and occupy its axial coordination site, thus the Cu(II) is stabilized significantly and the formation of mixed ligand complex Cu(NH3)4S2O3 is prevented. As a result of this, the oxidation of thiosulfate by Cu(II) gets restrained. Senanayake [] concluded that the performances of anionic additives for the reduction of thiosulfate consumption are in the sequence of phosphate > sulfate > chloride > nitrate > sulfite > sulfide, because the coordinating abilities of these anions for cupric ions, i.e., their abilities to stabilize Cu(II), are exactly in this order based on the analysis of hard-soft and Lewis acid-base concept. Feng and Van Deventer [] confirmed that hexametaphosphate and orthophosphate both can decrease thiosulfate consumption and enhance gold dissolution, and hexametaphosphate has a better effect because it not only stabilizes Cu(II) via complexing with the cupric ion at the axial coordination site, but also disperses leach slurry by improving its rheology. The influence of cations, including calcium, sodium, and ammonium, on the leaching of a pyrite concentrate has been studied by Feng and Van Deventer [], and the results showed that calcium thiosulfate and sodium thiosulfate have the best effect on gold leaching and thiosulfate consumption, respectively.
S4O62− + SO32− + 2OH− → 2S2O32− + SO42− + H2O
S3O62− + S2− → 2S2O32−
4S4O62− + 2S2− + 6OH− → 9S2O32− + 3H2O
3.2.2. Organic Macromolecular Additives
In recent decades, some organic macromolecular compounds have also been found to weaken the interaction between Cu(II) and thiosulfate and, thus, decrease thiosulfate consumption. As reported in the literature [], a natural guar type surfactant (Gempolym M47) reduced the detrimental effect of hematite on thiosulfate consumption because it could stabilize Cu(II) and minimize the catalytic effect of hematite on thiosulfate decomposition. Ethylene diamine tetraacetic acid (EDTA) is a widely used complexing reagent, and can also complex with Cu(II) and forms a more stable coordination compound. Thus, the addition of EDTA decreases thiosulfate consumption []. In addition to the coordination with Cu(II), carboxymethyl cellulose (CMC) changes the semiconductor characteristic of arsenopyrite surfaces and, thus, relieves the negative impact of arsenopyrite on thiosulfate decomposition []. Commercially-available amino acids, such as l-valine, glycine, dl-α-alanine, and l-histidine, can also reduce thiosulfate consumption []. Ammonium alcohol polyvinyl phosphate does not only decrease the consumption, but also markedly enhances gold leaching because the electronegative long-chain alcohol polyvinyl significantly improves the rheology of leach slurry [].
Humic acid (HA) is a mixture of macromolecular organics and can be easily extracted from peat, lignite and decomposed coal, whose molecules have abundant negatively-charged functional groups like carboxyl and hydroxyl groups []. The gold extraction from a refractory gold concentrate calcine was improved from 72.6% to 81.4% and thiosulfate consumption was reduced from 42.7% to 13.2% by the use of HA []. Compared with other additives, such as CMC, CMS (sodium carboxymethyl starch), NaCl, and Na2SO3, HA had the best performance on the decrease of consumption and improvement of gold leaching. Xu et al. [] further studied the role of HA on thiosulfate leaching in the presence of common associated sulfide minerals, and the results indicated that the HA effectively reduced the harmful effect of sulfide minerals. The acting mechanism of HA was studied in detail, mainly through the analyses of mixed pulp potential, zeta potential, and XPS, and was proposed as follows: humic acid radical ions not only weaken the interaction between Cu(II) and thiosulfate via complexing with the central cupric ion at its axial coordination site and relieve the catalytic effect of minerals on thiosulfate decomposition by making the affinity of mineral surfaces for S2O32− disappear, but also prevent passivation species from coating gold surfaces through electrostatic repulsion.
3.3. The Generation of Thiosulfate In Situ
Thiosulfate can be generated by the oxidation of elemental sulfur in alkaline media, and the generated thiosulfate is able to efficiently leach gold. For example, the S0 in an acid leaching residue was oxidized into thiosulfate during alkaline pressure oxidation under the condition of 85 °C, 0.1–0.3 MPa and n(OH−/S0) 0.90–1.25, and 90% of gold in the residue was simultaneously extracted by the formed thiosulfate []. In another study, more than 80% of S0 added at 0.8 M in lime milk solution in an autoclave was transformed into thiosulfate under 85 °C, 0.35 MPa and n(Ca/S0) 0.55, and the gold extraction from an oxide gold ore containing negligible copper (0.007%, mass percent) by generated thiosulfate reached 81.6% after 5 h of leaching []. This implies that gold can be efficiently leached at relatively high temperature and oxygen partial pressure without cupric-ammonia catalysis. Calcium thiosulfate can also be produced by the oxidation of S0 in lime milk solution with a two-step process []. At the first step, calcium polysulfide and calcium polythionate are formed by the reactions between the elemental sulfur, oxygen, and lime at 70–75 °C and 0.3–0.5 MPa, and at the second step these two products are both further converted into calcium thiosulfate at 80–95 °C and 0.3–0.5 MPa. The overall reaction for the generation of calcium thiosulfate is listed as Equation (17). The S0 can be obtained via the oxidation of sulfidic gold-bearing feed during acidic pressure oxidation pretreatment, and then the obtained S0 was further reacted with added sulfite to form thiosulfate. The gold occurring in sulfidic feed was efficiently leached by the formed thiosulfate [].
