Simple Summary
The bright red color of Erythraeid mites is conspicuous. The Erythraeid larvae are usually ectoparasitic on arthropods and easily observed. Both the genera Charletonia and Leptus are distributed worldwide. Charletonia has 86 species and Leptus has more than 240 species based on larvae, respectively. To date, two species of the genus Charletonia and 11 species of the genus Leptus have been reported from China. Here, four new species, Charletonia rectangia Xu and Jin sp. nov. collected from tropical rainforests in the Guangxi Province and Yunnan Province, Leptus (Leptus) bomiensis Xu and Jin sp. nov. from the Tibet Autonomous Region, where the altitude ranges from 2673 to 3374 m, Leptus (Leptus) longisolenidionus Xu and Jin sp. nov. from jungles in the Hainan Province (Hainan Island), and Leptus (Leptus) striatus Xu and Jin sp. nov. from Xishuangbanna tropical rainforests in the Yunnan Province. We believe that this study will contribute to further research on the taxonomy and phylogeny of the family.
Abstract
Four new species, Charletonia rectangia Xu and Jin sp. nov., Leptus (Leptus) bomiensis Xu and Jin sp. nov., Leptus (Leptus) longisolenidionus Xu and Jin sp. nov., and Leptus (Leptus) striatus Xu and Jin sp. nov. are described and illustrated based on larvae. All four new species are from biodiversity hotspots, L. (L.) bomiensis sp. nov. from the Eastern Himalayas biodiversity hotspot, while the other three species from the Indo–Burma biodiversity hotspot.
1. Introduction
The genus Charletonia Oudemans, 1910 is globally distributed and includes 86 species based on larvae [1]. The hosts of Charletonia larvae were recorded in three animal classes (Arachnida, Insecta, and Mammalia), 16 orders, and 45 families [2,3,4,5,6,7,8,9,10,11,12]. The most common hosts are arthropods, especially those of Araneae (Arachnida), Coleoptera (Insecta), Hemiptera (Insecta), Lepidoptera (Insecta), and Orthoptera (Insecta).
Nowadays, only two species of Charletonia are reported in China: C. banksi Southcott, 1966 (synonym C. hunanensis Zheng, 1996) based on the larval stage [1,13] and C. taiwanensis Tsai and Chow, 1988 based on the larvae and active post-larval instars [14]. The hosts of C. banksi are the dragonflies (Libellulidae) and damselflies (Megapodagriidae) in China [13], and in Australia, are orthopteran insects in Acrididae, Hepalieus gracilis, Goniaea vocans, Nomaducris guttulosa and Oedaleus australis [6,11]. The host of C. taiwanensis is also a species of Acrididae, Chondracris rosea [14].
The genus Leptus Latreille, 1796 was reported on all continents except for Antarctica [15], with more than 240 described species from the larval stage [15,16,17,18,19,20,21,22]. The hosts of this genus were reported from 101 families belonging to six classes (Insecta, Arachnida, Diplopoda, Entognatha, Aves, and Mammalia) and 28 Orders [2,3,4,18,23,24,25,26,27,28,29,30,31,32,33,34,35]. However, the common hosts of its larvae are arthropods, especially Araneae, Coleoptera, Diptera, Hemiptera, Lepidoptera, Opiliones, and Orthoptera [2,16].
To date, a total of 11 species of the genus Leptus have been reported based on larvae from China, of which only three species have host records and all the hosts are insects [13,36,37,38,39,40].
In this paper, Charletonia rectangia Xu and Jin sp. nov., Leptus (Leptus) bomiensis Xu and Jin sp. nov., Leptus (Leptus) longisolenidionus Xu and Jin sp. nov., and Leptus (Leptus) striatus Xu and Jin sp. nov. are described and illustrated based on larvae from China. All the specimens of the four new species were collected from biological hotspots [41,42,43].
2. Materials and Methods
Moths were collected by a light trip, harvestmen were captured on leaves or branches late at night, and other insects were collected using an insect net in the field and subsequently preserved in small vials containing 75% ethanol. Erythraeid larval specimens on insects or harvestmen were detached by a fine brush under a stereomicroscope. Then, the larval specimens were prepared with Oudemans’ fluid and mounted in Hoyer’s medium. Figures were drawn with the aid of a drawing tube attached to a Nikon Eclipse Ni-E microscope. The distribution map was prepared with Arcmap 10.3. The terminology and abbreviations are adapted from Bassini-Silva et al. [18,44], Jacinavicius et al. [45], Haitlinger and Saboori [46], Šundić et al. [47] and Xu et al. [48]. Measurements are expressed in micrometers (μm). The SD, standard deviation, keeps two decimal fractions.
