Pupal Age Estimation of Sarcophaga peregrina (Diptera: Sarcophagidae) at Different Constant Temperatures Utilizing ATR-FTIR Spectroscopy and Cuticular Hydrocarbons
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Sample Collection
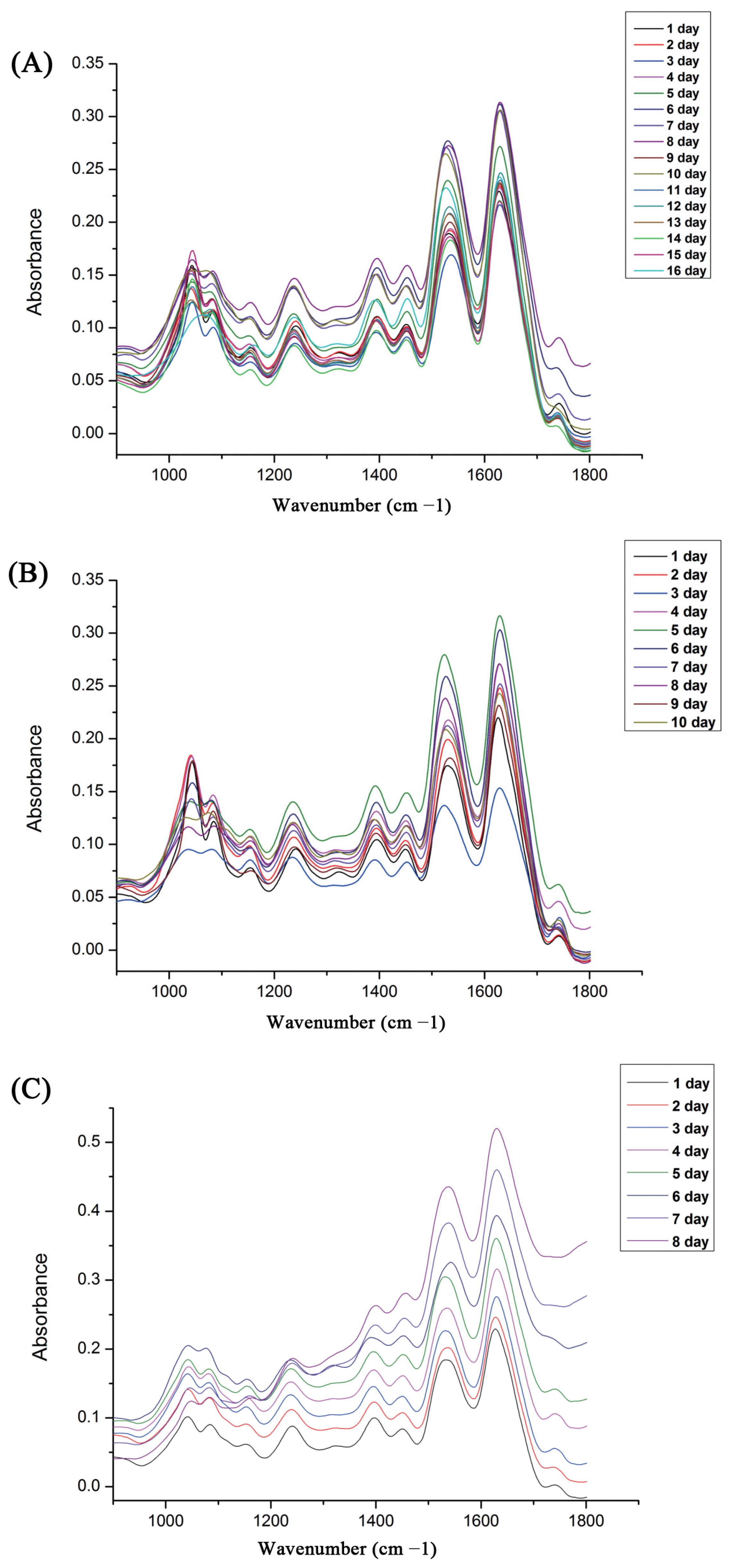
2.2. Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR) Analysis
2.3. Cuticular Hydrocarbon (CHC) Analysis
3. Results
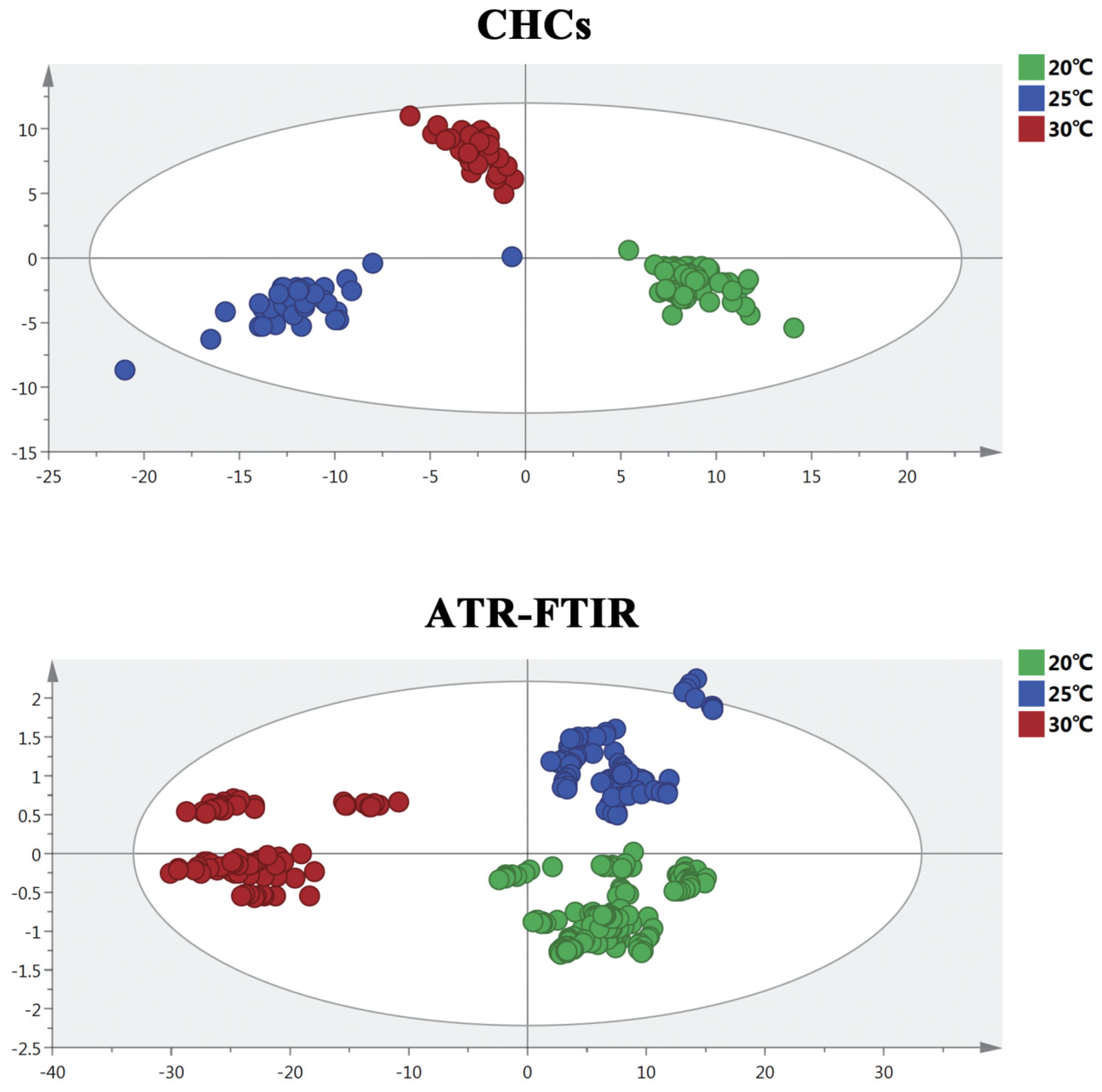
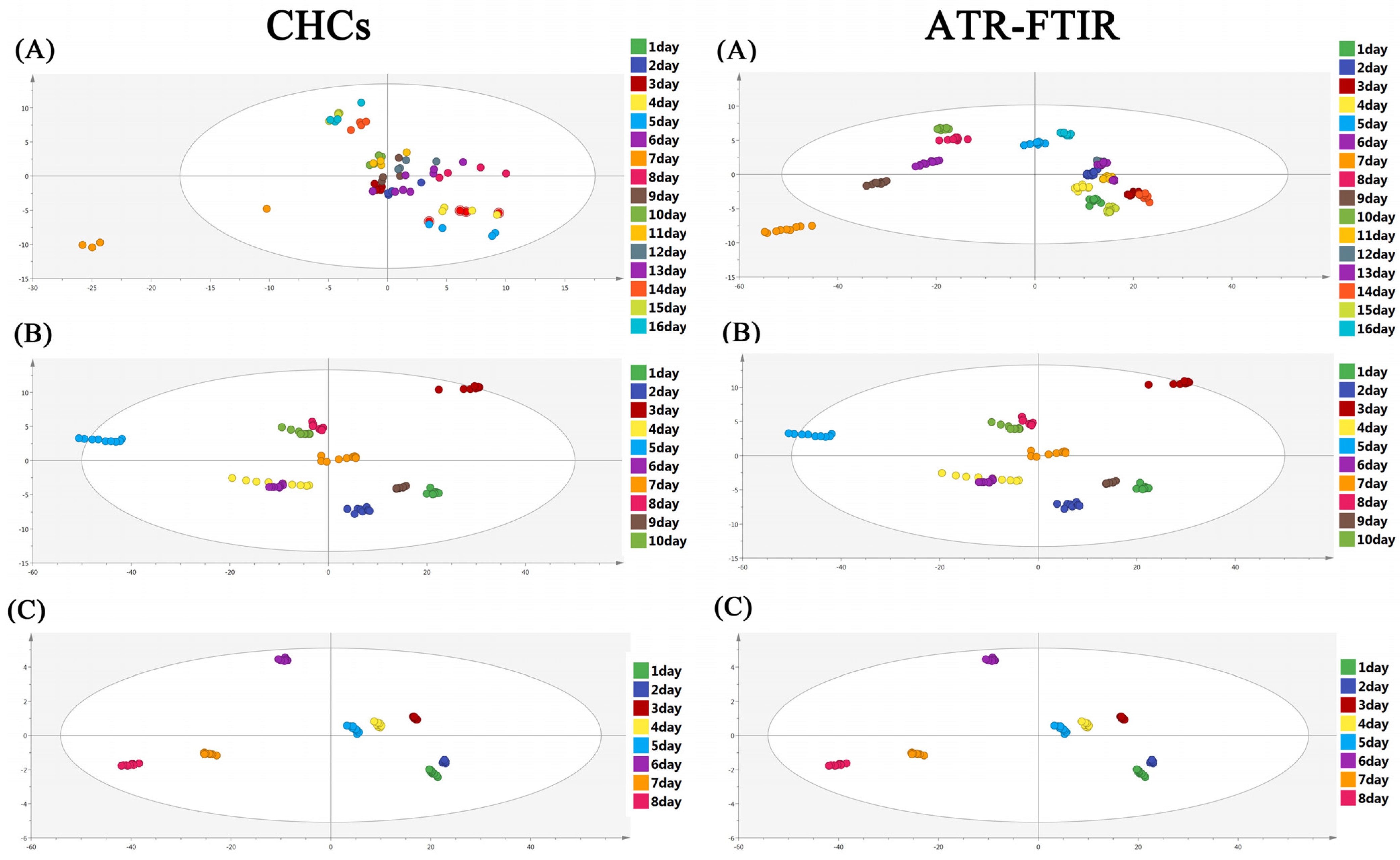
3.1. OPLS-DA Model Analysis
3.2. PLS Model Analysis
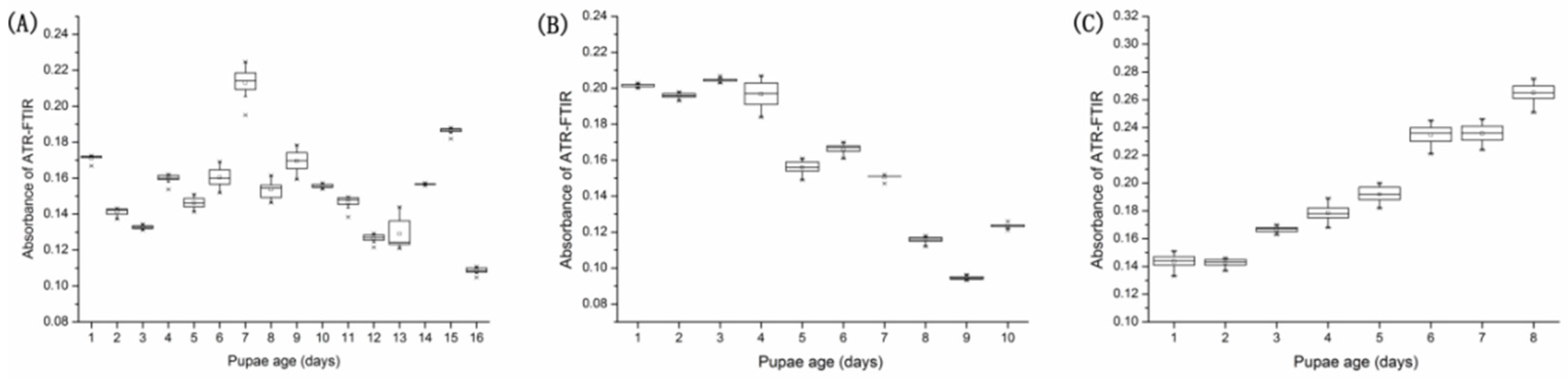
3.3. Variation Tendency with VIP Parameter > 1.0
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M.J. Best practice in forensic entomology--standards and guidelines. Int. J. Legal Med. 2007, 121, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J.R. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.H.; Castner, J.L. Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press: London, UK, 2010; pp. 153–200. [Google Scholar]
- Brown, K.; Thorne, A.; Harvey, M. Calliphora vicina (Diptera: Calliphoridae) pupae: A timeline of external morphological development and a new age and PMI estimation tool. Int. J. Legal Med. 2015, 129, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Ren, L.; Yang, L.; Wang, S.; Chen, W.; Dong, J.; Ma, H.; Qi, X.; Guo, Y. Differential Gene Expression for Age Estimation of Forensically Important Sarcophaga peregrina (Diptera: Sarcophagidae) Intrapuparial. J. Med. Entomol. 2020, 57, 65–77. [Google Scholar] [CrossRef]
- Bainbridge, S.P.; Bownes, M. Staging the metamorphosis of Drosophila melanogaster. J. Embryol. Exp. Morphol 1981, 66, 57–80. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Wang, M.; Wang, Y.; Xu, W.; Zhang, Y.; Wang, J. Intrapuparial Development and Age Estimation of Calliphora grahami (Diptera: Calliphoridae) for Postmortem Interval Estimation. J. Med. Entomol. 2022, 59, 454–466. [Google Scholar] [CrossRef]
- Boehme, P.; Spahn, P.; Amendt, J.; Zehner, R. Differential gene expression during metamorphosis: A promising approach for age estimation of forensically important Calliphora vicina pupae (Diptera: Calliphoridae). Int. J. Legal Med. 2013, 127, 243–249. [Google Scholar] [CrossRef]
