Abstract
The modification of eutectic silicon plays a crucial role in enhancing the mechanical properties of hypoeutectic Al–Si alloys. However, there is still limited understanding regarding the factors that affect the modification of eutectic silicon in hypoeutectic Al–Si alloys, particularly in terms of the role played by alloying elements during the modification process. In order to address this gap, this study aimed to investigate the influence of two key alloying elements, Mg and Cu, on the modification effect of Sr. To achieve this, the morphology of eutectic silicon in the as-cast and heat-treated states of Al–7Si alloy, modified with Sr, was examined using scanning electron microscopy (SEM). Different levels of magnesium and copper content were used to analyze their impact on the modification effect of Sr. The results obtained from the analysis revealed that both Mg and Cu significantly weaken the modification effect of strontium on eutectic silicon. Furthermore, it was observed that the degree of deterioration in the modification effect increases progressively with higher alloying element content. Interestingly, increasing the strontium content and adjusting the cooling rate were found to be ineffective in eliminating this weakening effect caused by Mg and Cu. These findings highlight the complex interplay between alloying elements and the modification process of eutectic silicon in hypoeutectic Al–Si alloys. A deeper understanding of these factors is essential for the development of effective strategies to optimize the mechanical properties of such alloys.
1. Introduction
Hypoeutectic Al–Si alloy, known for its exceptional casting and mechanical properties, is widely regarded as the most commonly used cast aluminum alloy. The morphology, size, and distribution of eutectic silicon play a crucial role in determining the alloy’s properties, particularly its elongation. Modification treatment has proven to be the most effective method for enhancing the eutectic structure and mechanical properties of hypoeutectic Al–Si alloys [1,2,3]. Unmodified eutectic silicon typically appears in the form of large plates, strips, or sticks, while fully modified eutectic silicon exhibits a fine pine needle or coral-like structure [4,5]. Currently, strontium is the most commonly used modifier in the casting aluminum industry. It is added in the form of an intermediate alloy, and the addition of 0.03 wt.% Sr achieves the desired modification effect [6,7,8].
The primary alloying elements in Al–Si casting alloys are Si, Mg, and Cu. The addition of Mg to Al–Si alloys improves the alloys’ strength through the precipitation of the Mg2Si aging phase [9,10,11]. However, Cu acts as a solid solution strengthener and aging strengthener, thereby enhancing the overall strength of the alloy [12,13,14,15]. Numerous studies have been conducted to investigate the influence of the Mg and Cu content on the mechanical properties of the alloys, as well as the composition and distribution of precipitated phases [16,17,18]. However, limited research has been conducted on the influence of alloying elements on the morphology of eutectic silicon. The mechanism by which alloying elements affect the morphology of eutectic silicon remains unclear, and conflicting results have been reported. Some researchers, such as Darlapudi [19], Li [20], Abedi [21], Fracchia [22], and Nafisi et al. [23], believe that in the case of unmodified Si in hypoeutectic Al–Si alloys, the addition of Mg can refine the eutectic silicon. Conversely, Cacerse et al. [24] proposed that under modification conditions, the eutectic silicon in A357 alloy with a higher Mg content appears coarser than that in A356 alloy with a lower Mg content. Joenoes et al. [25] argue that Mg weakens the modification of eutectic silicon, while Heusler et al. [26] discovered that Mg has a significant weakening effect on Sr modification at low cooling rates but not at high cooling rates. Zhang et al. [27] suggested that the addition of Mg worsens the modification of eutectic silicon in Al–Si alloys. Unfortunately, they did not conduct further research in this area. Mohamed et al. [28] proposed that Cu in Al–Si alloys reacts with Sr to form Al–Cu–Sr compounds, which reduces the effective Sr content in the melt and diminishes the modification effect on eutectic silicon. Garcia-Hinojosa et al. [29] discovered that the addition of 0.5 wt.% or 1.5 wt.% Cu to A356 alloy, followed by Sr modification, transforms the morphology of eutectic silicon into a fine fibrous structure, thereby improving the mechanical properties of the alloy. Zhang et al. [30] believed that the modification effect is determined by the element concentration in eutectic Si, which is mainly attributed to the solubility of the modifying element in Al and the interaction energy parameter of Al (or Si) and the modifying element. As is evident, there are numerous contradictions and discrepancies among these studies. In the light of these contradictions, this study focuses on investigating the influence of alloying elements on the morphology of eutectic silicon in hypoeutectic Al–Si alloys modified with Sr.
Al–7Si–0.3Mg alloy, also known as A356 alloy, is highly regarded for its exceptional casting and mechanical performance. It is considered the most extensively used alloy in the hypoeutectic aluminum–silicon system, particularly in the automotive component industry. Al–7Si–4Cu alloy, also known as ZL117 alloy (similar to A319 alloy), is frequently used in applications that involve high-temperature environments. Considering the significance of this alloy in such conditions, it is essential to conduct further research on modification process parameters in order to optimize its properties. To improve the properties of this alloy, it is important to focus on the modification of eutectic silicon. Modification refers to the process of transforming the structure of eutectic silicon particles into a more desirable form. Incomplete modification leads to the presence of large-size eutectic silicon particles, which negatively impact the alloy’s mechanical strength and other properties. To address this issue, the influence of two factors was investigated: the content of the modification element Sr and the cooling rate. Sr is commonly used as a modification element in Al–Si alloys due to its effectiveness in altering the morphology of eutectic silicon. Additionally, the cooling rate plays a significant role in the modification process. The cooling rate affects the nucleation and growth of eutectic silicon particles, thereby influencing their final size and morphology in the solidified alloy [31]. Additionally, we examined the effects of the Sr content and cooling rate on the modification of Al–7Si–0.3Mg and Al–7Si–4Cu alloys, thereby contributing to a deeper understanding of the underlying mechanisms involved.
2. Materials and Methods
The experiment used 99.7 wt.% Al, 99.7 wt.% Mg, 99.9 wt.% Si, Al–50Cu, and Al–10Sr intermediate alloys as raw materials to prepare Al–7Si–xMg (x = 0, 0.1, 0.2, 0.3, 0.4, 0.5) and Al–7Si–xCu (x = 0.5, 1, 2, 3, 4, 5) alloys with different Mg and Cu contents through smelting. Compositional details of the alloys are shown in Table 1. The chemical composition was determined using a SPECTROMAXx spark spectrometer (Spectro, Kleve, Germany).

Table 1.
The chemical compositions (wt.%) of alloys.
The pre-prepared Al–7Si–xMg and Al–7Si–xCu alloys (100 g each) were remelted and kept at 760 °C and afterward refined through salt fluxes. After removing the refined slag on the surface, 0.3 wt.% Al–10Sr intermediate alloy modifier was added to the melt for modification treatment. After an 8 min holding time and after removing the surface dross, the alloy melt was poured into steel molds for cooling.
Following the aforementioned experimental procedure, 0.03 wt.%, 0.06 wt.%, and 0.09 wt.% Sr was added to Al–7Si–0.3Mg and Al–7Si–4Cu alloys separately for modification treatment to study the effect of the Sr content on the modification of Al–Si alloys. To investigate the influence of the cooling rate on the modification of Al–Si alloys, the modified Al–7Si–0.3Mg and Al–7Si–4Cu alloys were poured into steel molds at room temperature, 200 °C, and 400 °C for solidification. In order to obtain different cooling rates, one K-type thermocouple was quickly immersed at the center of the mold containing ~100 g of the alloys. In order to ensure statistical accuracy, the thermal analysis experiments were repeated three times for each step. For the series of experiments, cooling rates above the liquidus were 7.06 °C, 5.70 °C, and 3.39 °C for the mold without preheating and preheated to 200 and 400 °C.
The alloys obtained from the aforementioned experiments were sectioned along the longitudinal center of the ingot and divided into two groups: one group for the as-cast state and the other group subjected to heat treatment. The heat treatment process consisted of a solution treatment at 530 °C for 2 h followed by an aging treatment at 175 °C for 2 h. The as-cast and heat-treated alloy samples were polished with sandpaper, polishing cloth, and diamond paste until a mirror-like finish was achieved, and then subjected to electrolytic polishing. The electrolytic polishing solution was a mixture of concentrated perchloric acid and anhydrous ethanol in a 1:9 ratio. The polishing was conducted at a voltage of 27 V for approximately 20 s. The microstructure of the samples was analyzed using a HITACHI SU8220 cold field emission scanning electron microscope (SEM) integrated with a flat-Quad EDX (Hitachi High-Tech Corporation, Tokyo, Japan).
3. Results and Discussion
3.1. The Influence of Alloying Element Mg on the Morphology of Eutectic Silicon in Al–7Si Alloy
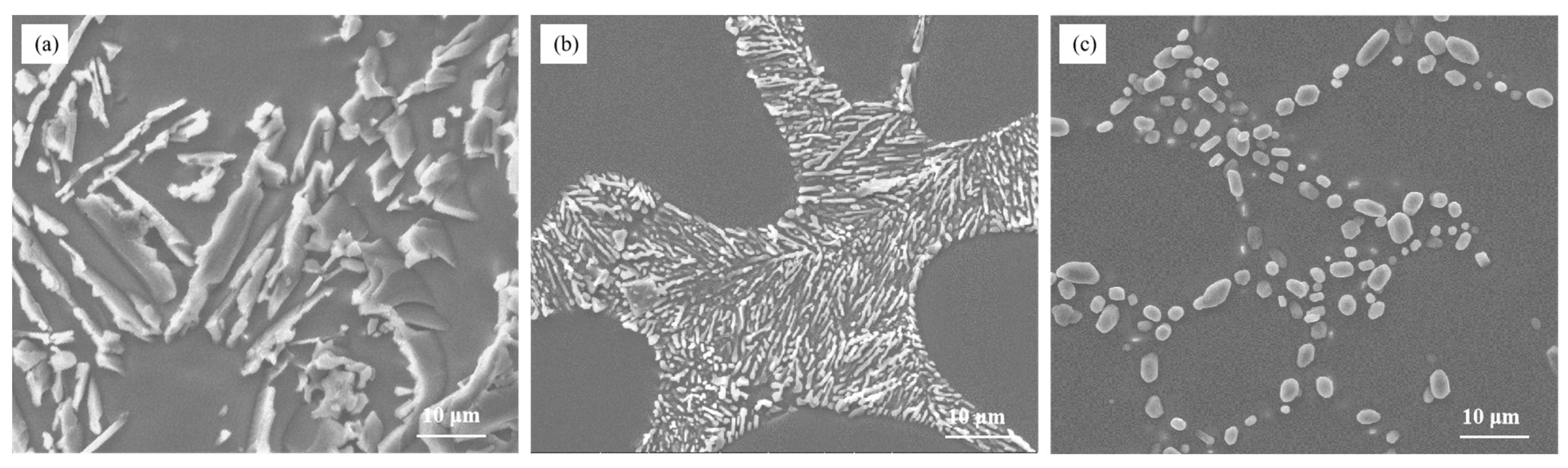
Figure 1 illustrates the morphology of eutectic silicon in Al–7Si alloy before and after modification. It can be observed from Figure 1a that unmodified eutectic silicon appeared in the form of large plates. The eutectic silicon in the as-cast alloy transformed into an ideal needle-like morphology, resembling pine trees, after modification (as shown in Figure 1b). Upon heat treatment, the eutectic silicon morphology in the alloy changed into fine and uniform spherical shapes. This indicates that the addition of 0.03 wt.% Sr leads to the desired morphology of eutectic silicon in Al–7Si alloy, without the presence of alloying elements Mg and Cu.
