Beneficiation of Low-Grade Dilband Iron Ore by Reduction Roasting
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
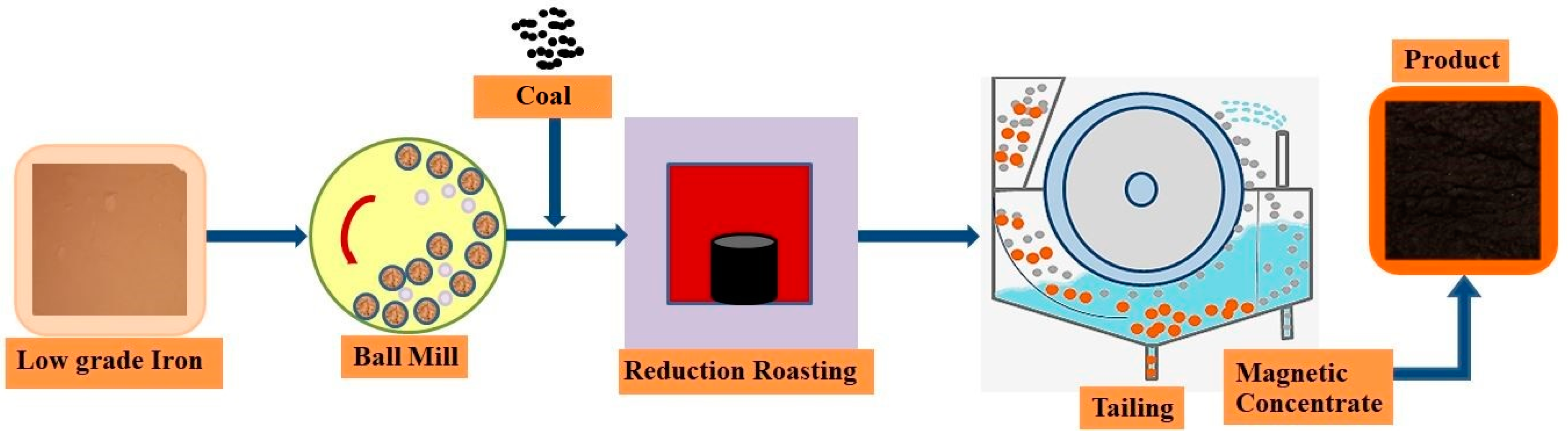
2.2. Methods
2.2.1. Crushing
2.2.2. High-Temperature Reduction Roasting
2.2.3. Magnetic Separation
2.3. Testing and Characterization
2.3.1. Sieving/Laser Particle Analysis
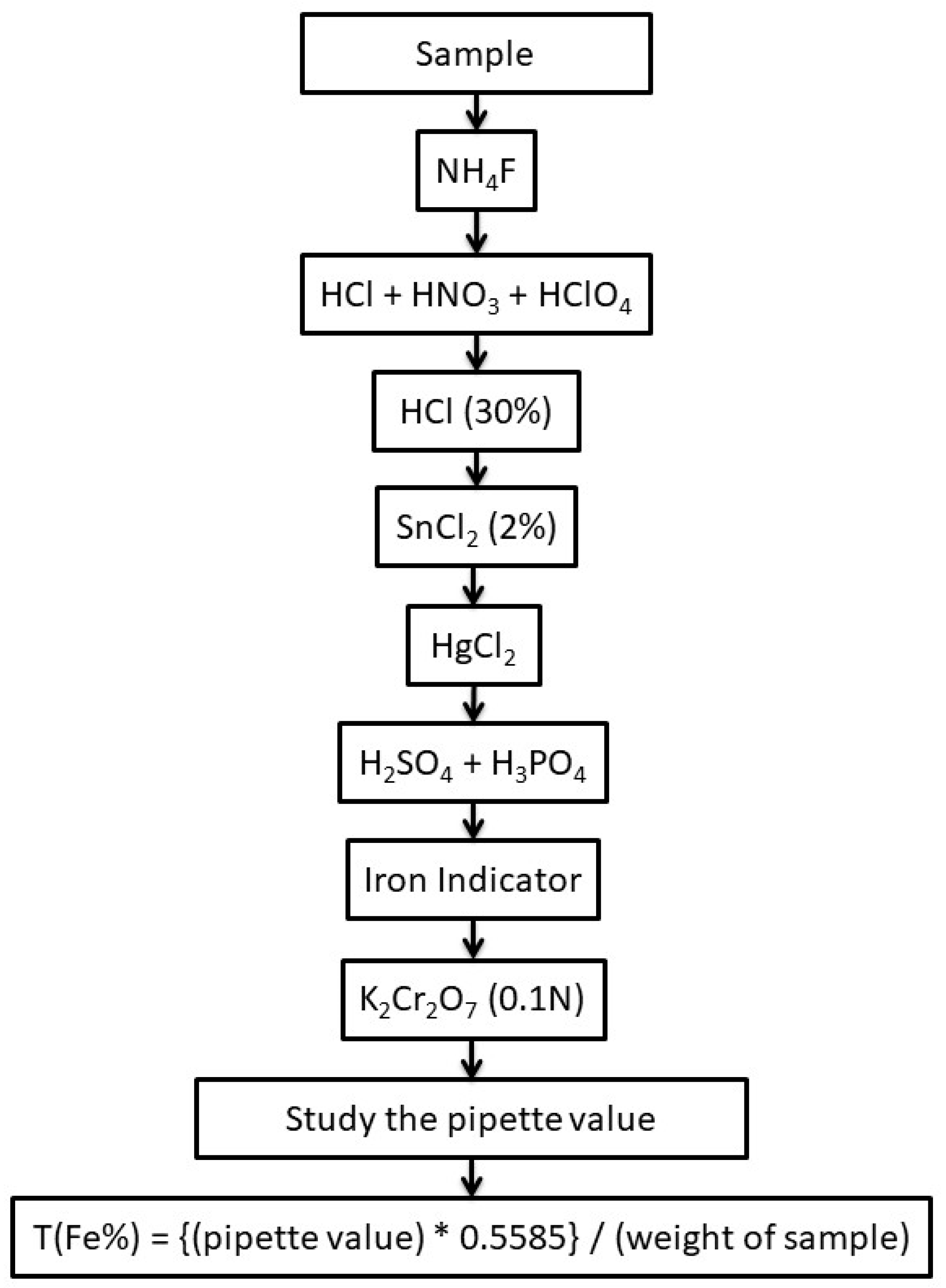
2.3.2. Chemical analysis
2.3.3. XRD
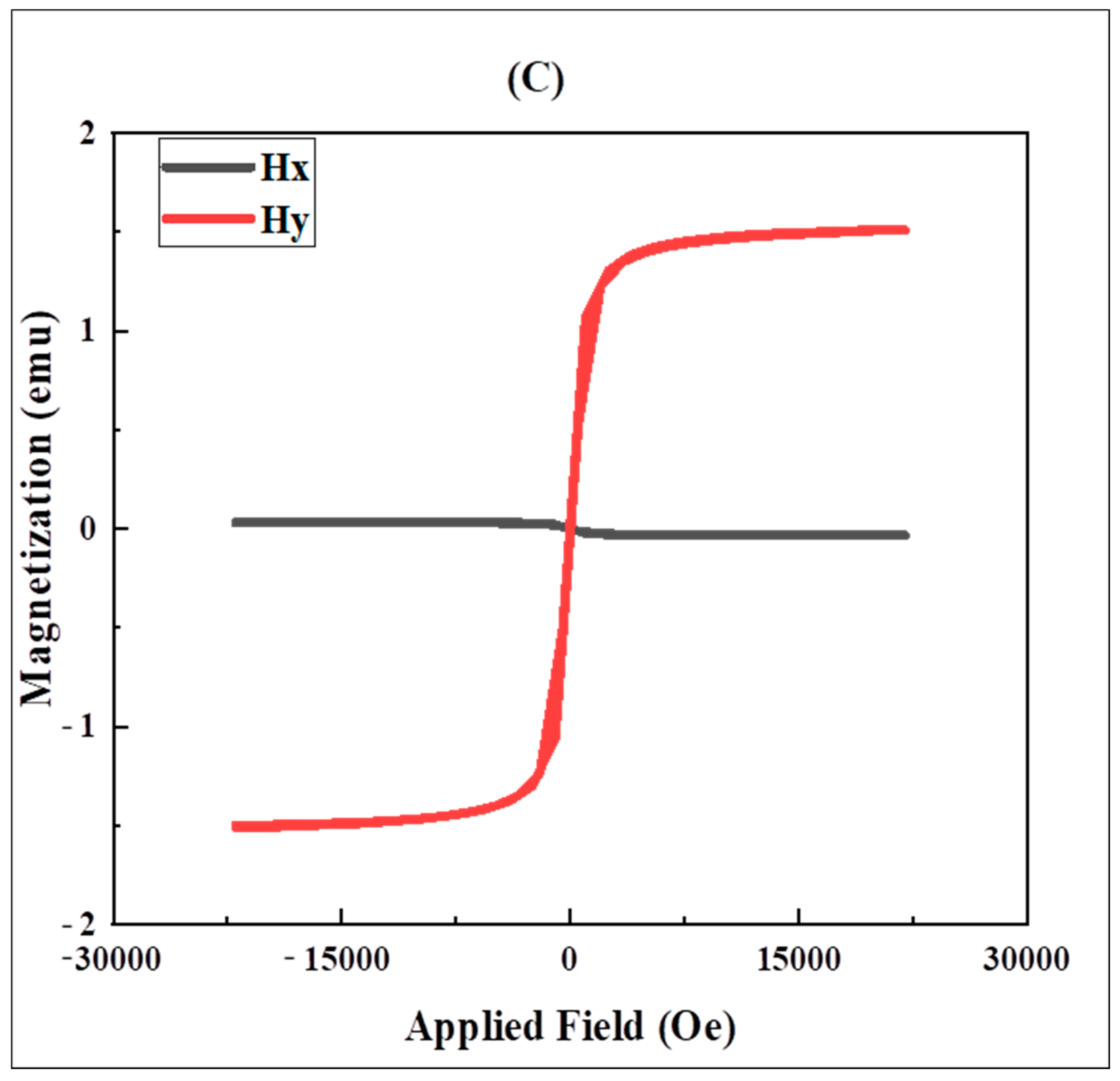
2.3.4. VSM
2.4. Recovery and Grade Calculations
3. Results and Discussion
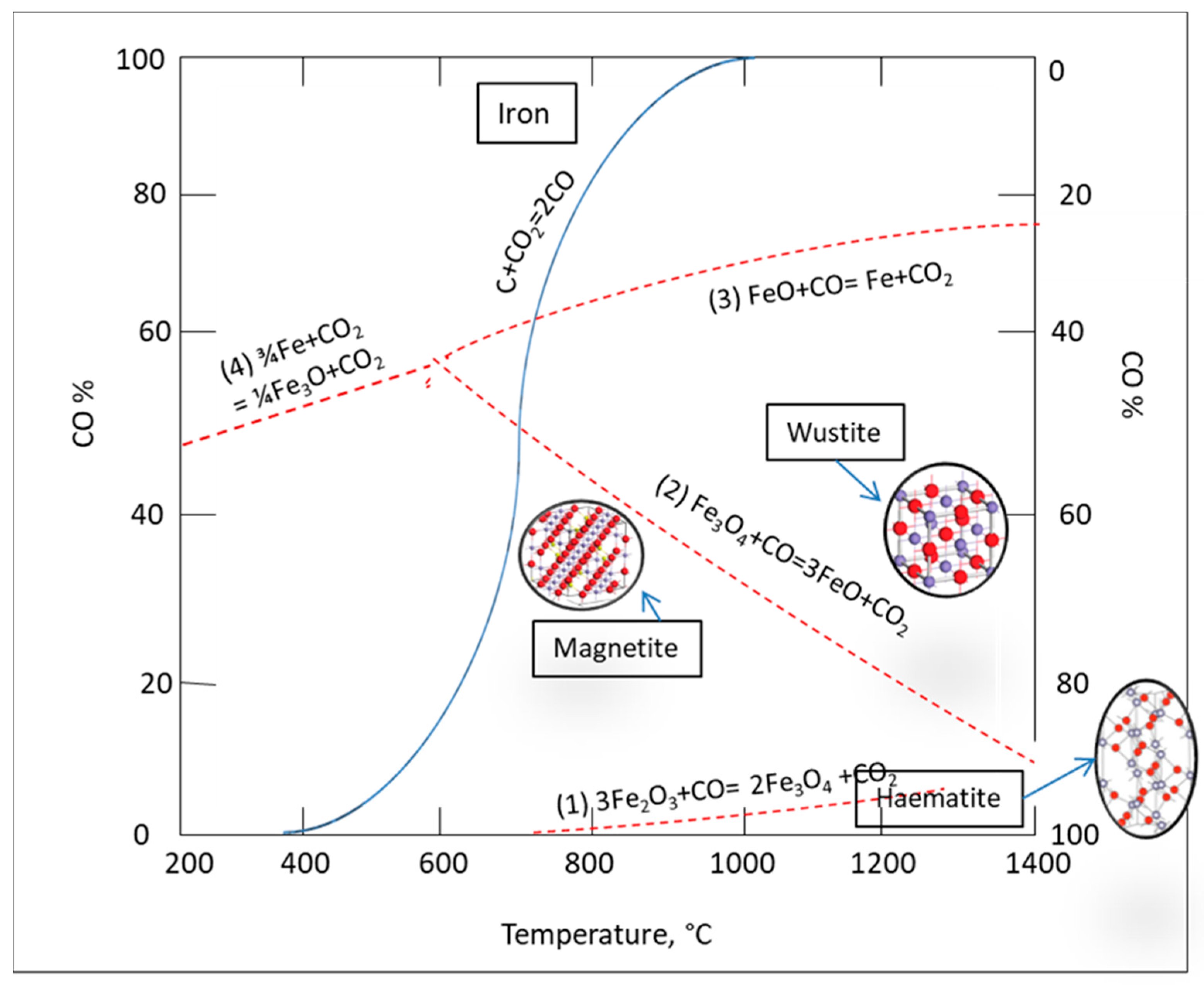
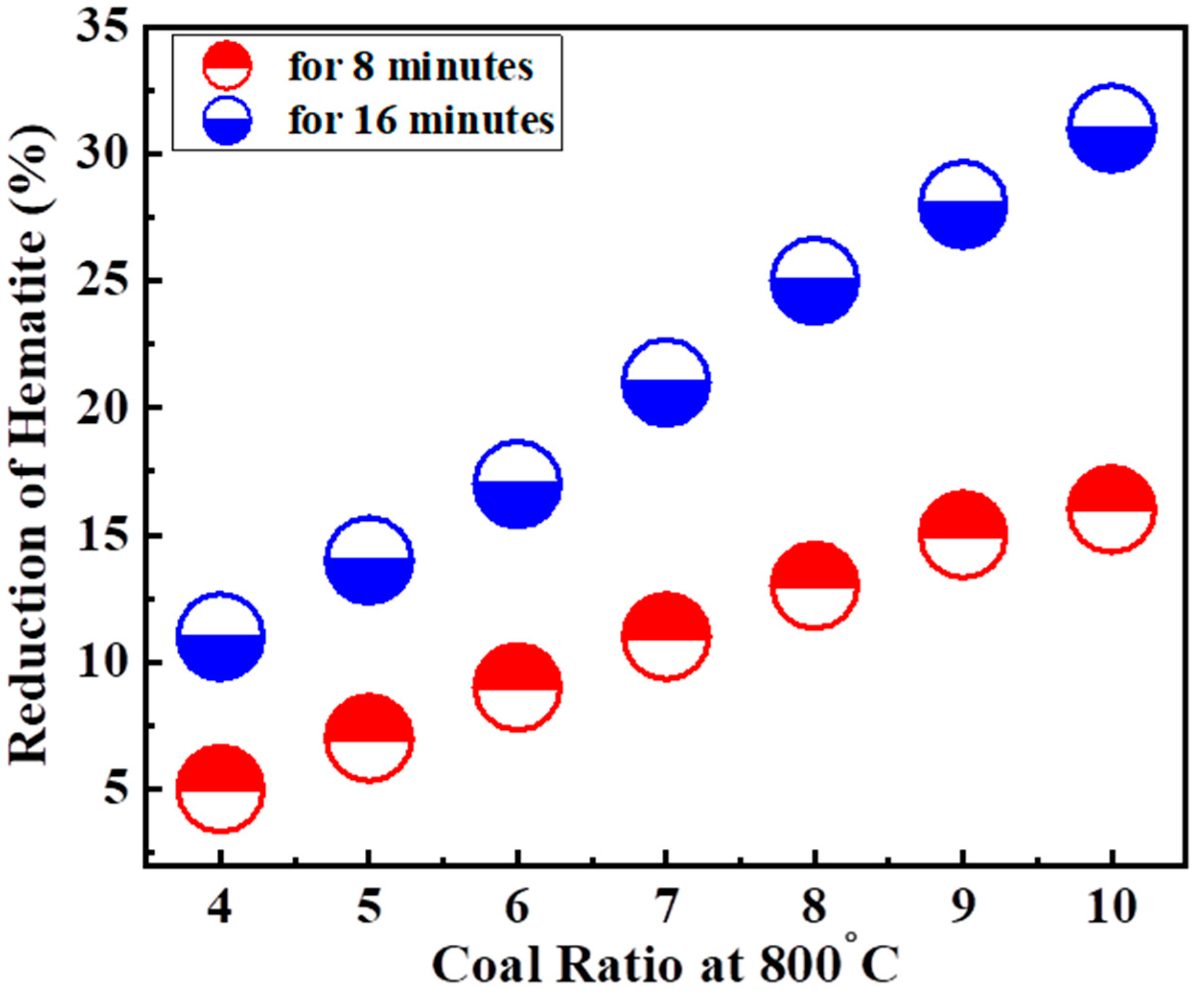
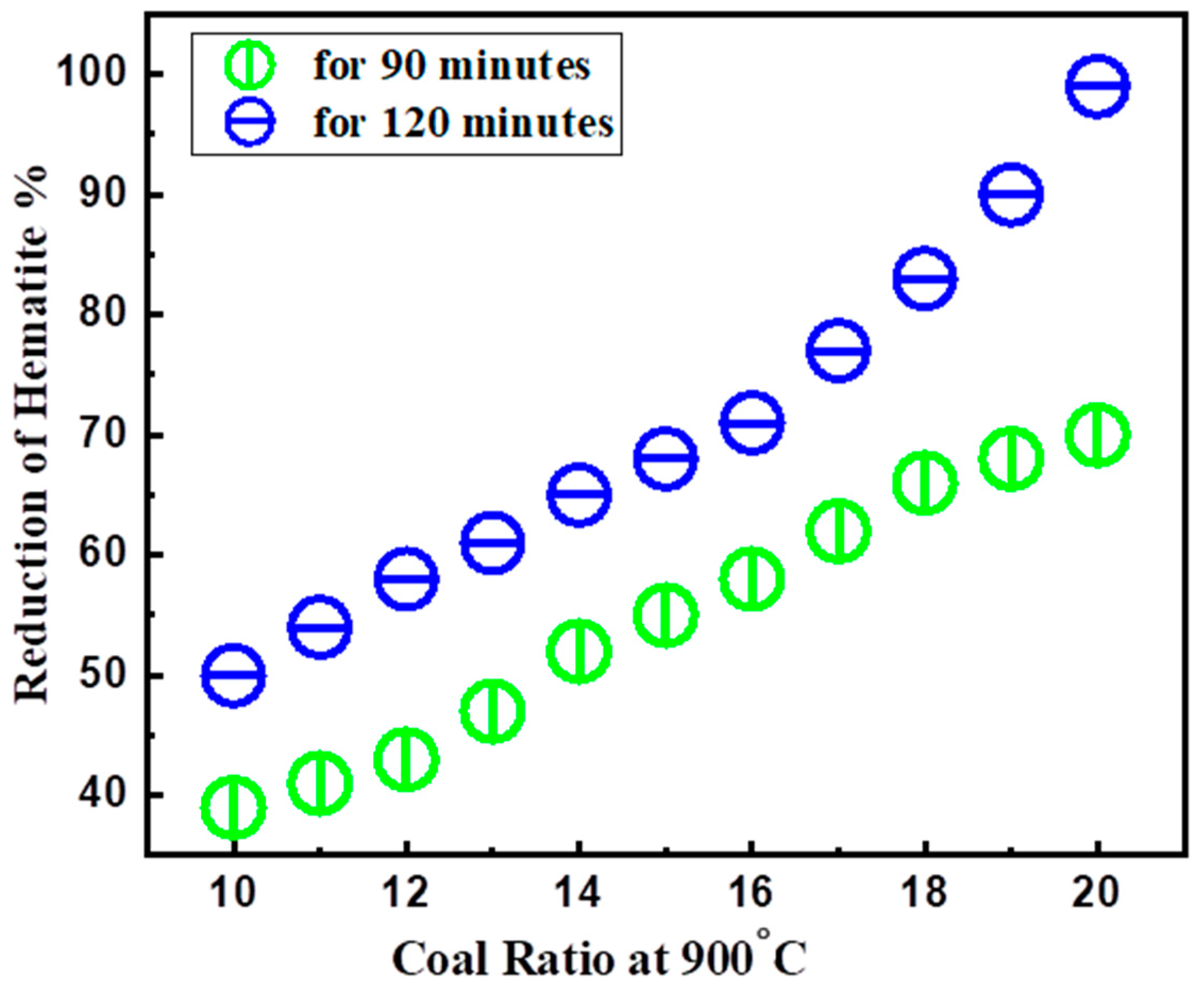
3.1. Reduction Roasting
Coal Ratios
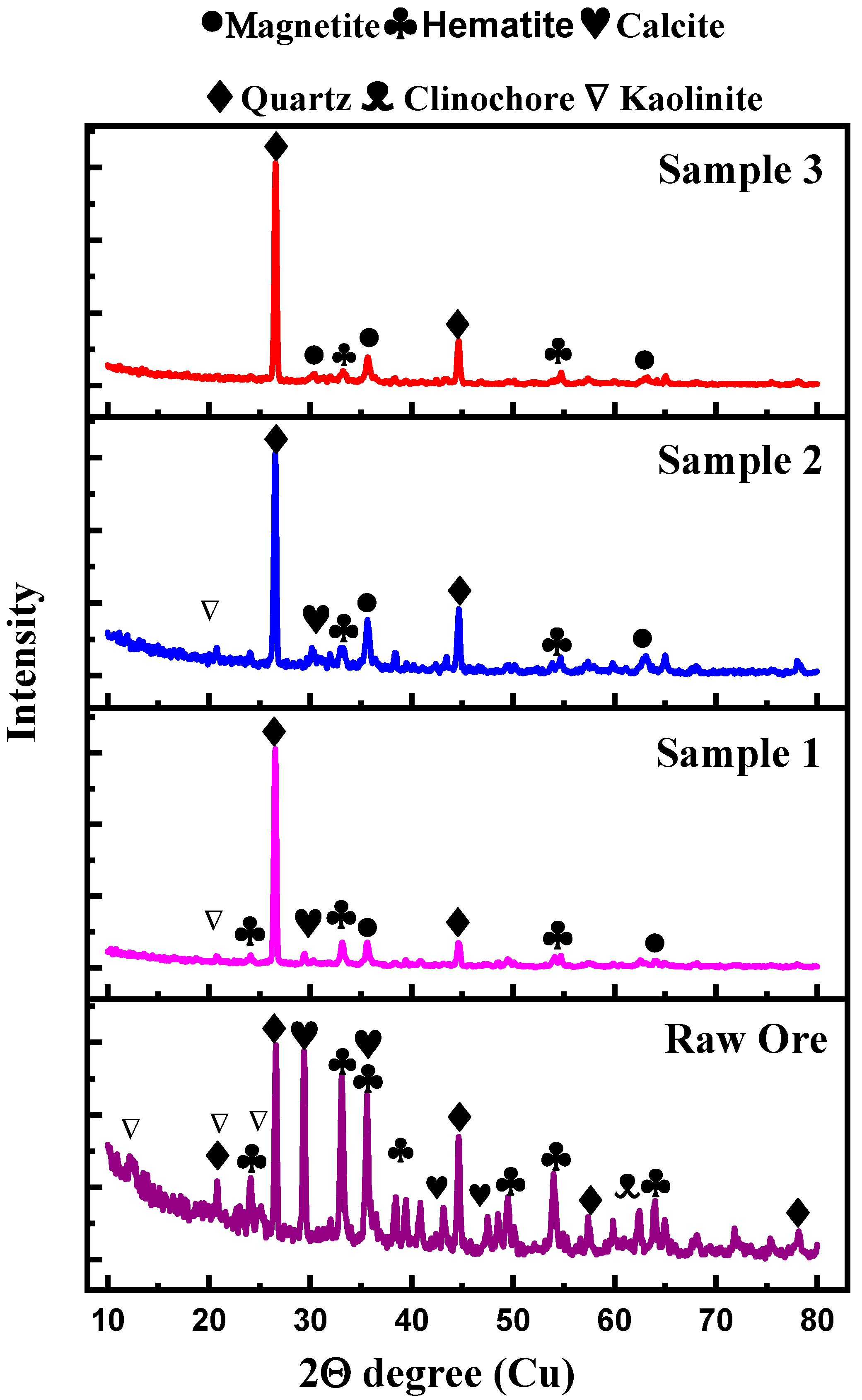
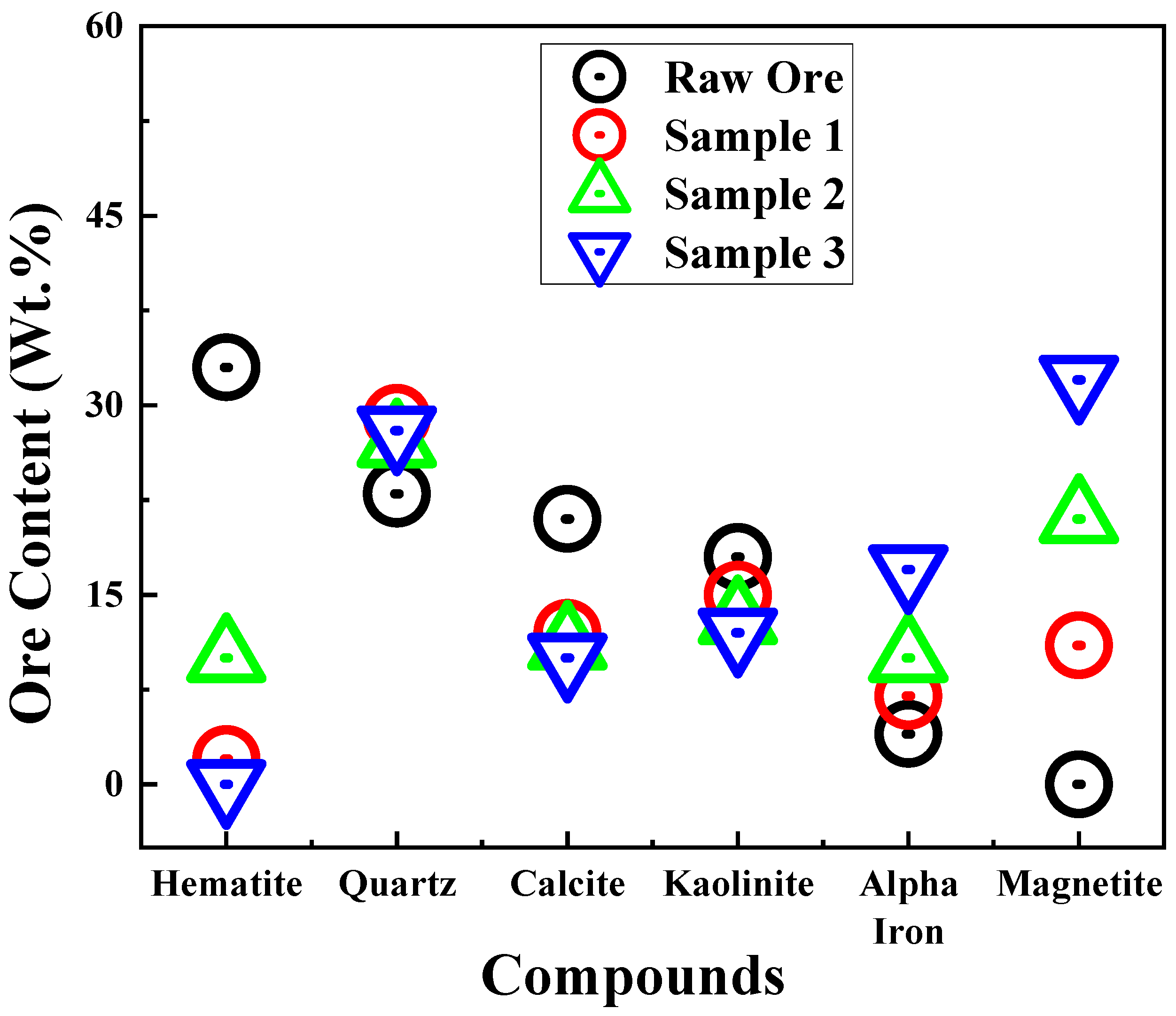
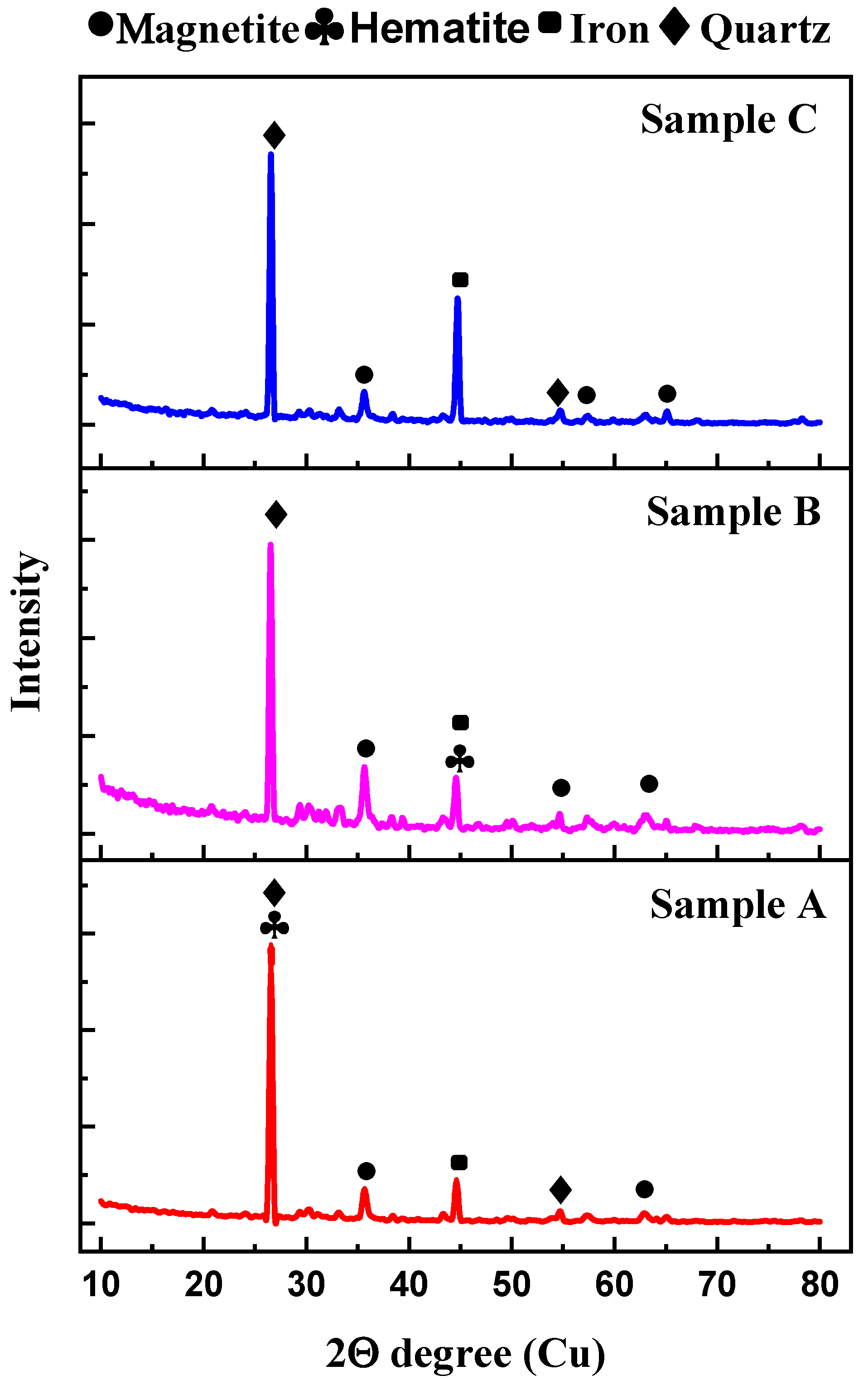
3.2. XRD
3.3. VSM
3.4. Recovery and Grade Calculations
3.5. Cost Estimation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, S.K.; Nayak, D.; Rath, S.S. A review on the enrichment of iron values of low-grade Iron ore resources using reduction roasting-magnetic separation. Powder Technol. 2020, 367, 796–808. [Google Scholar] [CrossRef]
- He, J.; Liu, C.; Hong, P.; Yao, Y.; Luo, Z.; Zhao, L. Mineralogical characterization of the typical coarse iron ore particles and the potential to discharge waste gangue using a dry density-based gravity separation. Powder Technol. 2019, 342, 348–355. [Google Scholar] [CrossRef]