- Schoborg, T.A.; Smith, S.L.; Smith, L.N.; Morris, H.D.; Rusan, N.M. Micro-computed tomography as a platform for exploring Drosophila development. Development 2019, 146, dev176685. [Google Scholar] [CrossRef]
- Voss, S.C.; Magni, P.; Dadour, I.; Nansen, C. Reflectance-based determination of age and species of blowfly puparia. Int. J. Legal Med. 2017, 131, 263–274. [Google Scholar] [CrossRef]
- Alkhuder, K. Attenuated total reflection-Fourier transform infrared spectroscopy: A universal analytical technique with promising applications in forensic analyses. Int. J. Legal Med. 2022, 136, 1717–1736. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, H.; Li, B.; Lin, H.; Zhang, Y.; Zhang, J.; Wang, Z. UV-Vis and ATR-FTIR spectroscopic investigations of postmortem interval based on the changes in rabbit plasma. PLoS ONE 2017, 12, e0182161. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R. Portable FTIR spectrometers get moving. Anal. Chem. 2004, 76, 369A–372A. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yu, K.; Wu, D.; Huang, P.; Sun, Q.; Wang, Z. Species identification of semen stains by ATR-FTIR spectroscopy. Int. J. Legal Med. 2021, 135, 73–80. [Google Scholar] [CrossRef]
- Mistek, E.; Lednev, I.K. Identification of species’ blood by attenuated total reflection (ATR) Fourier transform infrared (FT-IR) spectroscopy. Anal. Bioanal. Chem. 2015, 407, 7435–7442. [Google Scholar] [CrossRef] [PubMed]
- Manheim, J.; Doty, K.C.; McLaughlin, G.; Lednev, I.K. Forensic Hair Differentiation Using Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopy. Appl. Spectrosc. 2016, 70, 1109–1117. [Google Scholar] [CrossRef]
- Cai, W.; Wang, G.; Wu, H.; Li, H.; Shen, C.; Wei, X.; Yu, K.; Sun, Q.; Wang, Z. Identifying traumatic brain injury (TBI) by ATR-FTIR spectroscopy in a mouse model. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 274, 121099. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.L.; Hands, J.R.; Fullwood, L.M.; Smith, J.A.; Baker, M.J. Rapid discrimination of maggots utilising ATR-FTIR spectroscopy. Forensic. Sci. Int. 2015, 249, 189–196. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Ginzel, M.D. Chemical Ecology, Biochemistry, and Molecular Biology of Insect Hydrocarbons. Annu. Rev. Entomol. 2021, 66, 45–60. [Google Scholar] [CrossRef]