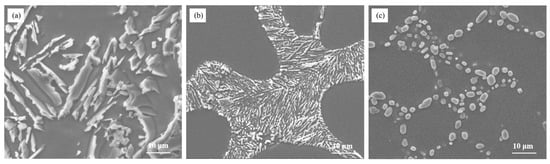
Figure 1.
The microstructure of Al–7Si alloy (a) without modification; (b) with 0.03 wt.% Sr, as-cast state; and (c) with 0.03 wt.% Sr, heat-treated state.
These observations suggest that Sr, as a modifier, has a significant impact on the morphology of eutectic silicon in Al–7Si alloy. The addition of 0.03 wt.% Sr results in the transformation of eutectic silicon from a plate-like morphology in the as-cast alloy into a fine spherical morphology, which is more uniform and stable. The size of eutectic silicon is about 3–6 μm. This is of great importance in improving the mechanical properties of the alloy and controlling the casting process.
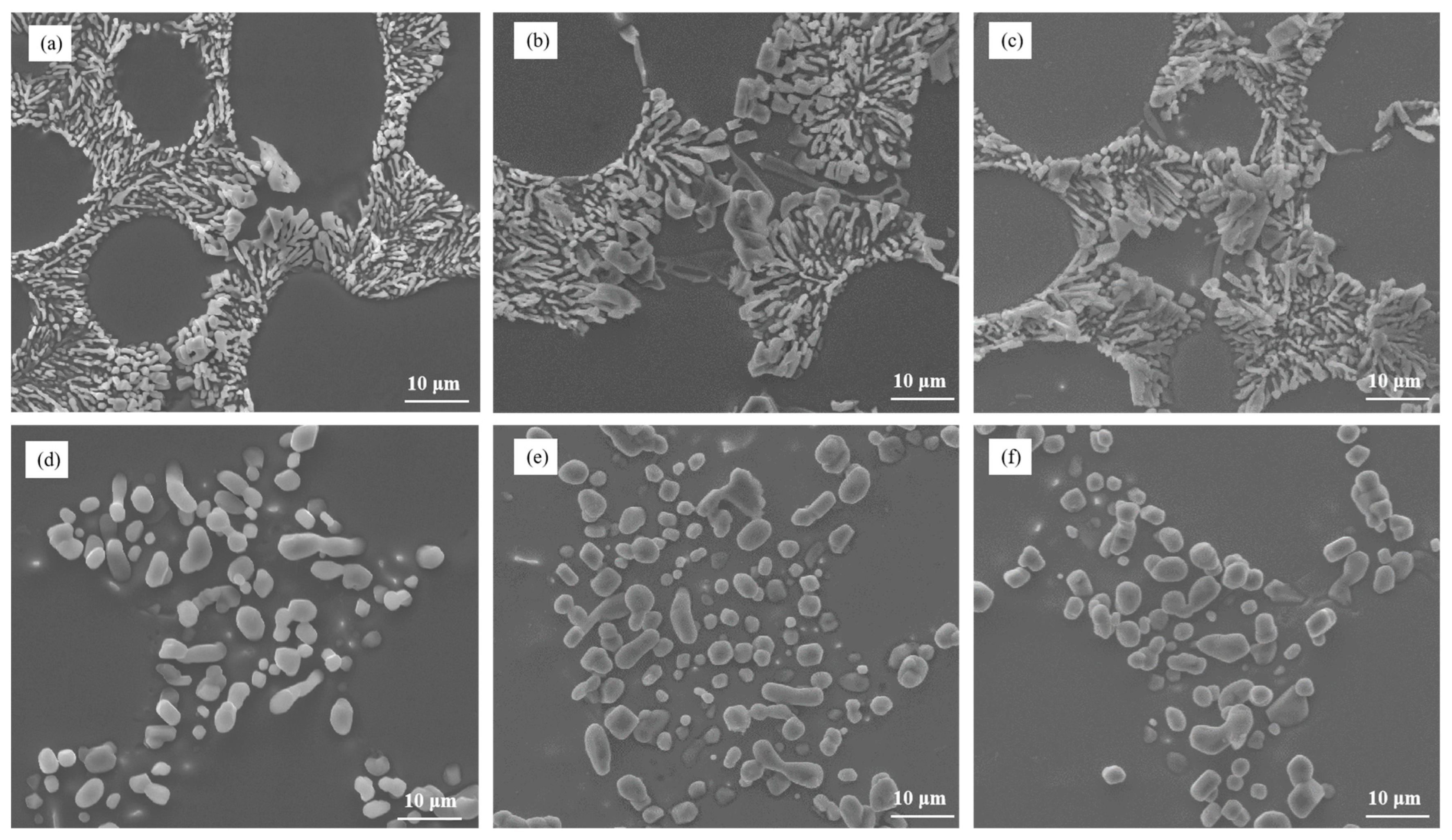
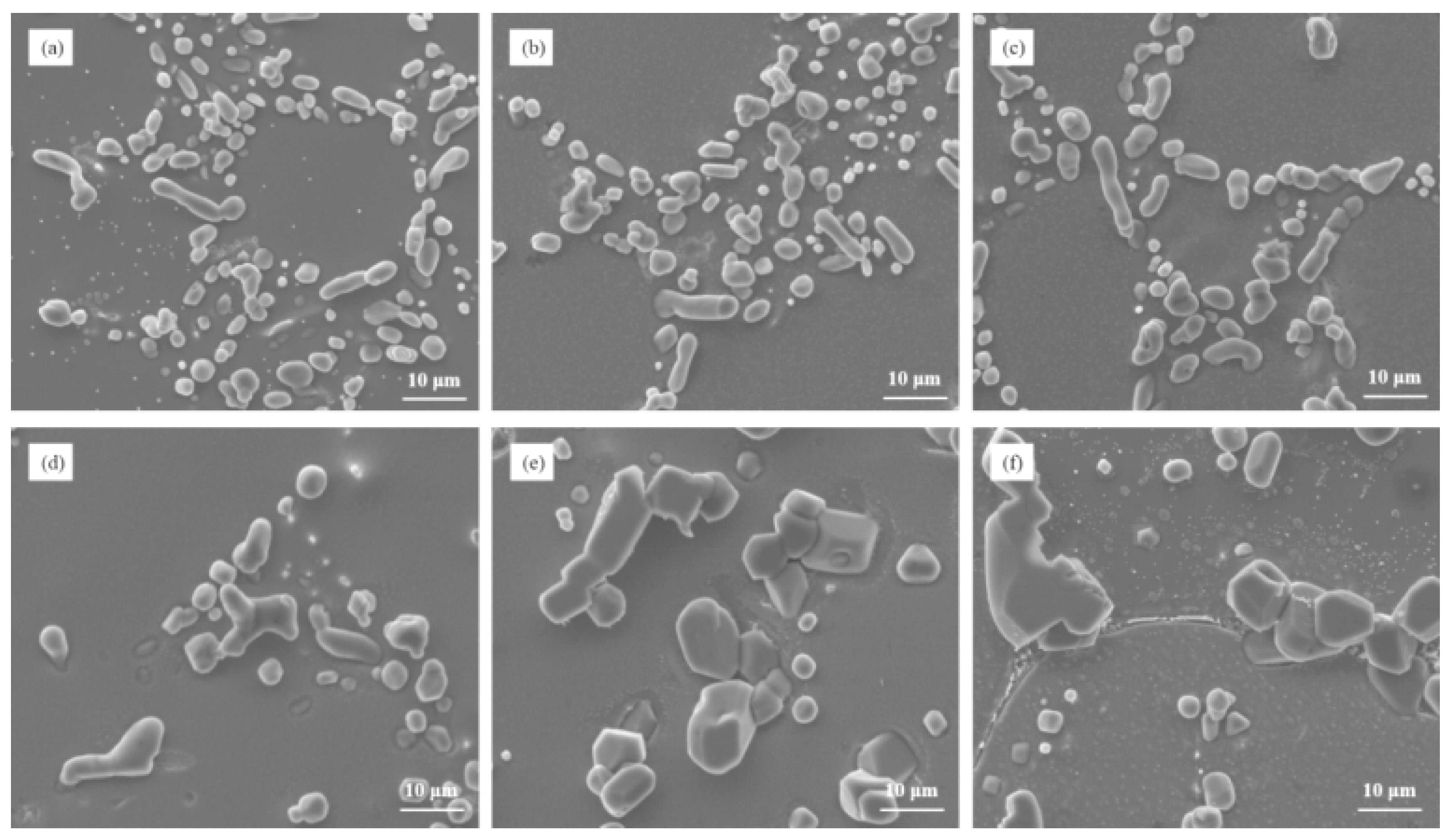
Figure 2 depicts the microstructure of Al–7Si–xMg alloys in the as-cast state after modification with 0.03 wt.% Sr. It is evident that the addition of even a small amount of Mg, such as 0.1 wt.%, resulted in the presence of partially modified blocky eutectic silicon in the alloy. As the Mg content increased, both the size and the quantity of unmodified eutectic silicon gradually increased, leading to a decrease in the effectiveness of silicon modification.