- Khoso, S.A.; Lyu, F.; Meng, X.; Hu, Y.; Sun, W. Selective separation of chalcopyrite and pyrite with a novel and non-hazardous depressant reagent scheme. Chem. Eng. Sci. 2019, 209, 115204. [Google Scholar] [CrossRef]
- Khoso, S.A.; Abro, M.I.; Agheem, M.H. Evaluation of Mesh of Liberation of Zard Koh and Kulli Koh Iron Ores of Pakistan. Mehran Univ. Res. J. Eng. Technol. 2018, 37, 569–580. [Google Scholar] [CrossRef]
- Agrawal, A.; Das, K.; Singh, B.K.; Singh, R.S.; Tripathi, V.R.; Kundu, S.; Padmapal; Ramna, R.; Singh, M.K. Means to cope with the higher alumina burden in the blast furnace. Ironmak. Steelmak. 2020, 47, 238–245. [Google Scholar] [CrossRef]
- Hanumanthappa, H.; Vardhan, H.; Mandela, G.R.; Kaza, M.; Sah, R.; Shanmugam, B.K. A comparative study on a newly designed ball mill and the conventional ball mill performance with respect to the particle size distribution and recirculating load at the discharge end. Miner. Eng. 2020, 145, 106091. [Google Scholar] [CrossRef]
- Hanumanthappa, H.; Vardhan, H.; Mandela, G.R.; Kaza, M.; Sah, R.; Shanmugam, B.K.; Pandiri, S. Investigation on Iron Ore Grinding based on Particle Size Distribution and Liberation. Trans. Indian Inst. Met. 2020, 73, 1853–1866. [Google Scholar] [CrossRef]
- Wasim, M.; Tariq, A.; Shafique, M.A.; Qureshi, R.N. Characterization and differentiation of iron ores using X-ray diffractometry, k0 instrumental neutron acti-vation analysis and inductively coupled plasma optical emission spectrometry. J. Radioanal. Nucl. Chem. 2020, 323, 179–187. [Google Scholar] [CrossRef]