Ca(OH)2 + 6S + 3O2 → 3CaS2O3 + 3H2O
Thiosulfate can also be directly generated by the oxidation of element S in sulfide minerals in alkaline media. Melashvili et al. [] studied the equation for thiosulfate yield during pyrite oxidation, and found that the sulfur in pyrite can be directly oxidized into thiosulfate under the condition of 80 °C, 0.07–0.28 MPa, and pH 12.5. As shown in Figure 5, the oxidation of sulfur in pyrite is a quasi-chain reaction process, where the valence of S is gradually increasing along the direction from left to right. Melashvili et al. [] further investigated the simultaneous dissolution of gold by in situ formed thiosulfate during pyrite oxidation, and the best result was that 96% of gold and 75% of silver were leached from a pyrite concentrate whilst 60% of sulfide sulfur is oxidized. Xu et al. [] found that the S in sulfidic gold-bearing ores/concentrates was partially oxidized into thiosulfate during alkaline pressure oxidation pretreatment under the condition of CaO 30–80 kg/t-feed, 140–160 °C and 0.3–0.4 MPa, and a part of gold in the feed was leached by the generated thiosulfate. From the above, it is worth noting that the generations of thiosulfate from elemental sulfur or sulfide minerals are both under high temperature and oxygen concentration. Thus, the solution composition is very complex due to the decomposition of formed thiosulfate under the strong oxidizing environment, which is detrimental to the gold recovery by resin adsorption [].
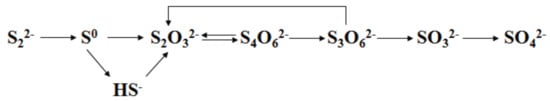
Figure 5.
Oxidation route of sulfur in pyrite.
3.4. The Replacement of Traditional Cupric-Ammonia Catalysis
Traditional cupric-ammonia catalysis has three inherent drawbacks [,]. The first one is that the thiosulfate decomposition is also catalyzed by Cu(II). The second one is the negative influence of copper on gold recovery from pregnant leach liquor. Copper ions are co-precipitated with dissolved gold during cementation using pulverized metal such as zinc and iron, and also are co-deposited on cathode surfaces during electrowinning. [Cu(S2O3)3]5− that is the predominant form of Cu(I) in ammoniacal thiosulfate solution seriously interferes with the adsorption or extraction of [Au(S2O3)2]3− by ion exchange resins or extractants due to the same ligand of these two coordination ions. The last one is that ammonia is poisonous and likely to pollute the atmosphere and water by volatilization and drainage. Thus, there have been some studies about the replacement of cupric-ammonia catalysis.
Fe3+ was considered to replace Cu2+ because it has also enough high potential to oxidize gold in ammoniacal thiosulfate solution, based on the thermodynamic knowledge that E0(Fe3+/Fe2+) (0.77 V) is higher than E0(Cu2+/Cu+) (0.15 V) []. Oxalate and EDTA both can stabilize iron ions in alkaline media and, thus, were adopted to substitute ammonia [,]. Although negligible thiosulfate consumption was achieved by the catalysis of ferric-oxalate or ferric-EDTA, the gold leaching rate was low without the extra addition of a probable carcinogen of thiourea. Furthermore, the Fe(III) was rapidly reduced to Fe(II) with the presence of sulfide minerals in actual leach slurry, likely because these minerals catalyzed the oxidation of thiosulfate by Fe(III), which notably impeded gold leaching. Ethanediamine can also complex with Cu2+ and forms stable cupric-ethanediamine complex []. The cupric-ethanediamine catalyzed thiosulfate leaching using a synergistic additive of cetyltrimethyl ammonium bromide was applied for a gold bearing ore containing high limonite, in which the gold leaching percentage reached up to 94.3% whilst thiosulfate consumption was as low as 1.12 kg/t-ore []. However, the development of cupric-ethanediamine catalysis is limited because ethanediamine is poisonous, corrosive, and expensive.
In the last ten years, nickel- and cobalt-based catalysts have been developed. Ninety-five percent of gold in a silicate-type ore was extracted by nickel-ammonia-catalyzed thiosulfate leaching, and thiosulfate consumption was only 1.2 kg/t-ore []. The dissolved gold in nickel-ammonia-catalyzed ammonium thiosulfate solution can be efficiently recovered by a strongly basic anion exchange resin, and the percentages of gold adsorption and elution are both over 99% because of the lack of interference of [Cu(S2O3)3]5− []. The extractions of gold and silver from a complex polymetallic sulfide concentrate by copper- and nickel-catalyzed ammoniacal thiosulfate leaching were comparatively studied in our previous work []. The results showed that thiosulfate consumption during nickel-catalyzed leaching was evidently lower than that of copper catalyzed leaching, and the gold and silver extractions with the nickel catalysis were also comparable. The construction of Eh–pH diagram of Ni–NH3–S2O32−–H2O system depicted in Figure 6 was carried out using Outokumpu HSC Chemistry 5.0 (Outotec Oyj, Espoo, Finland), and thermodynamic data were obtained from the database []. Based on the analysis of this figure in the pH range for thiosulfate leaching (9–11), the catalytic mechanism of nickel-ammonia is proposed as follows: [Ni(NH3)6]2+ is first oxidized into Ni3O4 then Ni3O4 acts as an oxidant for Au leaching, whilst Ni3O4 itself is reduced back to [Ni(NH3)6]2+ during the oxidation of Au. The corresponding chemical reactions are indicated as Equations (18) and (19). Ni3O4 particles originating from the oxidation of [Ni(NH3)6]2+ ions are likely to exist in the form of colloidal sol because of their extremely fine size and hydrophilia, thus, solid-state Ni3O4 can easily interact with gold in pulp owing to Brownian motion [,].