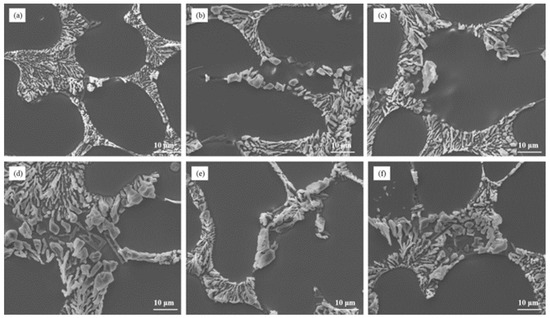
Figure 2.
The microstructure of Al–7Si–xMg alloys modified with Sr in the as-cast state: (a) 0.1 wt.% Mg, (b) 0.2 wt.% Mg, (c) 0.3 wt.% Mg, (d) 0.4 wt.% Mg, (e) 0.5 wt.% Mg, and (f) 0.6 wt.% Mg.
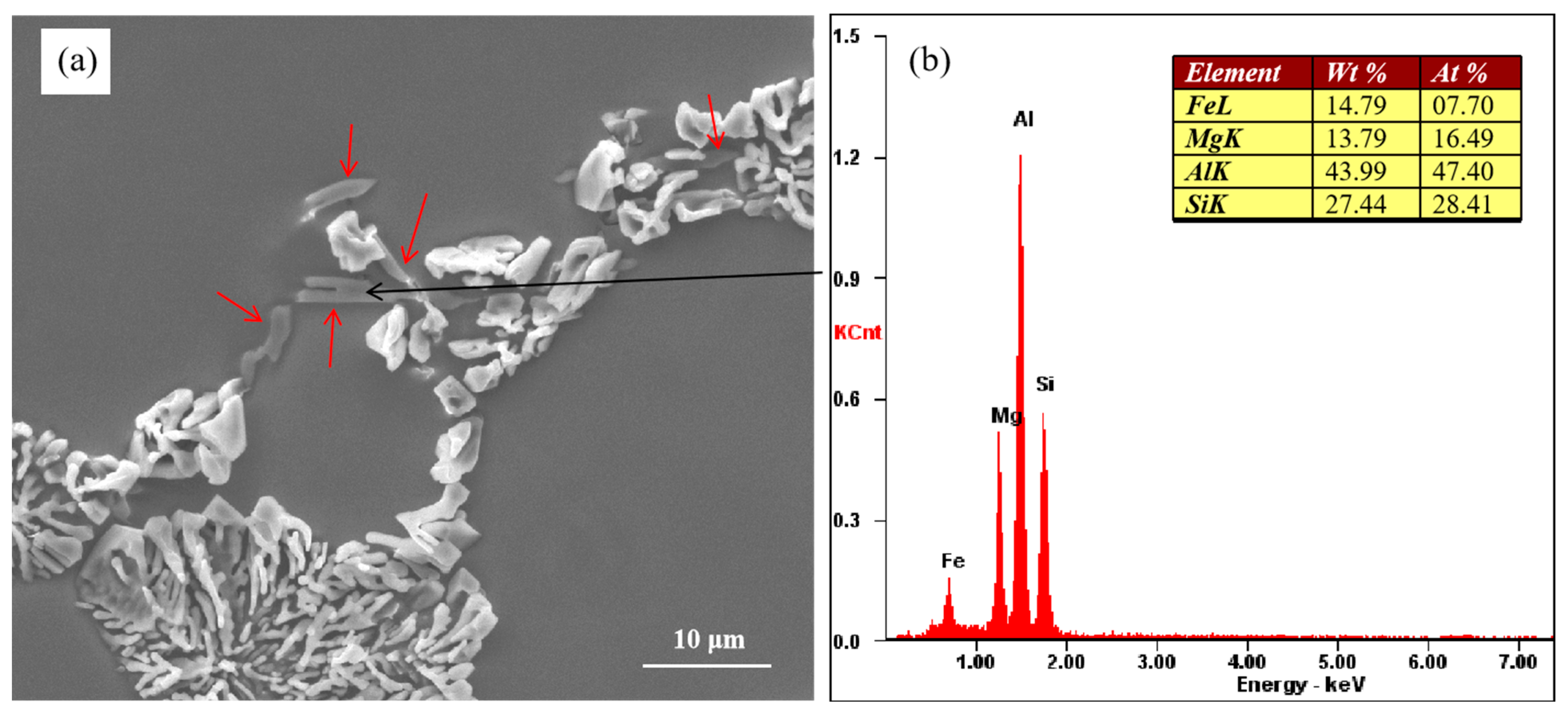
Figure 3a shows the morphology of unmodified eutectic Si and the Mg-rich phase. The Mg-rich phase is indicated by the red arrows in Figure 3a The energy spectrum of the Mg-rich phase indicated by the black arrow is shown in Figure 3b. Blocky Si was observed along with the Mg-rich phase at the eutectic grain boundaries. The results of the energy spectrum analysis showed that the Mg-rich phase contained Al, Mg, Fe, and Si elements. Figure 3 shows the as-cast microstructure of the alloy, and the Mg-rich phase was formed during the solidification process of the alloy.
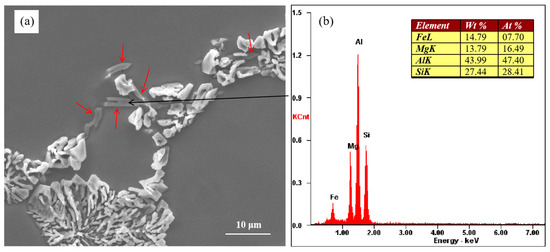
Figure 3.
(a) Morphology of partially modified eutectic Si and Mg-rich phase and (b) corresponding EDX results.
According to the research conducted by Joenoes et al. [25], this weakening effect of Mg on strontium modification can be attributed to the formation of Al–Mg–Sr–Si quaternary compounds. These compounds subsequently reduce the effective strontium content available in the melt. Unfortunately, we did not find the presence of this compound under the existing experimental conditions. In a study by Huang et al. [32], it was proposed that the formation of partially modified eutectic silicon occurs during the final stages of solidification. We believe that eutectic silicon is formed through the binary eutectic reaction in binary Al–Si alloy, and after the addition of the alloying element Mg to Al–Si alloy, eutectic silicon and the Mg2Si phase can be formed through the ternary eutectic reaction. As can be seen from Figure 3a, eutectic silicon in the ternary eutectic structure shows incomplete modification. The unmodified eutectic silicon has an adhering relationship with the Mg-rich phase. The Mg-rich phase is formed through the ternary eutectic reaction at the last stage of solidification but is not precipitated during subsequent cooling. So, the incomplete modification of eutectic silicon is closely related to the ternary eutectic reaction. During the solidification process of the alloy, the Mg element is continuously enriched in the remaining liquid phase, which leads to the transformation of the binary Al–Si eutectic reaction into the Al–Si–Mg2Si ternary eutectic reaction at the last stage of solidification. The addition of Mg can make the eutectic form over a temperature range [16], that is, the growth time of eutectic silicon becomes longer, which leads to the coarsening of eutectic silicon. As the content of the alloying element magnesium increases gradually, the ternary eutectic molten pool formed by the alloying element increases gradually, which leads to a gradual increase in the size of the incompletely modified eutectic silicon.
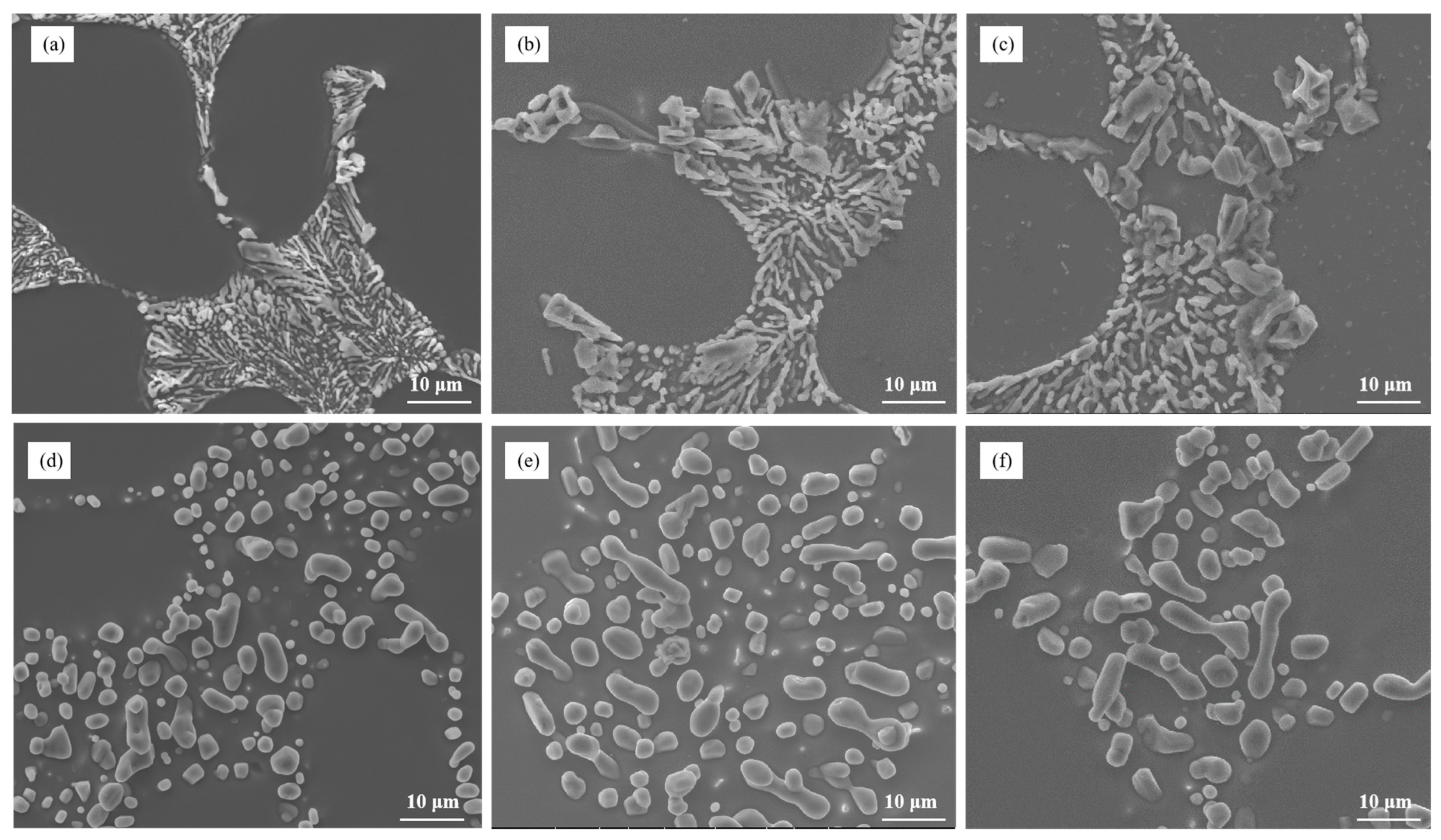
Figure 4 describes the microstructure of Al–7Si–xMg alloys after being heat-treated and modified with 0.03 wt.% Sr. The large size eutectic silicon is indicated by the red arrows in Figure 4. It suggests that the size and morphology of eutectic silicon in the alloys are influenced by the Mg content. Upon heat treatment, the eutectic silicon tended to spheroidize, which means it became more rounded in shape. However, the overall size of the eutectic silicon increased with increasing Mg content. This implies that the addition of Mg promotes the growt.h of eutectic silicon. At a Mg content of 0.3 wt.%, a significant portion of the eutectic silicon particles in the alloy started to coarsen, meaning they increased in size, while still maintaining a granular shape. This indicates that 0.3wt.% Mg content is a critical value beyond which the coarsening of eutectic silicon becomes noticeable. Furthermore, when the Mg content reached 0.4 wt.%, larger and longer elongated eutectic silicon particles appeared in the alloy, as indicated by the red arrows in the figure. This suggests that beyond 0.3 wt.% Mg content, the eutectic silicon particles continue to grow and change in shape, resulting in elongated structures [33].