- Moore, H.E.; Adam, C.D.; Drijfhout, F.P. Identifying 1st instar larvae for three forensically important blowfly species using "fingerprint" cuticular hydrocarbon analysis. Forensic. Sci. Int. 2014, 240, 48–53. [Google Scholar] [CrossRef]
- Moore, H.E.; Butcher, J.B.; Day, C.R.; Drijfhout, F.P. Adult fly age estimations using cuticular hydrocarbons and Artificial Neural Networks in forensically important Calliphoridae species. Forensic. Sci. Int. 2017, 280, 233–244. [Google Scholar] [CrossRef]
- Zhu, G.H.; Jia, Z.J.; Yu, X.J.; Wu, K.S.; Chen, L.S.; Lv, J.Y.; Eric Benbow, M. Predictable weathering of puparial hydrocarbons of necrophagous flies for determining the postmortem interval: A field experiment using Chrysomya rufifacies. Int. J. Legal Med. 2017, 131, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.; Lutz, L.; Bernhardt, V.; Drijfhout, F.P.; Cody, R.B.; Amendt, J. Cuticular hydrocarbons for the identification and geographic assignment of empty puparia of forensically important flies. Int. J. Legal Med. 2022, 136, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Shang, Y.; Zhang, X.; Chen, S.; Zheng, Y.; Zou, Y.; Qu, Y.; Cai, J.; Zhang, C.; Guo, Y. Temporal Expression Profiles Reveal Potential Targets during Postembryonic Development of Forensically Important Sarcophaga peregrina (Diptera: Sarcophagidae). Insects 2022, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.F.; Zhang, Y.N.; Tao, L.Y.; Wang, M. Forensically Important Boettcherisca peregrina (Diptera: Sarcophagidae) in China: Development Pattern and Significance for Estimating Postmortem Interval. J. Med. Entomol. 2017, 54, 1491–1497. [Google Scholar] [CrossRef]
- Rolff, J.; Johnston, P.R.; Reynolds, S. Complete metamorphosis of insects. Philos. Trans. R Soc. Lond. B Biol. Sci. 2019, 374, 20190063. [Google Scholar] [CrossRef]