6Ni(NH3)62+ + 12OH− + O2 → 2Ni3O4 + 36NH3 + 6H2O
2Au + Ni3O4 + 4S2O32− + 18NH3 + 4H2O → 2Au(S2O3)23− + 3Ni(NH3)62+ + 8OH−
4Co(NH3)52+ + 4NH3 + O2 + 2H2O → 4Co(NH3)63+ + 4OH−
Au + Co(NH3)63+ + 2S2O32− → Au(S2O3)23− + Co(NH3)52+ + NH3
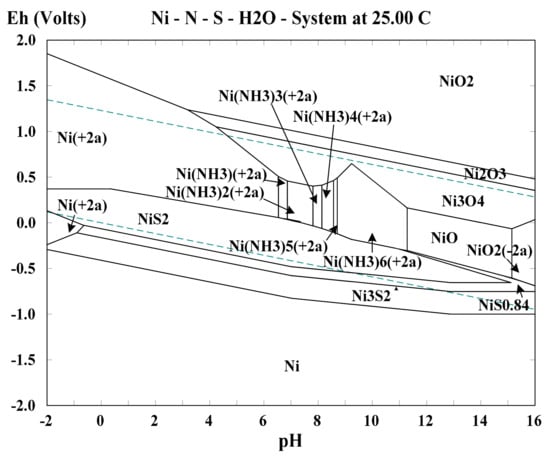
Figure 6.
Eh–pH diagram of Ni–NH3–S2O32−–H2O system under the conditions of 0.05 M Ni, 1.0 M S2O32−, 1.0 M NH3, 25 °C, and 101.3 kPa pressure.
The Eh–pH diagram of Co–NH3–S2O32−–H2O system is shown in Figure 7 drawn with the same way described in the above paragraph. Based on the analysis of this figure in the pH range of 9–11, it is concluded that cobalt-ammonia can also catalyze thiosulfate leaching of gold via the conversion between [Co(NH3)5]2+ and [Co(NH3)6]3+ with the aid of O2, and the related reactions are listed as Equations (20) and (21). The conclusion is supported by the literature []. Additionally, nickel-citrate and cobalt-EDTA are also found to be able to catalyze thiosulfate leaching through the transformation between the bivalent and trivalent nickel-citrate/cobalt-EDTA complex ions [,]. Compared with traditional cupric-ammonia catalysis, the consumptions with the nickel and cobalt catalysts are observably lower because the redox potential of the solution is significantly decreased as a result of the absence of [Cu(NH3)4]2+ and, furthermore, there is no ammonia pollution. In addition, nickel-citrate and cobalt-EDTA are, respectively, the dominant forms of nickel and cobalt complex ions, which are very different with [Au(S2O3)2]3− at the aspects of size and structure. Thus, the complex ions of nickel and cobalt have little interference with gold recovery by resin adsorption or solvent extraction. From the above, the replacement of traditional cupric-ammonia catalysis with nickel- and cobalt-based catalysts can not only reduce thiosulfate consumption, but is also beneficial to gold recovery from pregnant thiosulfate solution.
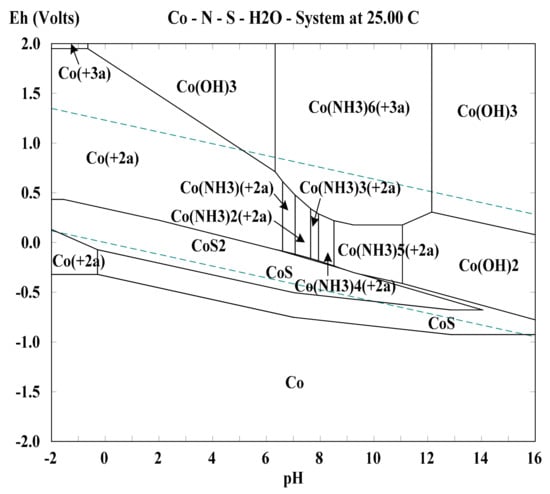
Figure 7.
Eh–pH diagram of Co–NH3–S2O32−–H2O system under the conditions of 0.05 M Co, 1.0 M S2O32−, 3.5 M NH3, 25 °C, and 101.3 kPa pressure.
4. Gold Recovery from Pregnant Thiosulfate Leach Solution
4.1. Activated Carbon Adsorption, Cementation, Electrowinning, and Solvent Extraction
Compared with gold leaching, there are fewer studies focusing on gold recovery from aurothiosulfate solution, but these works have indicated that activated carbon adsorption, cementation, electrowinning, and solvent extraction are all likely inapplicable [,,,,].
Activated carbon adsorption is a mature and widely applied technology in cyanidation, which is generally used in the form of carbon-in-pulp or carbon-in-leach. However, unfortunately [Au(S2O3)2]3− is very difficult to be adsorbed by activated carbon []. The low affinity of activated carbon for [Au(S2O3)2]3− may be due to the high negative charge and large size of this ion and weak interaction between carbon active sites and ionic ligand of S2O32− []. Adding cyanide into the pregnant solution can improve the gold recovery by this technique [], but this is unfavorable from an environmental perspective.