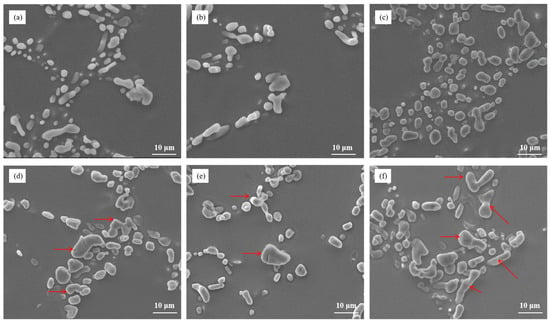
Figure 4.
The microstructure of Al–7Si–xMg alloys modified with Sr in the heat-treated state: (a) 0.1 wt.% Mg, (b) 0.2 wt.% Mg, (c) 0.3 wt.% Mg, (d) 0.4 wt.% Mg, (e) 0.5 wt.% Mg, and (f) 0.6 wt.% Mg.
In summary, the microstructure analysis revealed that the Mg content in Al–7Si–xMg alloys affects the size and morphology of eutectic silicon. The addition of Mg leads to increased overall size and coarsening of eutectic silicon, with a critical value of 0.3 wt.% Mg content where significant coarsening occurs.
The presence of large-size eutectic silicon particles in Al–Si–Mg alloys can have detrimental effects on the mechanical properties of the alloys [24]. Therefore, it is crucial to find ways to eliminate incomplete modification of eutectic silicon in these alloys. In this regard, the influence of the Sr content and solidification cooling rate on the modification of Al–7Si–0.3Mg alloy was analyzed.
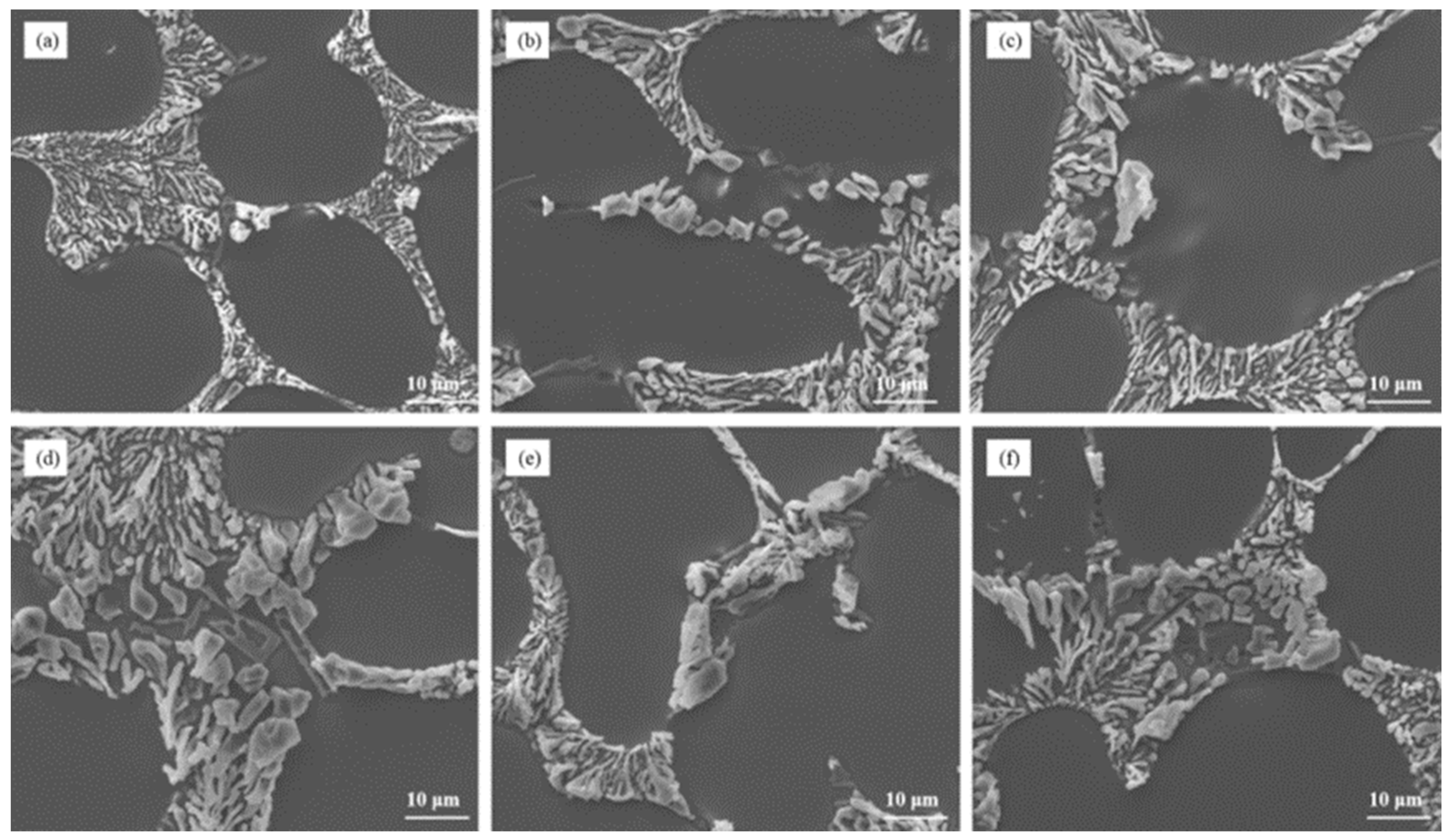
The Sr content plays a crucial role in the modification of eutectic silicon in Al–7Si–0.3Mg alloy. The microstructure analysis, as depicted in Figure 4a–c, illustrated the influence of different Sr contents on the as-cast state of the alloy. It was observed that as the Sr content increased, the incomplete modification of eutectic silicon was not completely eliminated. Some blocky eutectic silicon particles still persisted in the microstructure of the as-cast alloy. Furthermore, even after heat treatment, the size of eutectic silicon in Al–7Si–0.3 Mg alloy remained significantly larger compared to Al–7Si alloy, as shown in Figure 5d–f and Figure 1c. This indicates that increasing the Sr content, the modification element, in the alloy does not effectively solve the issue of incomplete modification caused by the alloying element Mg.
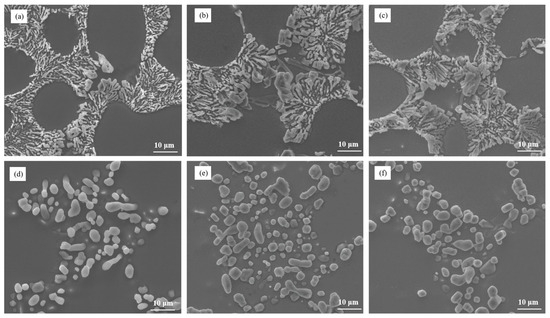
Figure 5.
The microstructural morphology of Al–7Si–0.3Mg alloy with different Sr contents: (a) as-cast/0.03 wt.% Sr, (b) as-cast/0.06 wt.% Sr, (c) as-cast/0.09 wt.% Sr, (d) heat-treated state/0.03 wt.% Sr, (e) heat-treated state/0.06 wt.% Sr, and (f) heat-treated state/0.09 wt.% Sr.
These findings highlight the limitations of solely relying on the modification element Sr to achieve complete modification of eutectic silicon in Al–7Si–0.3Mg alloy. Although Sr can improve the modification to some extent, it is unable to fully overcome the incomplete modification caused by the presence of Mg.
The microstructure analysis presented in Figure 6 focused on the influence of different cooling rates on the modification of eutectic silicon in Al–7Si–0.3Mg alloy with 0.03 wt.% Sr. The results indicated that the cooling rate plays a role in the modification process of eutectic silicon. As the cooling rate increased, certain changes could be observed in the microstructure. Specifically, the quantity and size of partially unmodified eutectic silicon decreased at higher cooling rates. This suggests that increasing the cooling rate can contribute to a more effective modification of eutectic silicon in Al–7Si–0.3Mg alloy. However, it is important to note that the presence of the alloying element Mg still poses challenges in achieving complete modification. Despite the influence of the cooling rate, partially modified eutectic silicon particles remain in the alloy. Therefore, solely relying on increasing the cooling rate is not sufficient to eliminate the weakening effect of Mg on the modification of eutectic silicon. To address this issue, alternative modification strategies need to be explored. This may involve a combination of modification elements, adjustment of the solidification process, or other techniques to overcome the incomplete modification caused by Mg.