- Hu, C. Forensic Entomology; Chongqing Publishing House: Chongqing, China, 2000. [Google Scholar]
- Zhang, J.; Wei, X.; Huang, J.; Lin, H.; Deng, K.; Li, Z.; Shao, Y.; Zou, D.; Chen, Y.; Huang, P.; et al. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectral prediction of postmortem interval from vitreous humor samples. Anal. Bioanal. Chem. 2018, 410, 7611–7620. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Wang, Q.; Li, C.; Zhang, Y.; Lin, H.; Wang, Z. Characterization of postmortem biochemical changes in rabbit plasma using ATR-FTIR combined with chemometrics: A preliminary study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, J.; Wang, Z.; Zhang, J.; Huang, P. An investigation on annular cartilage samples for post-mortem interval estimation using Fourier transform infrared spectroscopy. Forensic. Sci. Med. Pathol. 2019, 15, 521–527. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, Y.; Ngando, F.J.; Qu, H.; Shang, Y.; Ren, L.; Guo, Y. Predicting the Weathering Time by the Empty Puparium of Sarcophaga peregrina (Diptera: Sarcophagidae) with the ANN Models. Insects 2022, 13, 808. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Wang, Q.; Wei, X.; Feng, W.; Chen, Y.; Huang, P.; Wang, Z. Application of Fourier transform infrared spectroscopy with chemometrics on postmortem interval estimation based on pericardial fluids. Sci. Rep. 2017, 7, 18013. [Google Scholar] [CrossRef]
- Butcher, J.B.; Moore, H.E.; Day, C.R.; Adam, C.D.; Drijfhout, F.P. Artificial neural network analysis of hydrocarbon profiles for the ageing of Lucilia sericata for post mortem interval estimation. Forensic. Sci. Int. 2013, 232, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shang, Y.; Ren, L.; Qu, H.; Zhu, G.; Guo, Y. A Study of Cuticular Hydrocarbons of All Life Stages in Sarcophaga peregrina (Diptera: Sarcophagidae). J. Med. Entomol. 2022, 59, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, M.; Xu, W.; Zhang, Y.; Wang, J. Forensic Entomology in China and Its Challenges. Insects 2021, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.-X.; Wu, J.; Sun, D.-P.; Hillyer, J. Intrapuparial Age Estimation of Forensically Important Dohrniphora cornuta (Diptera: Phoridae). J. Med. Entomol. 2021, 58, 616–624. [Google Scholar] [CrossRef]