Cementation is also a common technique for gold recovery in cyanidation. Pulverized metals, such as zinc, iron, and aluminum, can precipitate gold from thiosulfate leach solution by a displacement reaction. However, unwanted cations are introduced and, thus, complicate the recycle of the solution. Copper ions as a catalyzer for gold leaching are also precipitated out by these active metals. Copper powder cementation avoids the above problems, but the dissolution of copper raises the redox potential of solution and leads to the re-dissolution of precipitated gold and severe oxidation of thiosulfate [,,]. The dosages of pulverized metals used in cementation are far more than theoretical amounts because the metal surfaces are easily passivated in the solution and, hence, the gold content of precipitation product is low [].
Aurothiosulfate ions can migrate to cathode surface and are reduced into metallic deposit by direct current during electrowinning. However, some metal complex ions such as [Cu(S2O3)3]5− will also be reduced into metals on the surface and contaminate the deposition product. Thiosulfate not only is reduced on cathode, but also gets oxidized on anode []. Due to these unwanted side reactions, gold product purity and electrowinning efficiency are dramatically decreased, and electric energy input is markedly increased. Furthermore, the recyclable copper ions and thiosulfate in the solution are severely depleted during electrowinning.
It has been proved that aurothiosulfate ions can be enriched in organic phase during solvent extraction employing the extractants of primary, secondary, and tertiary alkylamines, tertiary amine oxides, phosphines, phosphine oxides, phosphate esters, etc. [,,,]. However, the residual of extractant and organic phase in the solution is detrimental to its cycle use, and an enriching operation is required when Au(I) concentration is not high enough. Furthermore, the solution must be clarified to prevent the formation of the third phase that is the immiscible second organic phase between aqueous phase and organic phase, thus, additional plant equipment and processing time are necessary to achieve a complete solid-liquid separation []. This will bring a considerable increase in capital and operation costs.
4.2. Resin Adsorption
The technique of ion exchange resin adsorption has low demand for water quality, and it can be employed in the forms of resin-in-pulp and resin-in-leach [,]. The elution of loaded resin is at ambient temperature and its regeneration does not require the thermal reactivation and, furthermore, the regeneration procedure can be accomplished during the elution process through an elaborate choice of eluent [,]. In addition, the functional groups of resins may be designed and tailored to improve adsorption selectivity for aurothiosulfate []. Thus, resin adsorption technique has attracted increasing attention from researchers.
The ion exchange resins studied to recover gold from thiosulfate leach solution mainly involve weak-base anion resins and strong-base anion resins. The loading capacities of weak-base resins are usually less than 2 kg/t, and furthermore the capacities decrease markedly with the increase of pH in the range of 8–11 []. The reason for this is that the functional amine groups attached to the polymeric matrix tend to stay in the form of free base under relatively strong alkaline condition and are difficult to be protonated, thus, the ion-exchange ability of resins is damaged at a higher pH than 8. Thus, the weak base resins are not suitable to recover gold from ammoniacal thiosulfate solution whose pH is generally in the range of 9–11 [,].
The loading capacities of strong base resins usually reach up to 10–25 kg/t, and are independent of the solution pH. The high capacities make strong base resins more tolerant to competing anions and can efficiently adsorb the extremely low concentration aurothiosulfate []. However, the selectivity of these resins is poor, and inevitable anions such as [Cu(S2O3)3]5− and polythionate will strongly compete with [Au(S2O3)2]3− for the active sites of resin surface and are co-adsorbed with gold by resins. The co-adsorption of Cu(I) makes the adoption of a two-stage elution process necessary to separate the copper and gold. At first the copper can be selectively eluted by the solutions of oxygenated ammonia-ammonium sulfate, ammonium thiosulfate, etc., thus, a copper rich solution is produced. Then, the gold is able to be effectively eluted by the single component solutions of thiocyanate, polythionates, perchlorate, and nitrate or the two component solutions of thiourea + sulfuric acid and sulfite + chloride [,]. The elution with single component solutions requires an additional regeneration procedure for resins to regain their loading capacities and avoid the accumulation of elution reagents in leach solution. For the two component solutions, the synergistic reagents of thiourea (TU) and sulfite can form the mixed ligand complexes of [Au(S2O3)TU]− and [Au(S2O3)(SO3)]3− through reacting with [Au(S2O3)2]3−, and these two complexes have observably reduced affinity for the resins and, thus, are easily eluted by sulfate and chloride ions, respectively. The SO42− and Cl− on resin surface are easily substituted by [Au(S2O3)2]3− when the eluted resins are returned into pregnant leach solution. Furthermore, these ions have no obvious harmful impact on thiosulfate leaching, which is deduced from the fact that sulfate and chloride both can be used as additives to stabilize Cu(II) during leaching. Thus, the resins have regained their loading capacities during the gold elution process, and the additional regeneration procedure is omitted by use of the two component eluent solutions [].