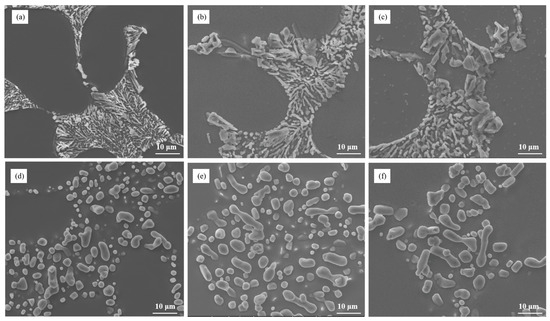
Figure 6.
The microstructural morphology of Al–7Si–0.3Mg alloy at different cooling rates: (a) as-cast/room-temperature steel mold, (b) as-cast/200 °C steel mold, (c) as-cast/400 °C steel mold, (d) heat-treated/room-temperature steel mold, (e) heat-treated/200°C steel mold, and (f) heat-treated/400 °C steel mold.
Mg additions provide an eutectic temperature interval; for a fairly constant eutectic temperature interval, the slower the cooling rate, the longer the time of eutectic silicon in the eutectic temperature range, the easier the coarsening. Therefore, Mg has a significant weakening influence on the Sr modification effect at low cooling rates but not at high cooling rates.
3.2. The Influence of Alloying Element Cu on the Morphology of Eutectic Silicon in Al–7Si Alloy
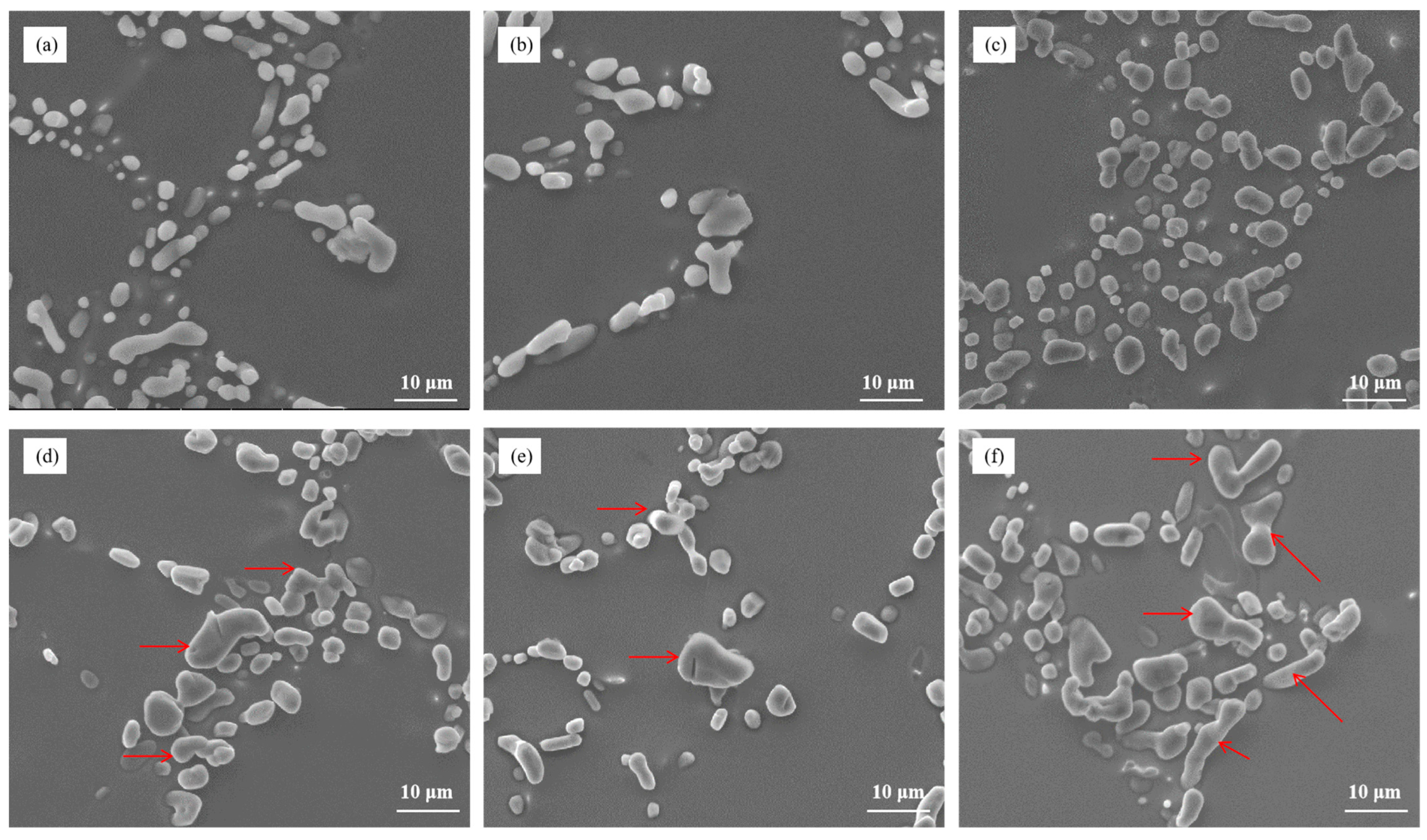
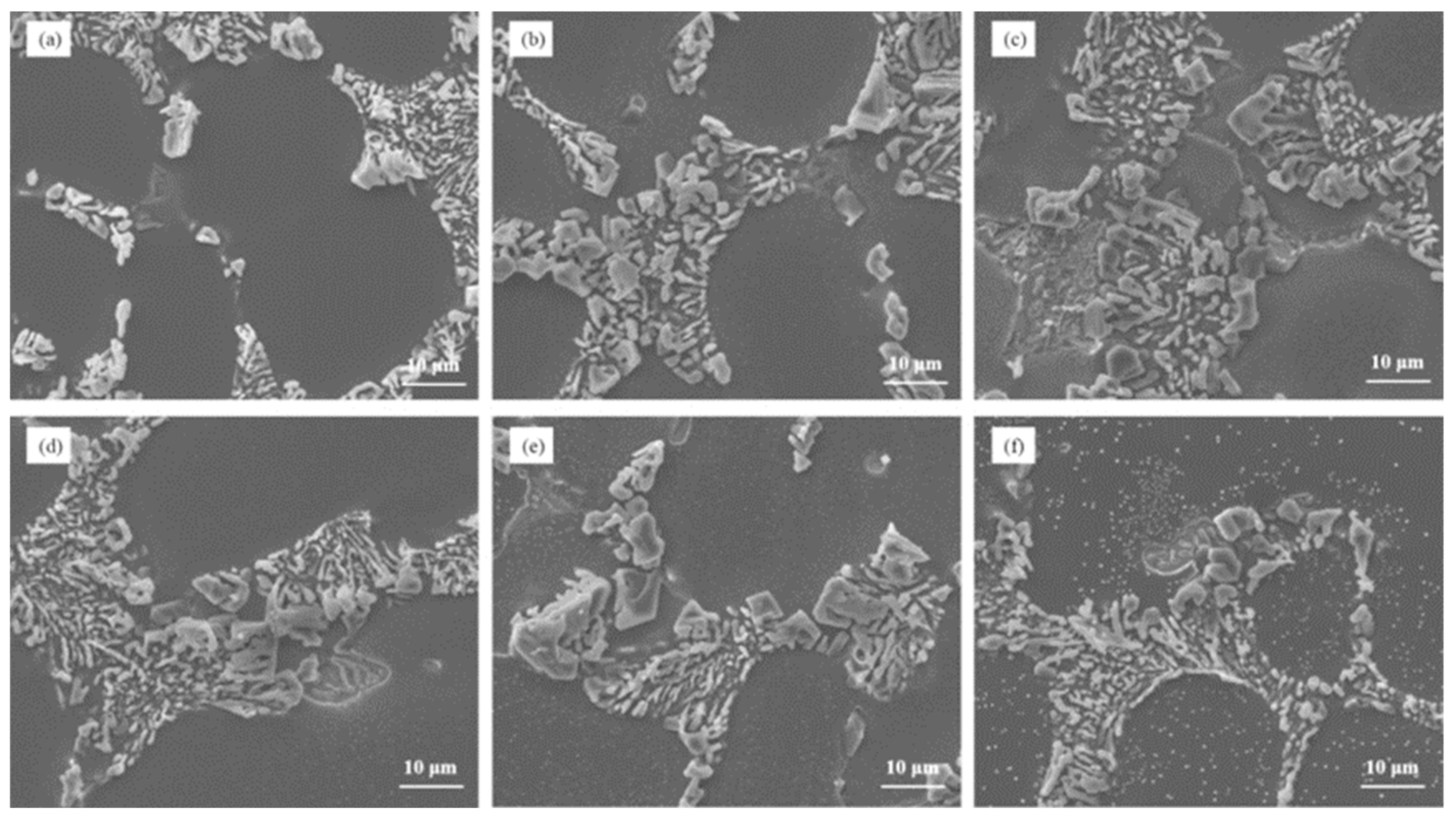
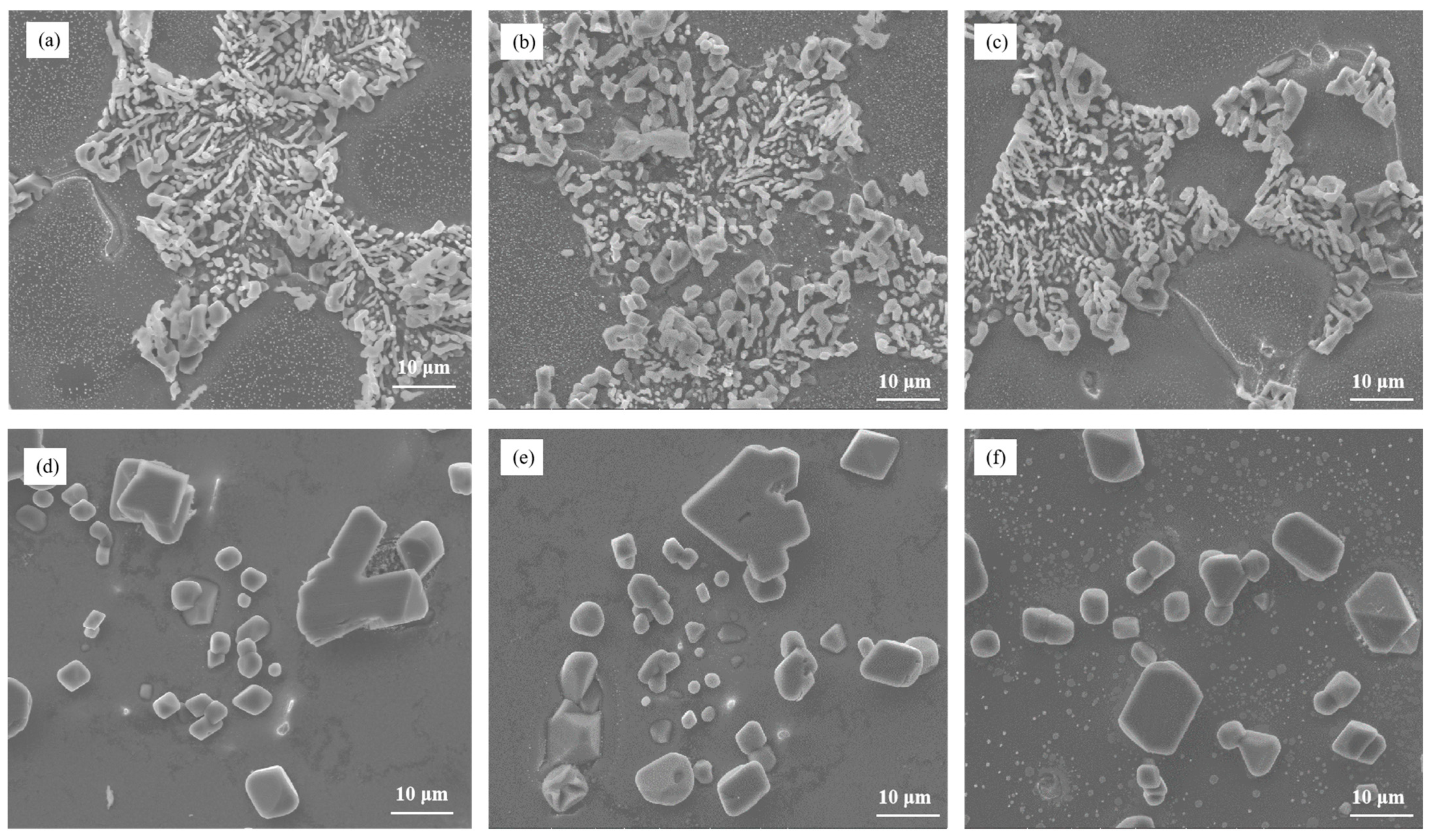
Figure 7 illustrates the microstructure of Al–7Si–xCu alloys with different Cu contents in the as-cast state after being modified with 0.03 wt.% Sr. Notably, the addition of merely 0.5 wt.% Cu to Al–7Si alloy resulted in the appearance of unmodified blocky eutectic silicon. As the Cu content increased, the eutectic silicon coarsened and the quantity of partially unmodified eutectic silicon grew, leading to a deterioration in the modification of eutectic silicon.
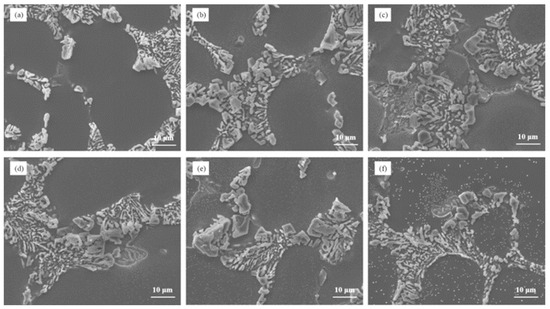
Figure 7.
The microstructure of Al–7Si–xCu alloys modified with Sr in the as-cast state: (a) 0.5 wt.% Cu, (b) 1 wt.% Cu, (c) 2 wt.% Cu, (d) 3 wt.% Cu, (e) 4 wt.% Cu, and (f) 5 wt.% Cu.