- Yu, J.; Han, Y.; Li, Y.; Gao, P. Recent Advances in Magnetization Roasting of Refractory Iron Ores: A Technological Review in the Past Decade. Miner. Process. Extr. Met. Rev. 2020, 41, 349–359. [Google Scholar] [CrossRef]
- Ponomar, V.P.; Brik, O.B.; Cherevko, Y.I.; Ovsienko, V.V. Kinetics of hematite to magnetite transformation by gaseous reduction at low concentration of coal mon-oxide. Chem. Eng. Res. Des. 2019, 148, 393–402. [Google Scholar] [CrossRef]
- Roy, S.; Nayak, D.; Dash, N.; Dhawan, N.; Rath, S.S. Microwave-assisted reduction roasting—Magnetic separation studies of two mineralogically different low-grade iron ores. Int. J. Miner. Met. Mater. 2020, 27, 1449–1461. [Google Scholar] [CrossRef]
- Chun, T.; Zhu, D.; Pan, J. Simultaneously Roasting and Magnetic Separation to Treat Low Grade Siderite and Hematite Ores. Miner. Process. Extr. Met. Rev. 2015, 36, 223–226. [Google Scholar] [CrossRef]
- Quast, K. A review on the characterisation and processing of oolitic iron ores. Miner. Eng. 2018, 126, 89–100. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Luo, L.; Yu, H. Behavior of Fe and P during reduction magnetic roasting-separation of phosphorus-rich oolitic hematite. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 47–64. [Google Scholar] [CrossRef]