The commercial competitiveness of resin adsorption technique is low due to the cost and time involving in operating this complicated two-stage elution process []. However, if the traditional cupric-ammonia catalysis is replaced by other metals such as nickel and cobalt based catalysts, whose complex ions have weak affinity for the resins, and the co-adsorption of base metals with gold on resin surface will not occur. Thus, the two-stage elution process can be substituted by a simple one-stage process, and the resin adsorption technique for pregnant thiosulfate solution become commercially viable.
5. Conclusions
Thiosulfate leaching is a promising alternative to cyanidation. Although there have been a great number of studies about thiosulfate leaching of gold in the past several decades, its successful commercial application is rare. The main reasons for this are as follows: thiosulfate consumption is high because thiosulfate itself is metastable and easily oxidized by Cu(II), which is a catalyst for gold leaching and, furthermore, certain associated minerals can accelerate the oxidation; the recovery of dissolved gold in pregnant leach solution is difficult because of the low affinity of activated carbon for [Au(S2O3)2]3−, the complex composition of the solution, and the interference of [Cu(S2O3)3]5−.
The adoption of low reagent concentrations under limited oxygen supply and the regulation of mixed pulp potential via the elaborate adjustment of reaction conditions are both beneficial to reduce thiosulfate consumption. Some additives can be dedicated to stabilize thiosulfate, especially certain organic macromolecular additives that decrease the oxidizing ability of Cu(II) and, furthermore, relieve the catalytic effect of associated minerals on thiosulfate oxidation. The generation of thiosulfate in situ deriving from the oxidation of sulfide minerals is also possible to solve the high consumption problem. However, the replacement of traditional cupric-ammonia catalysis with other metals, such as nickel- and cobalt-based catalysts is the most optimal approach, because it not only reduces thiosulfate consumption observably by decreasing the redox potential of the leach solution significantly, but also eliminates the interference of [Cu(S2O3)3]5− for gold recovery from pregnant thiosulfate solution.
The technique of ion-exchange resin adsorption is suitable for the gold recovery from thiosulfate leach solution because the resin can be employed in the form of resin-in-leach/pulp and be eluted and regenerated simultaneously at ambient temperature through the elaborate choice of eluent. Strong-base anion resins are preferred to weak-base anion resins because their loading capacities are markedly higher and independent of solution pH, but their selectivity for [Au(S2O3)2]3− against [Cu(S2O3)3]5− is low. Thus, a complex and high-cost two-stage elution process is required to separate the copper and gold loaded on the resins. If the traditional cupric-ammonia catalysis is replaced by other metal-based catalysts, whose complex ions have weak affinity for the resins, the two-stage process will be substituted by a simple and low-cost one-stage process.
Acknowledgments
Financial supports from the National Natural Science Foundation of China (grant Nos. 51504293 and 51574284), the China Postdoctoral Science Foundation (grant No. 2014M550422), the Hunan Provincial Natural Science Foundation of China (grant No. 2015JJ3149), the Fundamental Research Funds for the Central Universities of Central South University (No. 2017zzts799), and the Open-End Fund for the Valuable and Precision Instruments of Central South University (CSUZC201704) are all gratefully acknowledged.
Author Contributions
Bin Xu completed the main part of this review; Wenhao Kong and Yongbin Yang completed the other parts of this review; Qian Li and Tao Jiang offered the advice for writing and revision of this review; Xiaoliang Liu collected the references in the course of writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hilson, G.; Monhemius, A.J. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Muir, D.M. A review of the selective leaching of gold from oxidised copper-gold ores with ammonia-cyanide and new insights for plant control and operation. Miner. Eng. 2011, 24, 576–582. [Google Scholar] [CrossRef]
- Jiang, T. Chemistry of Extractive Metallurgy of Gold; Hunan Science and Technology Press: Changsha, China, 1998. [Google Scholar]
- Hasab, M.G.; Rashchi, F.; Raygan, S. Chloride-hypochlorite leaching and hydrochloric acid washing in multi-stages for extraction of gold from a refractory concentrate. Hydrometallurgy 2014, 142, 56–59. [Google Scholar] [CrossRef]
- Hasab, M.G.; Raygan, S.; Rashchi, F. Chloride-hypochlorite leaching of gold from a mechanically activated refractory sulfide concentrate. Hydrometallurgy 2013, 138, 59–64. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Li, G.; Jiang, T. Fluidized roasting-stage leaching of a silver and gold bearing polymetallic sulfide concentrate. Hydrometallurgy 2014, 147, 79–82. [Google Scholar] [CrossRef]