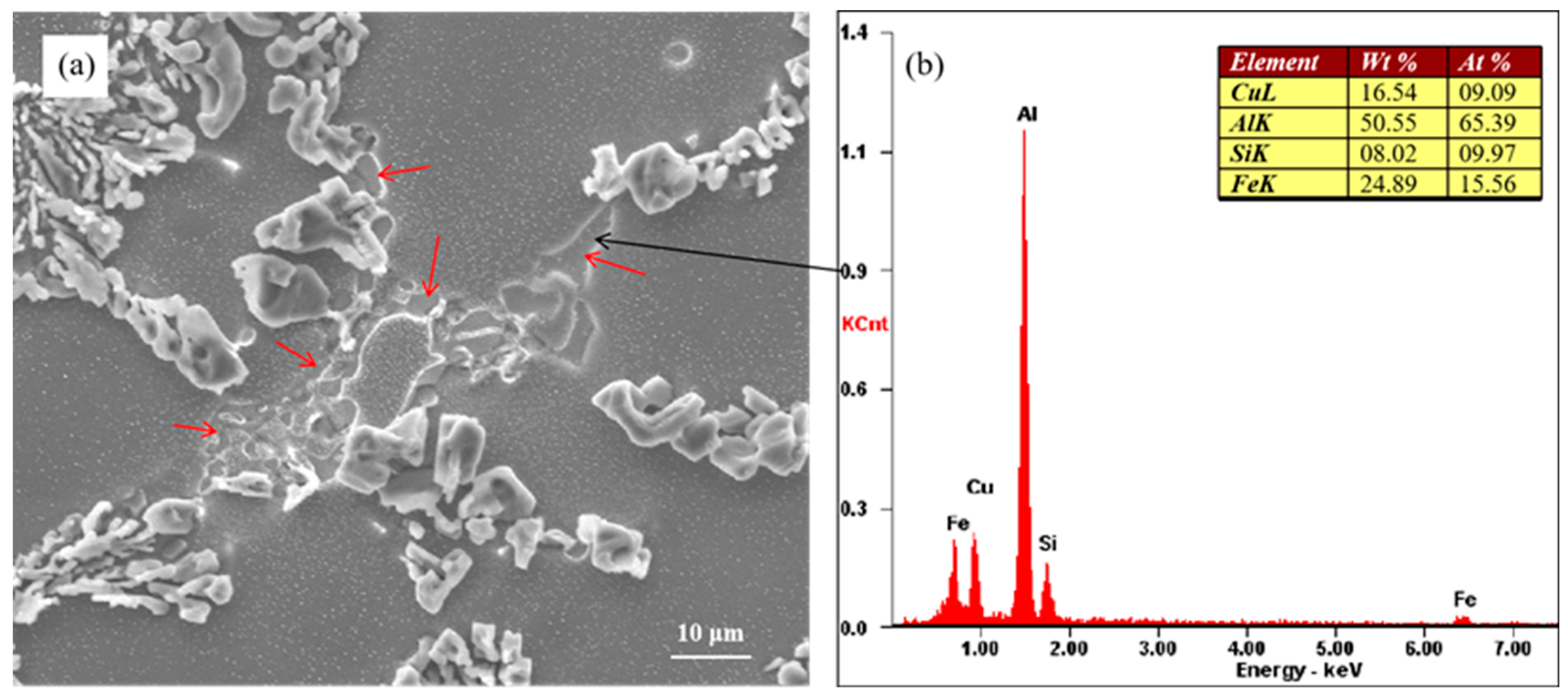
Figure 8 shows the morphology of unmodified eutectic Si and the Cu-rich phase. The Cu-rich phase is indicated by the red arrows in Figure 8a. The energy spectrum of the Cu-rich phase indicated by the black arrow is shown in Figure 8b. Blocky Si was observed along with the Cu-rich phase at the eutectic grain boundaries, that is, at the last stage of solidification. The results of energy spectrum analysis showed that the Cu-rich phase contained Al, Cu, Fe, and Si elements. Figure 8 shows the as-cast microstructure of the alloy, and the Cu-rich phase was formed during the solidification process of the alloy.
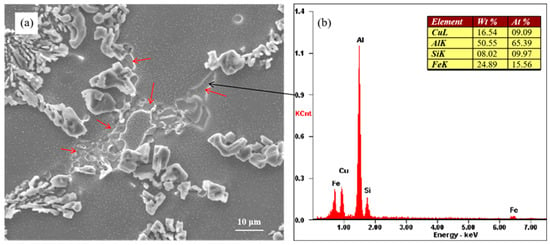
Figure 8.
(a) Morphology of partially modified eutectic Si and Cu-rich phase and (b) corresponding EDX results.
Mohamed et al. [28] proposed that Cu reacts with Sr to form Al–Cu–Sr compounds, which reduces the effective Sr content in the Al–Si alloy melt and diminishes the modification of eutectic silicon. However, the presence of Al–Cu–Sr compounds in the alloy was not detected. The addition of the alloying element Cu transforms the eutectic reaction in the alloy from the original binary reaction into the ternary eutectic reaction. The addition of Cu can make the eutectic form over a temperature range [16], that is, the growth time of eutectic silicon becomes longer, which leads to the coarsening of eutectic silicon. As the content of the alloying element Cu increases gradually, the ternary eutectic molten pool formed by the alloying element increases gradually, which leads to a gradual increase in the size of incomplete modification eutectic silicon.