- Dauce, P.D.; de Castro, G.B.; Lima, M.M.F.; Lima, R.M.F. Characterisation and magnetic concentration of an iron ore tailings. J. Mater. Res. Technol. 2019, 8, 1052–1059. [Google Scholar] [CrossRef]
- Sahu, S.N.; Sharma, K.; Barma, S.D.; Pradhan, P.; Nayak, B.K.; Biswal, S.K. Utilization of low-grade BHQ iron ore by reduction roasting followed by magnetic separation for the pro-duction of magnetite-based pellet feed. Metall. Res. Technol. 2019, 116, 611. [Google Scholar] [CrossRef]
- Ponomar, V.; Dudchenko, N.; Brik, A. Synthesis of magnetite powder from the mixture consisting of siderite and hematite iron ores. Miner. Eng. 2018, 122, 277–284. [Google Scholar] [CrossRef]
- Yu, J.; Han, Y.; Li, Y.; Gao, P. Beneficiation of an iron ore fines by magnetization roasting and magnetic separation. Int. J. Miner. Process. 2017, 168, 102–108. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, P.; Li, Y.; Han, Y.; Li, W.; Butt, S.; Zhang, Y. Recovery of iron from hazardous tailings using fluidized roasting coupling technology. Powder Technol. 2020, 361, 591–599. [Google Scholar] [CrossRef]
- Oritola, S.F.; Saleh, A.L.; Mohd Sam, A.R. Characterization of Iron Ore Tailings as Fine Aggregate. ACI Mater. J. 2020, 117, 125–134. [Google Scholar]
- Valerevich Lvov, V.; Sergeevich Chitalov, L. Sergeevich Chitalov, and Technology, Comparison of the different ways of the ball Bond work index determining. Int. J. Mech. Eng. Technol. 2019, 10, 1180–1194. [Google Scholar]