- Chen, X. Associated Sulfide Minerals in Thiosulfate Leaching of Gold: Problems and Solutions. Ph.D. Thesis, Queen’s University, Kingston, ON, Canada, September 2008. [Google Scholar]
- Öncel, M.S.; İnce, M.; Bayramoǧlu, M. Leaching of silver from solid waste using ultrasound assisted thiourea method. Ultrason. Sonochem. 2005, 12, 237–242. [Google Scholar] [CrossRef]
- Örgül, S.; Atalay, Ü. Reaction chemistry of gold leaching in thiourea solution for a Turkish gold ore. Hydrometallurgy 2002, 67, 71–77. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Chai, L. Research status and prospect of gold leaching in alkaline thiourea solution. Miner. Eng. 2006, 19, 1301–1306. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Thiosulphate leaching of gold in the presence of carboxymethyl cellulose (CMC). Miner. Eng. 2011, 24, 115–121. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Breuer, P.L.; Chu, C.K. The importance of controlling oxygen addition during the thiosulfate leaching of gold ores. Int. J. Miner. Process. 2003, 72, 323–330. [Google Scholar] [CrossRef]
- Lampinen, M.; Laari, A.; Turunen, I. Ammoniacal thiosulfate leaching of pressure oxidized sulfide gold concentrate with low reagent consumption. Hydrometallurgy 2015, 151, 1–9. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Li, G. Stage leaching of a complex polymetallic sulfide concentrate: A focus on the extractions of Ag and Au. Hydrometallurgy 2016, 159, 87–94. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Yin, W.; Jiang, T.; Li, G. Thiosulfate leaching of Au, Ag and Pd from a high Sn, Pb and Sb bearing decopperized anode slime. Hydrometallurgy 2016, 164, 278–287. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Genik-Sas-Berezowsky, R.M.; Sefton, V.B.; Gormely, L.S. Recovery of Precious Metals from Metal Sulphides. U.S. Patent 4,070,182, 24 January 1978. [Google Scholar]
- Grosse, A.C.; Dicinoski, G.W.; Shaw, M.J.; Haddad, P.R. Leaching and recovery of gold using ammoniacal thiosulfate leach liquors (a review). Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]
- Muir, D.M.; Aylmore, M.G. Thiosulphate as an alternative to cyanide for gold processing–issues and impediments. Trans. Inst. Min. Metall. Sect. C 2004, 113, 2–12. [Google Scholar] [CrossRef]
- Zhang, X.M.; Senanayake, G. A review of ammoniacal thiosulfate leaching of gold: An update useful for further research in Non-cyanide gold lixiviants. Miner. Process Extr. Metall. Rev. 2016, 37, 385–411. [Google Scholar] [CrossRef]
- Fleming, C.A.; McMullen, J.; Thomas, K.G.; Wells, J.A. Recent advances in the development of an alternative to the cyanidation process: Thiosulfate leaching and resin in pulp. Miner. Metall. Proc. 2001, 20, 1–9. [Google Scholar]
- Marchbank, A.R.; Thomas, K.G.; Dreisinger, D.; Fleming, C. Gold Recovery from Refractory Carbonaceous Ores by Pressure Oxidation and Thiosulfate Leaching. U.S. Patent 5,536,297, 28 July 1996. [Google Scholar]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Liu, S.; Li, G. The development of an environmentally friendly leaching process of a high C, As and Sb bearing sulfide gold concentrate. Miner. Eng. 2016, 89, 138–147. [Google Scholar] [CrossRef]
- Wan, R.Y.; LeVier, K.M. Solution chemistry factors for gold thiosulfate heap leaching. Int. J. Miner. Process. 2003, 72, 311–322. [Google Scholar] [CrossRef]
- Yen, W.T.; Stogran, K.; Fujita, T. Gold extraction from a copper bearing ore by thiosulphate leaching. Resour. Process. 1996, 43, 83–87. [Google Scholar] [CrossRef]
- Black, S.B. The Thermodynamic Chemistry of the Aqueous Copper-Ammonia Thiosulfate System. Ph.D. Thesis, Murdoch University, Perth, Australia, 2006. [Google Scholar]
- Jiang, T.; Chen, J.; Xu, S. Electrochemistry and mechanism of leaching gold with ammoniacal thiosulfate. In Proceedings of the XVIII International Mineral Processing Congress, Australasian Institute of Mining and Metallurgy, Sydney, Australia, 23–28 May 1993. [Google Scholar]
- Jiang, T.; Chen, J.; Xu, S. A kinetic study of gold leaching with thiosulphate. Hydrometall. Fundam. Technol. Innov. 1993, 119–126. [Google Scholar]
- Ter-Arakelyan, K. On technological expediency of sodium thiosulphate usage for gold extraction from raw material. lzv. VUZ Tsvetn. Metall. 1984, 5, 72–76. [Google Scholar]
- Melashvili, M.; Fleming, C.; Dymov, I.; Matthews, D.; Dreisinger, D. Equation for thiosulphate yield during pyrite oxidation. Miner. Eng. 2015, 74, 105–111. [Google Scholar] [CrossRef]
- Senanayake, G. Gold leaching by copper (II) in ammoniacal thiosulphate solutions in the presence of additives. Part I: A review of the effect of hard–soft and Lewis acid-base properties and interactions of ions. Hydrometallurgy 2012, 115, 1–20. [Google Scholar] [CrossRef]
- Senanayake, G. The role of ligands and oxidants in thiosulfate leaching of gold. Gold Bull. 2005, 38, 170–179. [Google Scholar] [CrossRef]