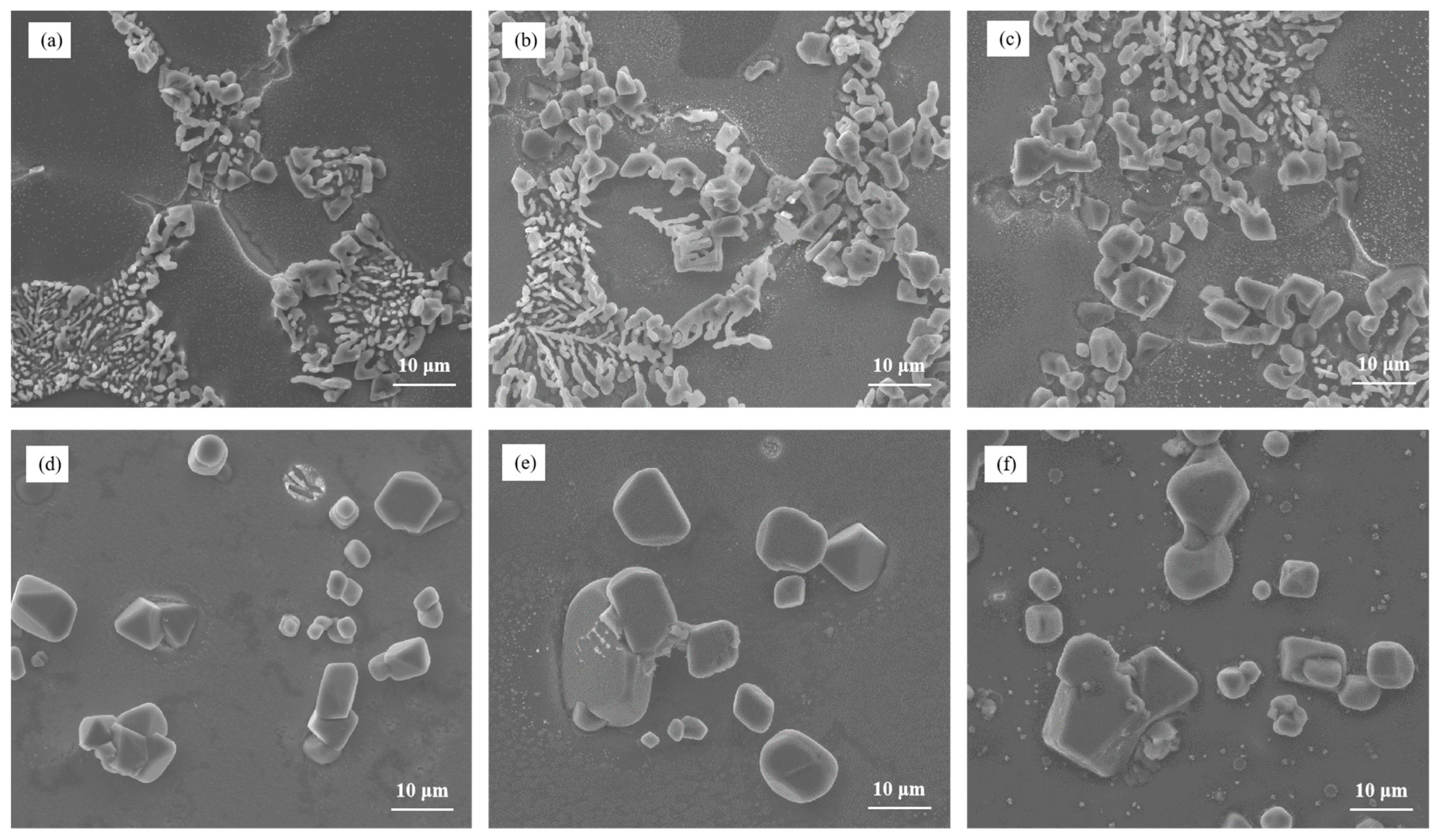
The microstructure analysis presented in Figure 9 examined the influence of different Cu contents on the heat-treated state of Al–7Si–xCu alloys after modification with 0.03 wt.% Sr. The results revealed a correlation between the Cu content and the size of eutectic silicon particles after heat treatment. As the Cu content increased, there was a noticeable increase in the size of eutectic silicon particles in the microstructure. This indicates that the addition of Cu, in combination with Sr modification, leads to the growth of eutectic silicon in the alloy. When the Cu content reached 3 wt.%, the microstructure showed the presence of large blocky eutectic silicon particles. This suggests that at this Cu content level, a significant portion of the eutectic silicon undergoes a transformation into larger block-like structures. Furthermore, when the Cu content reached 4 wt.%, most of the eutectic silicon particles in the alloy transformed into large blocks. The size of eutectic silicon is greater than 20 μm. This implies that at this higher Cu content, the majority of the eutectic silicon particles adopt a blocky morphology.
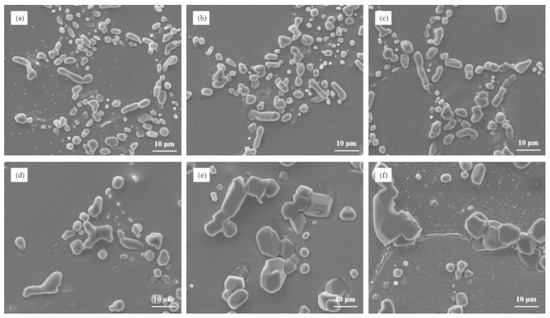
Figure 9.
The microstructure of Al–7Si–xCu alloys modified with Sr in the heat-treated state: (a) 0.5 wt.% Cu, (b) 1 wt.% Cu, (c) 2 wt.% Cu, (d) 3 wt.% Cu, (e) 4 wt.% Cu, and (f) 5 wt.% Cu.
The observed increase in the size and transformation of eutectic silicon into large blocks with higher Cu contents indicates the influence of Cu on the microstructure of Al–7Si–xCu alloys after heat treatment. These findings provide valuable insights into the relationship between the Cu content and the resulting morphology of eutectic silicon in the alloy. Understanding the impact of the Cu content on the microstructure is important for optimizing the composition and processing parameters of Al–7Si–xCu alloys. By controlling the Cu content, it is possible to manipulate the size and morphology of eutectic silicon, which can ultimately affect the mechanical properties and performance of the alloy in various applications.
Understanding the impact of the Sr content and solidification cooling rate on the modification effect of Al–7Si–4Cu alloy can provide valuable insights for optimizing the modification process parameters. By identifying the optimal Sr content and cooling rate, it becomes possible to achieve a more comprehensive and effective modification of eutectic silicon in the alloy.
Figure 10 illustrates the microstructure of the alloy with varying Sr contents, shedding light on the modification of eutectic silicon. The size of eutectic Si is about 10~20 μm. Upon analysis, it becomes evident that increasing the Sr content does not completely eliminate the phenomenon of incomplete modification of eutectic silicon. Partially blocky eutectic silicon particles remain in the microstructure of the as-cast alloy despite the higher Sr content. This indicates that the modification effect achieved with Sr is limited and unable to fully overcome the incomplete modification caused by the presence of Cu, the alloying element. Furthermore, in the heat-treated state, the size of eutectic silicon in the Al–7Si–4Cu alloy is significantly larger compared to the size observed in Al–7Si alloy. This observation suggests that increasing the Sr content does not effectively address the degraded modification effect caused by the alloying element Cu in Al–7Si–4Cu alloy.
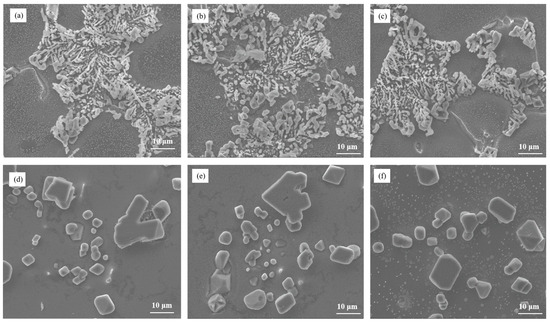
Figure 10.
The microstructural morphology of Al–7Si–4Cu alloy with different Sr contents: (a) as-cast/0.03 wt.% Sr, (b) as-cast/0.06 wt.% Sr, (c) as-cast/0.09 wt.% Sr, (d) heat-treated state/0.03 wt.% Sr, (e) heat-treated state/0.06 wt.% Sr, and (f) heat-treated state/0.09 wt.% Sr.
Continued research and development efforts are crucial to finding comprehensive solutions for overcoming the incomplete modification caused by the alloying element Cu in Al–7Si–4Cu alloy. Enhancing the modification process will contribute to the advancement and wider application of this alloy in various industries.
Figure 11 exhibits the microstructure of Al–7Si–4Cu alloy at different cooling rates in the heat-treated state after being modified with 0.03 wt.% Sr. It can be observed that at different solidification cooling rates, the modification of eutectic silicon did not exhibit significant changes. Even at high solidification cooling rates, large-sized unmodified eutectic silicon persisted in the as-cast microstructure. After heat treatment, the size of eutectic silicon remained large despite spheroidization. Therefore, the weakening effect of the alloying element Cu on the modification of eutectic silicon outweighs the positive effect of the solidification cooling rate.
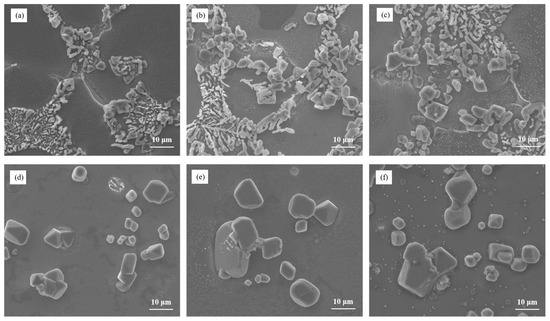
Figure 11.
The microstructural morphology of Al–7Si–4Cu alloy at different cooling rates: (a) as-cast/room-temperature steel mold, (b) as-cast/200 °C steel mold, (c) as-cast/400 °C steel mold, (d) heat-treated/room-temperature steel mold, (e) heat-treated/200°C steel mold, and (f) heat-treated/400 °C steel mold.
In summary, alloying elements Mg and Cu have a weakening or poisoning effect on eutectic silicon modification, with Cu having a more significant weakening or poisoning effect. This influence cannot be eliminated by increasing the content of the modifying element Sr. It is still a crucial issue that needs further research to enhance the modification of Al–Si–Mg and Al–Si–Cu alloys under slow solidification cooling conditions.
To address this, it is necessary to first find direct evidence of the weakening or poisoning effect of Mg and Cu on eutectic silicon modification, especially how Mg and Cu alter the growth mode of eutectic silicon and whether they change the mechanism of Sr inhibiting the Si phase growth, through SEM/TEM analysis techniques. We are considering the use of HRTEM analysis to investigate whether the added Mg and Cu have an impact on the lattice constant or arrangement of Si atoms in the near-surface silicon phase or whether there are other influencing factors, etc.