3. Results
3.1. New Species
Charletonia Oudemans, 1910

Figure 1.
Charletonia rectangia sp. nov., larva. (A). Dorsal view of idiosoma. (B). Ventral view of idiosoma.
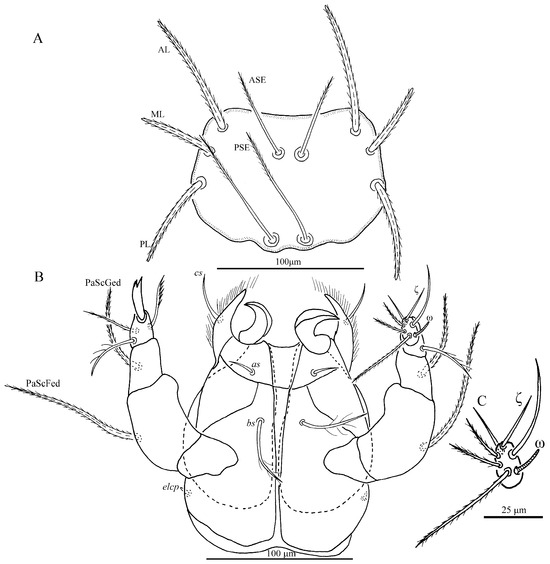
Figure 2.
Charletonia rectangia sp. nov., larva. (A). Scutum. (B). Ventral view of gnathosoma. (C). Ventral view of palptarsus.
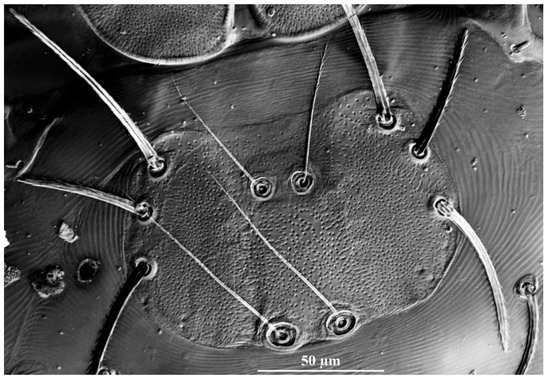
Figure 3.
Charletonia rectangia sp. nov., larva. Showing ASE and PSE shape and outline of scutum.
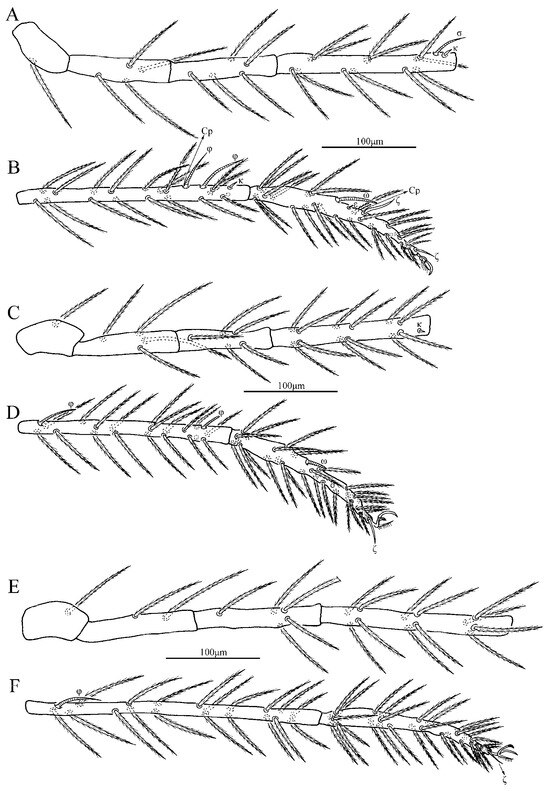
Figure 4.