- Ahmad, S.; Channa, I.A. Mathematical Relationship between Ferritic, Pearlitic and Average Grain Size of Steel. J. Mod. Sci. Technol. 2013, 1, 1–18. [Google Scholar]
- Peng, T.; Gao, X.; Li, Q.; Xu, L.; Luo, L.; Xu, L. Phase transformation during roasting process and magnetic beneficiation of oolitic-iron ores. Vacuum 2017, 146, 63–73. [Google Scholar] [CrossRef]
- Ravisankar, V.; Venugopal, R.; Bhat, H. Investigation on beneficiation of goethite-rich iron ores using reduction roasting followed by magnetic separation. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. 2019, 128, 175–182. [Google Scholar] [CrossRef]
- Pasechnik, L.; Skachkov, V.; Bogdanova, E.; Chufarov, A.Y.; Kellerman, D.; Medyankina, I.; Yatsenko, S. A promising process for transformation of hematite to magnetite with simultaneous dissolution of alumina from red mud in alkaline medium. Hydrometallurgy 2020, 196, 105438. [Google Scholar] [CrossRef]
- Abro, S.H.; Chandio, A.; Siddiqui, M.A.; Channa, I.A. Aluminum and aluminum nitrides effect on nucleation sites in micro-alloyed steel. Proc. Pak. Acad. Sci. Part B 2019, 56, 17–26. [Google Scholar]
- Maitlo, I.; Aftab, U.; Abro, M.I.; Baloch, M.M. Selective Leaching of Steel Pollutant Element from Dilband Iron Ore, Pakistan. Mehran Univ. Res. J. Eng. Technol. 2017, 36, 757–762. [Google Scholar] [CrossRef]