- Shang, Y.; Amendt, J.; Wang, Y.; Ren, L.; Yang, F.; Zhang, X.; Zhang, C.; Guo, Y. Multimethod combination for age estimation of Sarcophaga peregrina (Diptera: Sarcophagidae) with implications for estimation of the postmortem interval. Int. J. Legal Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lecheta, M.C.; Moura, M.O. Estimating the Age of Forensically Useful Blowfly, Sarconesia chlorogaster (Diptera: Calliphoridae), Using Larval Length and Weight. J. Med. Entomol. 2019, 56, 915–920. [Google Scholar] [CrossRef]
- Song, J.; Zhou, S. Post-transcriptional regulation of insect metamorphosis and oogenesis. Cell Mol. Life Sci. 2020, 77, 1893–1909. [Google Scholar] [CrossRef]
- Jales, J.T.; Barbosa, T.M.; de Medeiros, J.R.; de Lima, L.A.S.; de Lima, K.M.G.; Gama, R.A. Infrared spectroscopy and forensic entomology: Can this union work? A literature review. J. Forensic. Sci. 2021, 66, 2080–2091. [Google Scholar] [CrossRef]
- Barbosa, T.M.; de Lima, L.A.S.; Dos Santos, M.C.D.; Vasconcelos, S.D.; Gama, R.A.; Lima, K.M.G. A novel use of infra-red spectroscopy (NIRS and ATR-FTIR) coupled with variable selection algorithms for the identification of insect species (Diptera: Sarcophagidae) of medico-legal relevance. Acta Trop. 2018, 185, 1–12. [Google Scholar] [CrossRef]
- de Paula, M.C.; Michelutti, K.B.; Eulalio, A.; Mendonca, A.; Cardoso, C.A.L.; Andrade, L.H.C.; Lima, S.M.; Antonialli-Junior, W.F. New approach to application of mid-infrared photoacoustic spectroscopy in forensic analysis: Study with the necrophagous blow fly Chrysomya megacephala (Diptera: Calliphoridae). J. Photochem. Photobiol. B 2020, 209, 111934. [Google Scholar] [CrossRef]
- Baia, T.C.; Gama, R.A.; Silva de Lima, L.A.; Lima, K.M.G. FTIR microspectroscopy coupled with variable selection methods for the identification of flunitrazepam in necrophagous flies. Analytical. Methods 2016, 8, 968–972. [Google Scholar] [CrossRef]