Charletonia rectangia sp. nov., larva. (A). Leg I, trochanter—genu. (B). Leg I, tibia—tarsus. (C). Leg II, trochanter—genu. (D). Leg II, tibia—tarsus. (E). Leg III, trochanter—genu. (F). Leg III, tibia—tarsus.
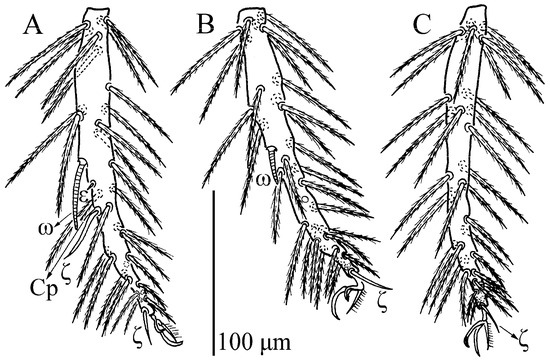
Figure 5.
Charletonia rectangia sp. nov., larva. (A). Leg I, tarsus. (B). Leg II, tarsus. (C). Leg III, tarsus.
Diagnosis (larva). Four setae between coxae II and III; gnathosoma with two hypostomalae; solenidion on Ge I distal to most distal normal setae; ASE posterior to ML bases and very close to ML bases, ASE and PSE with fine barbs on distal halves; Ti I 241–265; Ti III 305–331.
Description. Dorsum. Idiosoma with 54 (fD = 54–58 in paratypes) barbed setae, a pair of setae located between scutum and eyes at level with PSE bases (Figure 1A). Scutum about rectangular outline with rounded angles, anterior margin slightly concave, lateral margins almost straight, posterior margin wavy near the base of PSE (Figure 1A, Figure 2A and Figure 3). Scutum with three pairs of normal setae (AL, ML and PL), and two pairs of sensilla (ASE and PSE), AL, ML and PL are completely barbed, ASE and PSE with barbs in distal half. PSE is much longer than ASE, AL > PL > ML (Table 1). ASE bases are posterior but very close to the level of ML bases, PSE near the posterior margin of scutum.

Table 1.
Measurements of Charletonia rectangia sp. nov. (larvae, a–g = paratypes).
Venter (Figure 1B). All ventral setae, including coxal fields, are barbed and acute (Figure 1B). Five pairs of intercoxal setae (paired intercoxal setae 1a, 2a and 3a, and two pairs of unnamed setae between II and III), 1a posterior to level of the posterior edge of coxae I, 2a at a line with anterior edges of coxae II, 3a between coxae III; 25 setae behind coxae III (fV = 24–26 in paratypes). Intercoxal setae II (2a) is slightly longer than intercoxal setae III (3a), 2a and 3a both longer than 1a. Five pairs of coxalae (1b, 2b1, 2b2, 3b1 and 3b2), 1b longest, 2b1 longer than 3b1, 2b2 and 3b2 subequal, 2b1 longer than 2b2, 3b1 longer than 3b2 (Table 1).
Gnathosoma (Figure 2B). With two nude galealae (cs), two nude anterior hypostomalae (as) and two subcapitular setae (bs) with few setules; bs longer than cs and much longer than as (Table 1). Hypostomal lip with fimbriated. Palpfemur and palpgenu each with one barbed, pointed dorsal seta (PaScFed and PaScGed). Palptibia with one seta on ventral surface, this seta with few setules; palptibia with one barbed dorsal seta, and one brush-like dorsal seta; odontus bifid. Palptarsus with seven setae, of which, three barbed, two nude, one solenidion (ω) and one eupathidium (ζ). fPp = 0-B-B-3B2-3B2Nωζ. Palpal supracoxal seta (elcp) peg-like.
Legs (Figure 4 and Figure 5). With seven segments (femora divided). IP = 2901–3081 (Holotyp and seven paratypes). Dorsum of coxa I with a supracoxal seta (eI) which is peg-like with a rounded tip. Anterior claw hook-like, posterior claw pulvilliform with few ciliations, and empodium claw-like or falciform. Normal setae on legs barbed and pointed. Leg setal formula: leg I: Cx—1n; Tr—1n; Bfe—4n; Tfe—5n; Ge—1σ, 1κ, 12n; Ti—2φ, 1κ, 1Cp, 18n; Ta—1ω, 1ε, 2ζ, 29n (27n in the paratype numbered g, both sides). leg II: Cx—2n; Tr—1n; Bfe—4n; Tfe—5n; Ge—1κ, 12n; Ti—2φ, 19n; Ta—1ω, 1ζ, 30n. leg III: Cx—2n; Tr—1n; Bfe—2n; Tfe—5n; Ge—12n; Ti—1φ, 19n; Ta—1ζ, 30n. The lengths of the legs were measured from coxae to tarsus (Table 1).