- Chu, C.K.; Breuer, P.L.; Jeffrey, M.I. The impact of thiosulfate oxidation products on the oxidation of gold in ammonia thiosulfate solutions. Miner. Eng. 2003, 16, 265–271. [Google Scholar] [CrossRef]
- Ahern, N. Thiosulfate Degradation during Gold Leaching in Ammoniacal Thiosulfate Solutions: A Focus Trithionate. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, October 2005. [Google Scholar]
- Feng, D.; Van Deventer, J.S.J. Ammoniacal thiosulphate leaching of gold in the presence of pyrite. Hydrometallurgy 2006, 82, 126–132. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Effect of hematite on thiosulphate leaching of gold. Int. J. Miner. Process. 2007, 82, 138–147. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Leaching behaviour of sulphides in ammoniacal thiosulphate systems. Hydrometallurgy 2002, 63, 189–200. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A. The stability of thiosulfate in the presence of pyrite in low-temperature aqueous solutions. Geochim. Cosmochim. Acta 1995, 59, 4605–4622. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhang, X.; Li, G. Effect of common associated sulfide minerals on thiosulfate leaching of gold and the role of humic acid additive. Hydrometallurgy 2017, 171, 44–52. [Google Scholar] [CrossRef]
- Yen, W.T.; Aghamirian, M.; Deschenes, G.; Theben, S. Gold extraction from mild refractory ore using ammonium thiosulfate. In Proceedings of the International Symposium on Gold Recovery, Montreal, QC, Canada, 3–6 May 1998. [Google Scholar]
- Chen, X. Thiosulfate Stability in Gold Leaching Process. Master’s Thesis, Queen’s University, Kingston, ON, Canada, May 2001. [Google Scholar]
- Feng, D.; Van Deventer, J.S.J. The role of oxygen in thiosulphate leaching of gold. Hydrometallurgy 2007, 85, 193–202. [Google Scholar] [CrossRef]
- Molleman, E.; Dreisinger, D. The treatment of copper–gold ores by ammonium thiosulfate leaching. Hydrometallurgy 2002, 66, 1–21. [Google Scholar] [CrossRef]
- Arima, H.; Fujita, T.; Yen, W.T. Using nickel as a catalyst in ammonium thiosulfate leaching for gold extraction. Mater. Trans. 2004, 45, 516–526. [Google Scholar] [CrossRef]
- Ha, V.H.; Lee, J.C.; Huynh, T.H.; Jeong, J.; Pandey, B.D. Optimizing the thiosulfate leaching of gold from printed circuit boards of discarded mobile phone. Hydrometallurgy 2014, 149, 118–126. [Google Scholar] [CrossRef]
- Liu, X.; Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhang, X.; Zhang, Y. Effect of galena on thiosulfate leaching of gold. Hydrometallurgy 2017, 171, 157–164. [Google Scholar] [CrossRef]
- Sun, W. Mechanism and Applications of Potential-Controlled Flotation in Lime Adjust High Alkali Pulp. Ph.D. Thesis, Central South University, Changsha, China, December 2001. [Google Scholar]
- Feng, D.; Van Deventer, J.S.J. Thiosulphate leaching of gold in the presence of orthophosphate and polyphosphate. Hydrometallurgy 2011, 106, 38–45. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Effect of thiosulphate salts on ammoniacal thiosulphate leaching of gold. Hydrometallurgy 2010, 105, 120–126. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Thiosulphate leaching of gold in the presence of ethylenediaminetetraacetic acid (EDTA). Miner. Eng. 2010, 23, 143–150. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. The role of amino acids in the thiosulphate leaching of gold. Miner. Eng. 2011, 24, 1022–1024. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, B.; Li, Q.; Jiang, T.; Zhang, X.; Li, G.; Guo, Y.; Chen, X.; Fan, X.; Huang, Z.; et al. A Method of Thiosulfate Leaching of Gold with the Additive of Ammonium Alcohol Polyvinyl Phosphate. China Patent No. 201510223605.1, 12 April 2017. [Google Scholar]
- Allard, B. A comparative study on the chemical composition of humic acids from forest soil, agricultural soil and lignite deposit: Bound lipid, carbohydrate and amino acid distributions. Geoderma 2006, 130, 77–96. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Jiang, T.; Li, Q.; Zhang, X.; Wang, D. Improved thiosulfate leaching of a refractory gold concentrate calcine with additives. Hydrometallurgy 2015, 152, 214–222. [Google Scholar] [CrossRef]
- Fang, Z.H.; Li, Z.J.; Shi, W.; Han, B.L. Gold leaching of a residue containing elemental sulfur with lime added under pressurized oxygen. Chin. J. Process Eng. 2002, 2, 17–20. [Google Scholar]
- Zhou, S. Research on the Alkaline Pressure Leaching of Gold Based on Situ Generated Thiosulfate. Master’s Thesis, Central South University, Changsha, China, May 2016. [Google Scholar]
- Hojjatie, M.M.; Lockhart, C.L.F.; Dimitriadis, A.; Van Cauwenbergh, J.; Van Dael, R. Continuous Process for Preparation of Calcium Thiosulfate Liquid Solution. U.S. Patent 8,454,929, 4 June 2013. [Google Scholar]
- Choi, Y.; Kondos, P.; Aylmore, M.G.; McMullen, J.; Van Weert, G. Thiosulfate Generation in situ in Precious Metal Recovery. U.S. Patent 7,572,317, 11 August 2009. [Google Scholar]