- Okude, G.; Moriyama, M.; Kawahara-Miki, R.; Yajima, S.; Fukatsu, T.; Futahashi, R. Molecular mechanisms underlying metamorphosis in the most-ancestral winged insect. Proc. Natl. Acad. Sci. USA 2022, 119, e2114773119. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Huang, L.X.; Gong, Y.J.; Zheng, S.C.; Liu, L.; Huang, L.H.; Feng, Q.L. De novo characterization of transcriptome and gene expression dynamics in epidermis during the larval-pupal metamorphosis of common cutworm. Insect. Biochem. Mol. Biol. 2013, 43, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.H.; Hou, Q.L.; Dou, W.; Yang, P.J.; Wang, J.J. Expression profiles of tyrosine metabolic pathway genes and functional analysis of DOPA decarboxylase in puparium tanning of Bactrocera dorsalis (Hendel). Pest Manag. Sci. 2022, 78, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.H.; Hou, Q.L.; Wei, D.D.; Dou, W.; Liu, Z.; Yang, P.J.; Smagghe, G.; Wang, J.J. Tyrosine hydroxylase coordinates larval-pupal tanning and immunity in oriental fruit fly (Bactrocera dorsalis). Pest Manag. Sci. 2018, 74, 569–578. [Google Scholar] [CrossRef]
- Chen, E.H.; Hou, Q.L.; Dou, W.; Wei, D.D.; Yue, Y.; Yang, R.L.; Yang, P.J.; Yu, S.F.; De Schutter, K.; Smagghe, G.; et al. Genome-wide annotation of cuticular proteins in the oriental fruit fly (Bactrocera dorsalis), changes during pupariation and expression analysis of CPAP3 protein genes in response to environmental stresses. Insect Biochem. Mol. Biol. 2018, 97, 53–70. [Google Scholar] [CrossRef]
- Alotaibi, F.; Alkuriji, M.; AlReshaidan, S.; Alajmi, R.; Metwally, D.M.; Almutairi, B.; Alorf, M.; Haddadi, R.; Ahmed, A. Body Size and Cuticular Hydrocarbons as Larval Age Indicators in the Forensic Blow Fly, Chrysomya albiceps (Diptera: Calliphoridae). J. Med. Entomol. 2021, 58, 1048–1055. [Google Scholar] [CrossRef]
- Sharma, A.; Drijfhout, F.P.; Tomberlin, J.K.; Bala, M. Cuticular hydrocarbons as a tool for determining the age of Chrysomya rufifacies (Diptera: Calliphoridae) larvae. J. Forensic. Sci. 2021, 66, 236–244. [Google Scholar] [CrossRef]
- Moore, H.E.; Pechal, J.L.; Benbow, M.E.; Drijfhout, F.P. The potential use of cuticular hydrocarbons and multivariate analysis to age empty puparial cases of Calliphora vicina and Lucilia sericata. Sci. Rep. 2017, 7, 1933. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef]