- Makhdoom, M.A.; Ahmed, F.; Channa, I.A.; Inam, A.; Riaz, F.; Siyal, S.H.; Shar, M.A.; Alhazaa, A. Effect of Multiple Thermal Cycles on the Microstructure and Mechanical Properties of AISI 1045 Weldments. ACS Omega 2022, 7, 42313–42319. [Google Scholar] [CrossRef]
- Ezhov, A.; Shvaljov, Y. Dry Magnetic Separation of Iron Ore of the Bakchar Deposit. Procedia Chem. 2015, 15, 160–166. [Google Scholar] [CrossRef]
- Xiao, J.; Zhou, L. Increasing Iron and Reducing Phosphorus Grades of Magnetic-Roasted High-Phosphorus Oolitic Iron Ore by Low-Intensity Magnetic Separation–Reverse Flotation. Processes 2019, 7, 388. [Google Scholar] [CrossRef]
- Dash, N.; Rath, S.S.; Angadi, S.I. Thermally assisted magnetic separation and characterization studies of a low-grade hematite ore. Powder Technol. 2019, 346, 70–77. [Google Scholar] [CrossRef]
- Harano, T.; Nemoto, Y.; Murao, R.; Kimura, M. Accuracy Improvement of the XRD-Rietveld Method for the Quantification of Crystalline Phases in Iron Sintered Ores through the Correction of Micro-absorption Effects. ISIJ Int. 2020, 60, 2851–2858. [Google Scholar] [CrossRef]
- Ardiani, N.R.; Setianto, S.; Santosa, B.; Wibawa, B.M.; Panatarani, C.; Joni, I.M. Quantitative analysis of iron sand mineral content from the south coast of Cidaun, West Java using rietveld refinement method. In Proceedings of the AIP Conference Proceedings 2nd International Conference and Exhibition on Powder Technology (ICePTi), Solo, Indonesia, 20–21 August 2019; AIP Publishing LLC: Bandung, Indonesia. [Google Scholar] [CrossRef]