- Melashvili, M.; Fleming, C.; Dymov, I.; Matthews, D.; Dreisinger, D. Dissolution of gold during pyrite oxidation reaction. Miner. Eng. 2016, 87, 2–9. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Liu, S.; Zhou, S.; Li, G.; Guo, Y.; Chen, X.; Fan, X.; et al. A Technology of Gold Leaching from Sulfide Gold Ores/Concentrates. China Patent 201510223325.0, 5 April 2017. [Google Scholar]
- Chandra, I.; Jeffrey, M.I. A fundamental study of ferric oxalate for dissolving gold in thiosulfate solutions. Hydrometallurgy 2005, 77, 191–201. [Google Scholar] [CrossRef]
- Heath, J.A.; Jeffrey, M.I.; Zhang, H.G.; Rumball, J.A. Anaerobic thiosulfate leaching: Development of in situ gold leaching systems. Miner. Eng. 2008, 21, 424–433. [Google Scholar] [CrossRef]
- Yu, H.; Zi, F.; Hu, X.; Zhong, J.; Nie, Y.; Xiang, P. The copper–ethanediamine–thiosulphate leaching of gold ore containing limonite with cetyltrimethyl ammonium bromide as the synergist. Hydrometallurgy 2014, 150, 178–183. [Google Scholar] [CrossRef]
- Arima, H.; Fujita, T.; Yen, W.T. Gold recovery from nickel catalyzed ammonium thiosulfate solution by strongly basic anion exchange resin. Mater. Trans. 2003, 44, 2099–2107. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E.; Motekaitis, R.J. Critically Selected Constants of Metal Complexes Database; Version 5.0 Software; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Zhou, Z. Fundament of Colloid Chemistry; Beijing University Press: Beijing, China, 1987. [Google Scholar]
- Feng, D.; Van Deventer, J.S.J. The role of heavy metal ions in gold dissolution in the ammoniacal thiosulphate system. Hydrometallurgy 2002, 64, 231–246. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhou, S.; Liu, X.; Li, G.; Guo, Y.; Chen, X.; Fan, X.; et al. A Technology of Thiosulfate Leaching of Gold with the Catalysis of Nickel-Citrate. China Patent 201510229799.6, 7 September 2016. [Google Scholar]
- Xu, B.; Li, Q.; Liu, X.; Jiang, T.; Yang, Y.; Min, X.; Li, G.; Guo, Y.; Fan, X.; Zhang, Y.; et al. A Method of Thiosulfate Leaching of Gold with the Catalysis of Cobalt-Ethylene Diamine Tetraacetic Acid. China Patent 201610018078.5, 15 May 2017. [Google Scholar]
- Gallagher, N.P.; Hendrix, J.L.; Milosavljevic, E.B.; Nelson, J.H.; Solujic, L. Affinity of activated carbon towards some gold(I) complexes. Hydrometallurgy 1990, 25, 305–316. [Google Scholar] [CrossRef]
- Lulham, J.; Lindsay, D. Separation Process. International Patent No. WO/1991/011539, 8 August 1991. [Google Scholar]
- Guerra, E.; Dreisinger, D.B. A study of the factors affecting copper cementation of gold from ammoniacal thiosulphate solution. Hydrometallurgy 1999, 51, 155–172. [Google Scholar] [CrossRef]
- Choo, W.L.; Jeffrey, M.I. An electrochemical study of copper cementation of gold(I) thiosulfate. Hydrometallurgy 2004, 71, 351–362. [Google Scholar] [CrossRef]
- Hiskey, J.B.; Lee, J. Kinetics of gold cementation on copper in ammoniacal thiosulfate solution. Hydrometallurgy 2003, 69, 45–56. [Google Scholar] [CrossRef]
- Lee, J. Gold Cementation on Copper in Thiosulfate Solution: Kinetic, Electrochemical, and Morphological Studies. Ph.D. Thesis, The University of Arizona, Tucson, AZ, USA, December 2003. [Google Scholar]
- Zhao, J.; Wu, Z. Extraction of gold from thiosulfate solutions with alkyl phosphorus esters. Hydrometallurgy 1997, 46, 363–372. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Z. Extraction of gold from thiosulfate solutions using amine mixed with neutral donor reagents. Hydrometallurgy 1998, 48, 133–144. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Z.; Chen, J. Solvent extraction of gold in thiosulfate solutions with amines. Solvent Extr. Ion Exch. 1998, 16, 527–543. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Z.; Chen, J. Gold extraction from thiosulfate solutions using mixed amines. Solvent Extr. Ion Exch. 1998, 16, 1407–1420. [Google Scholar] [CrossRef]
- Mohansingh, R. Adsorption of Gold from Gold Copper Ammonium Thiosulfate Complex onto Activated Carbon and Ion Exchange Resins. Master’s Thesis, University of Nevada, Reno, NV, USA, May 2000. [Google Scholar]
- Zhang, H.; Dreisinger, D.B. The recovery of gold from ammoniacal thiosulfate solutions containing copper using ion exchange resin columns. Hydrometallurgy 2004, 72, 225–234. [Google Scholar] [CrossRef]
- Fleming, C.A. Hydrometallurgy of precious metals recovery. Hydrometallurgy 1992, 30, 127–162. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Hewitt, D.M.; Dai, X.; Brunt, S.D. Ion exchange adsorption and elution for recovering gold thiosulfate from leach solutions. Hydrometallurgy 2010, 100, 136–143. [Google Scholar] [CrossRef]
- Zhang, H.; Dreisinger, D.B. The adsorption of gold and copper onto ion-exchange resins from ammoniacal thiosulfate solutions. Hydrometallurgy 2002, 66, 67–76. [Google Scholar] [CrossRef]
- Kononova, O.N.; Kholmogorov, A.G.; Kononov, Y.S.; Pashkov, G.L.; Kachin, S.V.; Zotova, S.V. Sorption recovery of gold from thiosulphate solutions after leaching of products of chemical preparation of hard concentrates. Hydrometallurgy 2001, 59, 115–123. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).