- Sterkel, M.; Ons, S.; Oliveira, P.L. DOPA decarboxylase is essential for cuticle tanning in Rhodnius prolixus (Hemiptera: Reduviidae), affecting ecdysis, survival and reproduction. Insect Biochem. Mol. Biol. 2019, 108, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, L.; Ren, L.; Shang, Y.; Wang, S.; Guo, Y. Impact of Constant Versus Fluctuating Temperatures on the Development and Life History Parameters of Aldrichina grahami (Diptera: Calliphoridae). Insects 2019, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, I.; McCalla, K.A.; Ratkowsky, D.A.; Hoddle, M.S. Effects of Constant and Fluctuating Temperatures on Development Rates and Longevity of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- McCalla, K.A.; Kececi, M.; Milosavljevic, I.; Ratkowsky, D.A.; Hoddle, M.S. The Influence of Temperature Variation on Life History Parameters and Thermal Performance Curves of Tamarixia radiata (Hymenoptera: Eulophidae), a Parasitoid of the Asian Citrus Psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef]
- Niederegger, S.; Pastuschek, J.; Mall, G. Preliminary studies of the influence of fluctuating temperatures on the development of various forensically relevant flies. Forensic. Sci. Int. 2010, 199, 72–78. [Google Scholar] [CrossRef]


| Baseline Points (cm−1) | Wavenumber (cm−1) | Infrared Band |
|---|---|---|
| 1760~1730 | 1741 | Lipid (C = O stretching vibration) |
| 1680~1610 | 1647 | Amide I (C = O stretching) |
| 1580~1510 | 1540 | Amide II (N-H bending coupled to C-N stretching) |
| 1480~1420 | 1454 | C-H bending from CH2 and CH3 |
| 1420~1350 | 1398 | C=O vibrations of COO− from free fatty acids, free amino acids, and polypeptides |
| 1330–1277 | 1313 | Amide III |
| 1180~1100 | 1145 | C-O(H) stretching vibration |
| 1100~1000 | 1040 | C-O(H) or C-C vibrations and PO2- symmetric stretching |
| OPLS-DA | Permutation Test | |||||
|---|---|---|---|---|---|---|
| Temperatures | R2X (cum) | R2Y (cum) | Q2 (cum) | R2 | Q2 | |
| CHCs | 20 °C | 0.965 | 0.946 | 0.863 | 0.272 | −0.681 |