- Zhu, X.; Han, Y.; Sun, Y.; Li, Y.; Wang, H. Siderite as a novel reductant for clean utilization of refractory iron ore. J. Clean. Prod. 2020, 245, 118704. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Z.; Pan, Y.; Ge, Y.; Feng, C.; Chu, M.; Tang, J. Separation and Recovery of Iron and Nickel from Low-Grade Laterite Nickel Ore Using Reduction Roasting at Rotary Kiln Followed by Magnetic Separation Technique. Min. Metall. Explor. 2018, 36, 375–384. [Google Scholar] [CrossRef]
- Campos, T.M.; Bueno, G.; Rodriguez, V.A.; Boettcher, A.C.; Kwade, A.; Mayerhofer, F.; Tavares, L.M. Relationships between particle breakage characteristics and comminution response of fine iron ore con-centrates. Miner. Eng. 2021, 164, 106818. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Sun, Y.; Lv, Y.; Li, Y.; Tang, Z. An Novel Method for Iron Recovery from Iron Ore Tailings with Pre-Concentration Followed by Magnetization Roasting and Magnetic Separation. Miner. Process. Extr. Met. Rev. 2020, 41, 117–129. [Google Scholar] [CrossRef]
- Purohit, S.; Ekman, B.; Mejias, R.; Brooks, G.; Rhamdhani, M.A. Solar processing of composite iron ore pellets: Preliminary assessments. J. Clean. Prod. 2018, 205, 1017–1028. [Google Scholar] [CrossRef]