4. Conclusions
- (1)
- Alloying elements Mg and Cu weaken the modification of eutectic silicon in hypoeutectic Al–7Si alloy. Even with the addition of only 0.1wt.% Mg or 0.5wt.% Cu, unmodified blocky eutectic silicon appears in certain fraction in the alloy. The weakening effect increases with the increase in the alloying element content.
- (2)
- Under the experimental conditions in this paper, increasing the content of the modification element Sr or improving the solidification cooling rate cannot eliminate the weakening effect of alloying elements Mg and Cu on the modification of eutectic silicon.
Author Contributions
Conceptualization, L.H. and X.D.; methodology, Q.Z.; software, C.H.; validation, L.H., X.D. and Q.Z.; formal analysis, C.H.; investigation, L.H.; resources, J.L.; data curation, X.D.; writing—original draft preparation, L.H.; writing—review and editing, L.H.; visualization, C.H.; supervision, J.L.; project administration, J.L.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
Author Qianyu Zhuang was employed by the company Hangcai Guochuang (Qingdao) High-Speed Railway Material Research Institute Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Flood, S.C.; Hunt, J.D. Modification of Al-Si eutectic alloys with Na. Met. Sci. J. 1981, 15, 287–294. [Google Scholar] [CrossRef]
- Ravi, M.; Pillai, U.T.S.; Pai, B.C.; Damodaran, A.D.; Dwarakadasa, E.S. Mechanical properties of cast Al-7Si-0.3Mg (LM 25/356) alloy. Int. J. Cast Met. Res. 1998, 11, 113–125. [Google Scholar] [CrossRef]
- Faraji, M.; Katgerman, L. Microstructural analysis of modification and grain refinement in a hypoeutectic Al–Si alloy. Cast Met. 2013, 22, 108–110. [Google Scholar] [CrossRef]
- Ganesh, M.R.S.; Reghunath, N.J.; Levin, M.; Prasad, A.; Doondi, S.; Shankar, K.V. Strontium in Al–Si–Mg Alloy: A Review. Met. Mater. Int. 2022, 28, 1–40. [Google Scholar] [CrossRef]
- Pan, Y.P.; Zhang, Z.F.; Li, B.; Xu, J. Effect of Sr Modification on Eutectic Silicon Morphology in Al-6Si-3Cu-0.3Mg Alloy. Spec. Cast. Nonferrous Alloys 2015, 35, 322–324. [Google Scholar]
- Sui, Y.D.; Wang, Q.D.; Wang, G.L.; Liu, T. Effects of Sr content on the microstructure and mechanical properties of cast Al–12Si–4Cu–2Ni–0.8Mg alloys. J. Alloys Compd. 2015, 622, 572–579. [Google Scholar] [CrossRef]
- Sigworth, G.; Campbell, J.; Jorstad, J. The Modification of Al-Si Casting Alloys: Important Practical and Theoretical Aspects. Int. J. Metal Cast. 2009, 3, 65–78. [Google Scholar] [CrossRef]
- Dong, X.G.; Zhou, J.; Jia, Y.J.; Liu, B.; Lei, Z.Z.; Wang, W.H.; Bi, H. Influence of Sr on microstructure and mechanical properties of ZL114 cast alloy. China Foundry 2011, 8, 401–406. [Google Scholar]
- Yamamoto, K.; Takahashi, M.; Kamikubo, Y.; Sugiura, Y.; Wasawa, S.I.; Nakata, T.; Kamado, S. Effect of Mg content on age-hardening response, tensile properties, and microstructures of a T5-treated thixo-cast hypoeutectic Al-Si alloy. Mater. Sci. Eng. A. Struct. Mater. Prop. Misrostruct. Process 2020, 798, 140089. [Google Scholar] [CrossRef]
- Tavitas-Medrano, F.J.; Gruzleski, J.E.; Samuel, F.H.; Valtierra, S.; Doty, H.W. Effect of Mg and Sr-modification on the mechanical properties of 319-type aluminum cast alloys subjected to artificial aging. Mater. Sci. Eng. A 2008, 480, 356–364. [Google Scholar] [CrossRef]
- Pezda, J. Optimization of Heat Treatment Parameters of AlSi7Mg Alloy. Materials 2022, 15, 1163. [Google Scholar] [CrossRef]
- Stani´c, D.; Brodarac, Z.Z.; Li, L. Influence of Copper Addition in AlSi7MgCu Alloy on Microstructure Development and Tensile Strength Improvement. Metals 2020, 10, 1623. [Google Scholar] [CrossRef]
- Rashed, H.M.M.A.; Ashrafi, E.A.; Hasan, A. Modelling Effects of Copper on Microstructure and Properties of Al-Si-Cu Alloys. Mater. Trans. 2023, 64, 177–183. [Google Scholar] [CrossRef]
- Shivaprasad, C.G.; Aithal, K.; Narendranath, S.; Vijay, D.; Mukunda, P.G. Effects of 4.5% Copper Addition and Melt Treatment on Microstructure and Wear Properties of Al-7Si Alloy. Appl. Mech. Mater. 2015, 766–767, 410–415. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Timelli, G.; Fabrizi, A.; Bonollo, F. Effect of V and Zr microalloying, and heat treatment on microstructure and mechanical properties of secondary Al-7Si-3Cu-0.3Mg alloy. Int. J. Mater. Res. 2018, 109, 1099–1112. [Google Scholar] [CrossRef]
- Dons, A.L.; Heiberg, G.; Voje, J.; Maland, J.S.; Loland, J.O.; Prestmo, A. On the effect of additions of Cu and Mg on the ductility of AlSi foundry alloys cast with a cooling rate of approximately 3 K/s. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process 2005, 413–414, 561–566. [Google Scholar] [CrossRef]
- Alessandro, D.M.; Giulio, T.; Alberto, F.; Berto, F. Influence of Cu content on the microstructure and high-temperature tensile and fatigue properties of secondary AlSi7Mg0.3VZr alloys. Mater. Sci. Eng. A 2021, 816, 141310. [Google Scholar]
- Callegari, B.; Lima, T.N.; Coelho, R.S. The Influence of Alloying Elements on the Microstructure and Properties of Al-Si-Based Casting Alloys: A Review. Metals 2023, 13, 1174. [Google Scholar] [CrossRef]
- Darlapudi, A.; McDonald, S.D.; Terzi, S.; Prasad, A.; Felberbaum, M.; StJohn, D.H. The influence of ternary alloying elements on the Al–Si eutectic microstructure and the Si morphology. J. Cryst. Growth 2016, 433, 63–73. [Google Scholar] [CrossRef]
- Li, Q.L.; Li, B.Q.; Li, J.B.; Xia, J.B.; Lan, T.D.; Guo, Y.F.; Biao, T. Effects of the Addition of Mg on the Microstructure and Mechanical Properties of Hypoeutectic Al-7%Si Alloy. Int. J. Met. Cast. 2017, 11, 823–830. [Google Scholar] [CrossRef]
- Abedi, A.; Shahmiri, M.; Esgandari, B.; Nami, B. Microstructural Evolution during Partial Remelting of Al-Si Alloys Containing Different Amounts of Magnesium. J. Mater. Sci. Technol. 2013, 29, 971–978. [Google Scholar] [CrossRef]
- Fracchia, E.; Gobber, F.S.; Rosso, M. Effect of Alloying Elements on the Sr Modification of Al-Si Cast Alloys. Metals 2021, 11, 342. [Google Scholar] [CrossRef]
- Nafisi, S.; Emadi, D.; Ghomashchi, R. Impact of Mg addition on solidification behaviour of Al–7%Si alloy. Mater. Sci. Technol. 2008, 24, 718–724. [Google Scholar] [CrossRef]
- Caceres, C.H.; Davidson, C.J.; Griffiths, J.R.; Wang, Q.G. The effect of Mg on the microstructure and mechanical behavior of Al-Si-Mg casting alloys. Metall. Mater. Trans. A 1999, 30, 2611–2618. [Google Scholar] [CrossRef]
- Joenoes, A.T.; Gruzleski, J.E. Magnesium Effects on the Microstructure of Unmodified and Modified Al-Si Alloys. Cast Met. 1991, 4, 62–71. [Google Scholar] [CrossRef]
- Heusler, L.; Schneider, W. Influence of alloying elements on the thermal analysis results of Al–Si cast alloys. J. Light Met. 2002, 2, 17–26. [Google Scholar] [CrossRef]
- Zhang, W.D.; Ma, S.X.; Wei, Z.H.; Bai, P.K. The Relationship between Residual Amount of Sr and Morphology of Eutectic Si Phase in A356 Alloy. Materials 2019, 12, 3222. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M.A.; Samuel, A.M.; Samuel, F.H.; Doty, H.W. Influence of additives on the microstructure and tensile properties of near-eutectic Al–10.8%Si cast alloy. Mater. Des. 2009, 30, 3943–3957. [Google Scholar] [CrossRef]
- Garcia-Hinojosa, J.A.; Gonzalez, C.R.; Gonzalez, G.M.; Houbaert, Y. Structure and properties of Al–7Si–Ni and Al–7Si–Cu cast alloys nonmodified and modified with Sr. J. Mater. Process. Technol. 2003, 143–144, 306–310. [Google Scholar] [CrossRef]
- Zhang, L.L.; Ji, Z.W.; Zhao, J.Z.; He, J.; Jiang, H.X. Factors affecting eutectic Si modification in Al-Si hypoeutectic alloy with the addition of Na, Sr, Eu and Yb. Mater. Lett. 2021, 308, 131206. [Google Scholar] [CrossRef]
- Sebaie, O.E.; Samuel, A.M.; Samuel, F.H.; Doty, H.W. The effects of mischmetal, cooling rate and heat treatment on the eutectic Si particle characteristics of A319.1, A356.2 and A413.1 Al–Si casting alloys. Mater. Sci. Eng. A 2008, 480, 342–355. [Google Scholar] [CrossRef]
- Huang, C.F.; Liu, Z.G.; Li, J.G. Influence of Alloying Element Mg on Na and Sr Modifying Al-7Si Hypoeutectic Alloy. Materials 2022, 15, 1537. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jiang, Y.H.; Pan, L.X.; Jin, Q.L.; Zhou, R. Effect of Eutectic Silicon Morphology on Tensile Fracture Mechanism of ZL114A Alloy. Foundry Technol. 2010, 31, 612–615. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).