Etymology. The new species is named after the rectangular-like shape of the scutum.
Types. Holotype, larva, an unknown long-horned grasshopper of Tettigoniidae (Orthoptera), collected by Si-yuan Xu on 10 May 2016, from Xishuangbanna National Natural Reserve (Altitude: 672 m), Yunnan Province, China. Paratypes: two larvae, the same data as the holotype; one larva, an unidentified mantis (Mantodea), collected by Si-yuan Xu on 7 May 2016, from Xishuangbanna National Natural Reserve (Altitude: 627 m); one larva, an unknown grasshopper of Acrididae (Orthoptera), collected by Si-yuan Xu on 7 May 2016, from Xishuangbanna National Natural Reserve (Altitude: 627 m); one larva, an unidentified moth (Lepidoptera), collected by Si-yuan Xu on 24 April 2018, from Xishuangbanna National Natural Reserve (Altitude: 633 m); one larva, an unidentified stick (Phasmatodea), collected by Si-yuan Xu on 28 April 2018, from Xishuangbanna National Natural Reserve (Altitude: 732 m); one larva, an unknown beetle of Chrysomelidae (Coleoptera), collected by Yan Jiang on 18 April 2019, from Nonggang National Natural Reserve (Altitude: 293 m), Guangxi Province, China.
The holotype and paratypes are deposited in the Institute of Entomology, Guizhou University, Guiyang, China (GUGC).
Distribution. China: Guangxi and Yunnan Province.
Remarks. According to the keys by Hakimitabar and Saboori [1], C. rectangia sp. nov. falls into the brunni species group (Four setae between coxae II and III), and buforania species subgroup (Sigma (σ) on Ge I placed in the distal half of the segment after the most distal normal seta, ASE posterior to or the same level with ML). This subgroup includes nine species, of which, C. alvedae Haitlinger, 2000 was from Peru [49]; C. buforania (Womersley, 1934) [5] and C. striaticeps Southcott, 1991 [6] from Australia; C. lankensis Southcott, 1988 from India and Sri Lanka [50,51]; C. rajmundi Haitlinger, 2007 from South Africa [52]; C. stekolnikovi Hakimitabar and Saboori, 2011 [53] and C. terianae Hakimitabar, Saboori and Seiedy, 2013 [54] from Iran; C. villingensis Haitlinger, 2007 from Maldives [51]; C. womersleyi Southcott, 1966 from Belgium and Great Britain [5].
Charletonia rectangia sp. nov. differs from C. alvedae by the longer Ti I (241–265 vs. 192), Ti II (219–231 vs. 178), Ti III (305–331 vs. 220), Ge I (175–199 vs. 146), Ge II (152–168 vs. 140), Ge III (183–203 vs. 140), 1b (115–119 vs. 76).
Charletonia rectangia sp. nov. differs from C. buforania by the longer Ti I (241–265 vs. 155), Ti III (305–331 vs. 192), Ge I (175–199 vs. 125), Ge III (183–203 vs. 121), W (121–141 vs. 86–95), AL (80–96 vs. 34–42), PL (64–79 vs. 32–42).
Charletonia rectangia sp. nov. differs from C. lankensis by the longer Ti I (241–265 vs. 98–110), Ti II (219–231 vs. 82–92), Ti III (305–331 vs. 122–132), Ge I (175–199 vs. 84–92), Ge II (152–168 vs. 68–80), Ge III (183–203 vs. 80–92), W (121–141 vs. 92–108), AL (80–96 vs. 40–58), PL (64–79 vs. 42–52), ASE (60–71 vs. 40–49), 1b (115–119 vs. 50–64).