| 25 °C | 0.971 | 0.972 | 0.913 | 0.285 | −0.488 | |
| 30 °C | 0.928 | 0.982 | 0.957 | 0.308 | −0.83 | |
| ATR-FTIR | 20 °C | 1 | 0.97 | 0.964 | 0.0971 | −0.29 |
| 25 °C | 1 | 0.978 | 0.974 | 0.0545 | −0.229 | |
| 30 °C | 1 | 0.899 | 0.953 | 0.0805 | −0.275 | |
| PLS | Permutation Test | |||||
|---|---|---|---|---|---|---|
| Temperatures | Equation | R2 | RMSECV | R2 | Q2 | |
| CHCs | 20 °C | y = x − 1.316 × 10−7 | 0.9618 | 1.067 | 0.258 | −0.417 |
| 25 °C | y = x − 3.242 × 10−7 | 0.981 | 0.702 | 0.259 | −0.745 | |
| 30 °C | y = x − 2.37 × 10−7 | 0.9809 | 0.589 | 0.344 | −0.455 | |
| ATR-FTIR | 20 °C | y = x + 2.923 × 10−7 | 0.927 | 1.268 | 0.00486 | −0.267 |
| 25 °C | y = x + 1.74 × 10−8 | 0.988 | 0.364 | 0.0197 | −0.418 | |
| 30 °C | y = x + 8.175 × 10−8 | 0.9957 | 0.163 | 0.0353 | −0.344 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, Y.; Feng, Y.; Ren, L.; Zhang, X.; Yang, F.; Zhang, C.; Guo, Y. Pupal Age Estimation of Sarcophaga peregrina (Diptera: Sarcophagidae) at Different Constant Temperatures Utilizing ATR-FTIR Spectroscopy and Cuticular Hydrocarbons. Insects 2023, 14, 143. https://doi.org/10.3390/insects14020143
Shang Y, Feng Y, Ren L, Zhang X, Yang F, Zhang C, Guo Y. Pupal Age Estimation of Sarcophaga peregrina (Diptera: Sarcophagidae) at Different Constant Temperatures Utilizing ATR-FTIR Spectroscopy and Cuticular Hydrocarbons. Insects. 2023; 14(2):143. https://doi.org/10.3390/insects14020143
Chicago/Turabian StyleShang, Yanjie, Yakai Feng, Lipin Ren, Xiangyan Zhang, Fengqin Yang, Changquan Zhang, and Yadong Guo. 2023. "Pupal Age Estimation of Sarcophaga peregrina (Diptera: Sarcophagidae) at Different Constant Temperatures Utilizing ATR-FTIR Spectroscopy and Cuticular Hydrocarbons" Insects 14, no. 2: 143. https://doi.org/10.3390/insects14020143
APA StyleShang, Y., Feng, Y., Ren, L., Zhang, X., Yang, F., Zhang, C., & Guo, Y. (2023). Pupal Age Estimation of Sarcophaga peregrina (Diptera: Sarcophagidae) at Different Constant Temperatures Utilizing ATR-FTIR Spectroscopy and Cuticular Hydrocarbons. Insects, 14(2), 143. https://doi.org/10.3390/insects14020143