- Palacios, J.-L.; Fernandes, I.; Abadias, A.; Valero, A.; Valero, A.; Reuter, M.A. Avoided energy cost of producing minerals: The case of iron ore. Energy Rep. 2019, 5, 364–374. [Google Scholar] [CrossRef]





| Samples | Fe Content (Wt.%) | Standard Deviation |
|---|---|---|
| Sample A | 39.20 | ±1.0 |
| Sample B | 44.00 | ±1.0 |
| Sample C | 55.12 | ±1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandio, A.D.; Channa, I.A.; Shaikh, A.A.; Madad, S.; Rizvi, S.B.H.; Shah, A.A.; Ashfaq, J.; Ali Shar, M.; Alhazaa, A. Beneficiation of Low-Grade Dilband Iron Ore by Reduction Roasting. Metals 2023, 13, 296. https://doi.org/10.3390/met13020296
Chandio AD, Channa IA, Shaikh AA, Madad S, Rizvi SBH, Shah AA, Ashfaq J, Ali Shar M, Alhazaa A. Beneficiation of Low-Grade Dilband Iron Ore by Reduction Roasting. Metals. 2023; 13(2):296. https://doi.org/10.3390/met13020296
Chicago/Turabian StyleChandio, Ali Dad, Iftikhar Ahmed Channa, Asif Ahmed Shaikh, Shabbir Madad, Syed Bilal Hasan Rizvi, Aqeel Ahmed Shah, Jaweria Ashfaq, Muhammad Ali Shar, and Abdulaziz Alhazaa. 2023. "Beneficiation of Low-Grade Dilband Iron Ore by Reduction Roasting" Metals 13, no. 2: 296. https://doi.org/10.3390/met13020296
APA StyleChandio, A. D., Channa, I. A., Shaikh, A. A., Madad, S., Rizvi, S. B. H., Shah, A. A., Ashfaq, J., Ali Shar, M., & Alhazaa, A. (2023). Beneficiation of Low-Grade Dilband Iron Ore by Reduction Roasting. Metals, 13(2), 296. https://doi.org/10.3390/met13020296