Charletonia rectangia sp. nov. differs from C. rajmundi as follows: posterior half of scutum in C. rectangia sp. nov. polygonal with wavy edges, while semicircular in C. rajmundi; shape of ASE and PSE (barbed about distal half vs. nude), the number of setae in fD formula (54–58 vs. 148–152); the shorter Ti I (241–265 vs. 284–304) and Ti III (305–331 vs. 346–364), IP (2901–3081 vs. 3158–3444).
Charletonia rectangia sp. nov. differs from C. stekolnikovi by the longer Ti I (241–265 vs. 134–136), Ti II (219–231 vs. 129), Ti III (305–331 vs. 176), W (121–141 vs. 90–91), AL (80–96 vs. 48–50), PL (64–79 vs. 54–55), and 1b (115–119 vs. 77–79).
Charletonia rectangia sp. nov. differs from C. striaticeps as follows: the dorsal of cheliceral bases with striations (no vs. yes), scutum with a prominent reticular pattern (no vs. yes); the longer Ti I (241–265 vs. 120–169), Ti II (219–231 vs. 111–144), and Ti III (305–331 vs. 162–222).
Charletonia rectangia sp. nov. differs from C. terianae by the number of setae in fD formula (54–58 vs. 78), the longer Ti I (241–265 vs. 101–119), Ti II (219–231 vs. 94–109), Ti III (305–331 vs. 129–153), L (95–109 vs. 69–87), W (121–141 vs. 79–94), AL (80–96 vs. 45–54), and 1b (115–119 vs. 67–87).
Charletonia rectangia sp. nov. differs from C. villingensis by the longer Ti I (241–265 vs. 116), Ti II (219–231 vs. 100), Ti III (305–331 vs. 144), IP (2901–3081 vs. 1694), W (121–141 vs. 98), AL (80–96 vs. 52), ASE (60–71 vs. 32), and 1b (115–119 vs. 72).
Charletonia rectangia sp. nov. differs from C. womersleyi by the longer Ti I (241–265 vs. 110), Ti III (305–331 vs. 140), Ta I (181–198 vs. 98), Ta III (180–196 vs. 104), W (121–141 vs. 107), AW (84–91 vs. 66), PW (115–122 vs. 92).
Leptus Latreille, 1796
Leptus (Leptus) bomiensis Xu and Jin sp. nov. (Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10)
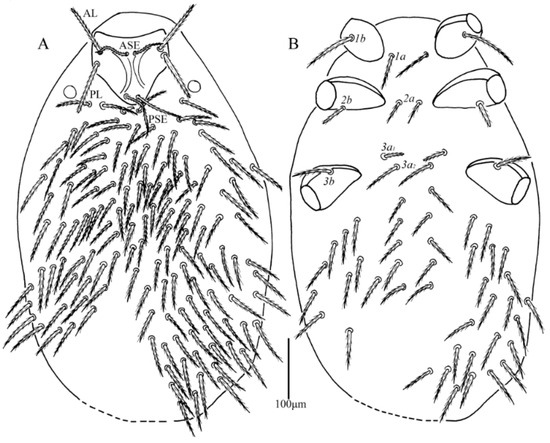
Figure 6.
Leptus (Leptus) bomiensis sp. nov., larva. (A). Dorsal view of idiosoma. (B). Ventral view of idiosoma.
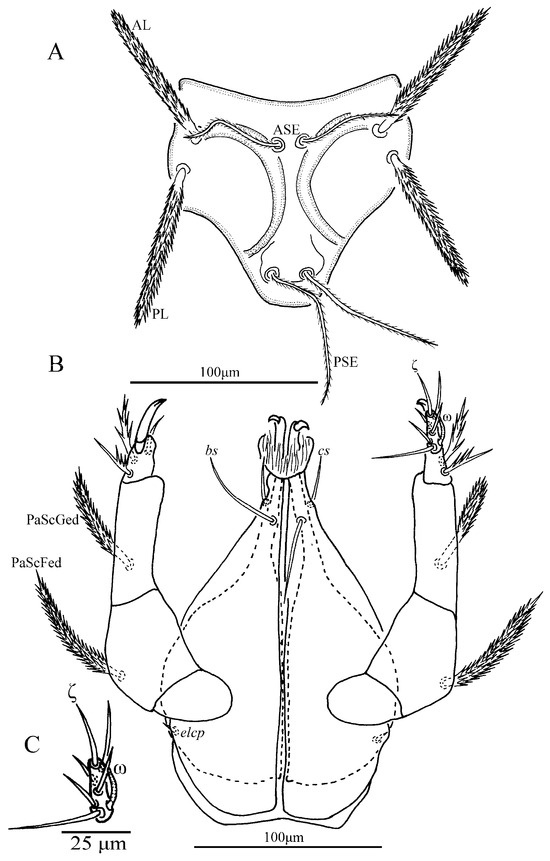
Figure 7.
Leptus (Leptus) bomiensis sp. nov., larva. (A). Scutum. (B). Ventral view of gnathosoma. (C). Ventral view of palptarsus.
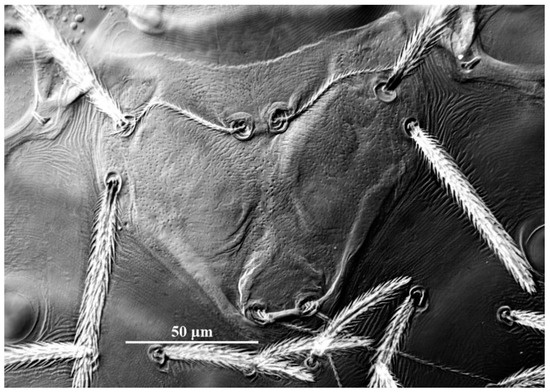
Figure 8.
Leptus (Leptus) bomiensis sp. nov., larva. Showing ASE and PSE shape and outline of scutum.
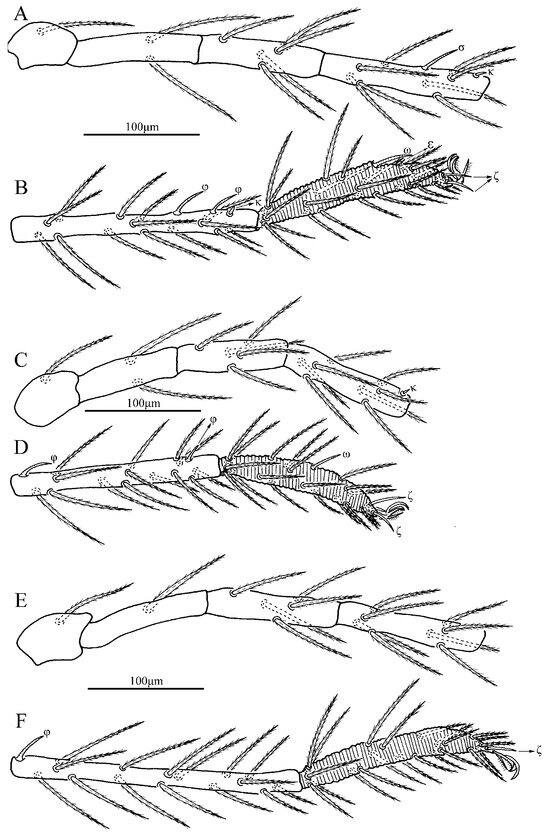
Figure 9.
Leptus (Leptus) bomiensis sp. nov., larva. (A). Leg I, trochanter—genu. (B). Leg I, tibia—tarsus. (C). Leg II, trochanter—genu. (D). Leg II, tibia—tarsus. (E). Leg III, trochanter—genu. (F). Leg III, tibia—tarsus.
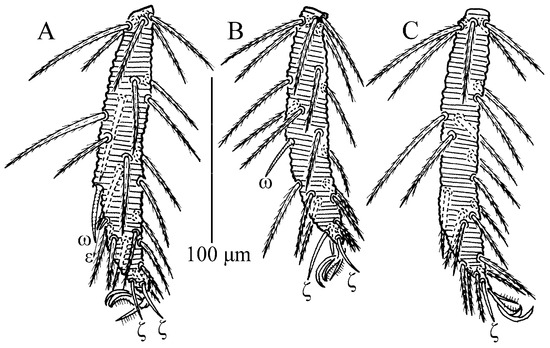
Figure 10.
Leptus (Leptus) bomiensis sp. nov., larva. (A). Leg I, tarsus. (B). Leg II, tarsus. (C). Leg III, tarsus.
Diagnosis (larva). ASE and PSE with fine barbs throughout the length; gnathosoma with two hypostomalae; palpfemur and palpgenu each with one barbed seta on the dorsal surface (PaScFed and PaScGed); fD = 148–150; Ti I 206–212; Ti III 251–264.
Description. Dorsum. Idiosoma is almost oval (the holotype was used for drawing and its posterior cuticle was broken during slide preparation), with 148 barbed setae (fD = 148–150 in paratypes) (Figure 6A). Scutum length slightly shorter than width, with two pairs of sensilla (ASE and PSE), and two pairs of scutalae (AL and PL); anterior margin concave, anterolateral margins slightly cambered, posterolateral margins sinuous, posterior margin somewhat convex (Figure 7A and Figure 8). ASE bases are slightly posterior to the level of AL bases, PSE near the posterior margin of the scutum. ASE, PSE, AL and PL are all entirely barbed, and PSE much longer than ASE, AL and PL subequal (Table 2).

Table 2.
Measurements of Leptus (Leptus) bomiensis sp. nov. (larvae, a and b = paratypes).
Venter. All ventral setae, including coxalae, barbed and acute (Figure 6B). Two intercoxal setae present between coxae I (1a) and between coxae II (2a), respectively. Four intercoxal setae (3a1 and 3a2) located between coxae II and III, 3a1 distinctly shorter than 3a2, 1a longer than 2a, and 2a subequal to 3a2 (Table 2). Three pairs of coxalae (1b, 2b and 3b) present, 1b much longer than 2b and 3b, 3b longer than 2b.
Gnathosoma (Figure 7B). With two nude galealae (cs), two nude hypostomalae (bs), bs longer than cs. Palpfemur and palpgenu with one barbed seta on the dorsal surface (PaScFed and PaScGed), respectively. Palptibia with two barbed setae and one nude seta, odontus simple. Palptarsus with seven setae, of which, five nude setae, one solenidion (ω) and one eupathidium (ζ). fPp = 0-B-B-2BN-5Nωζ. Palpal supracoxal seta (elcp) peg-like.
Legs (Figure 9 and Figure 10). With seven segments (femora divided). IP = 2548–2560 (Holotyp and two paratypes). Dorsum of coxa I with a supracoxal seta (eI) which is peg-like and apically rounded. Leg setal formula: leg I: Cx—1n; Tr—1n; Bfe—2n; Tfe—5n; Ge—1σ, 1κ, 8n; Ti—2φ, 1κ, 14n; Ta—1ω, 1ε, 2ζ, 23n. leg II: Cx—1n; Tr—1n; Bfe—2n; Tfe—5n; Ge—1κ, 8n; Ti—2φ, 14n; Ta—1ω, 2ζ, 20n. leg III: Cx—1n; Tr—1n; Bfe—1n; Tfe—5n; Ge—8n; Ti—1φ, 15n; Ta—1ζ, 22n. The lengths of the legs were measured from coxae to tarsus (Table 2).
Etymology. The name of the new species is derived from Bomi where it was collected.
Types. Holotype, larva, from an unidentified moth (Lepidoptera), collected by Si-yuan Xu on 17 July 2019, from Guxiang Town (Altitude: 2673 m), Bomi County, Tibet Autonomous Region, China. Paratypes: one larva, an unidentified Elateridae (Coleoptera), collected by Si-yuan Xu on 18 July 2019, from Bomi County (Altitude: 3084 m), Tibet Autonomous Region, China; one larva, an unidentified Pentatomidae (Hemiptera), collected by Si-yuan Xu on 20 July 2019, from Bomi County (Altitude: 3374 m), Tibet Autonomous Region, China.
The holotype and paratypes are deposited in the Institute of Entomology, Guizhou University, Guiyang, China (GUGC).
Distribution. China: Tibet Autonomous Region.
Remarks. Leptus (Leptus) bomiensis sp. nov. keyed to the phalangii species group and killingtoni species subgroup proposed by Saboori et al. [16]. The killingtoni species subgroup consists of 12 species, of which, L. (L.) albertensis Southcott, 1992 was from Canada [31]; L. (L.) brachypodos Zheng, 1996, L. (L.) dolichopodos Zheng, 1996, L. (L.) shimenensis Zheng, 1996 and L. (L.) sulciscutus Zheng, 1996 from China [36]; L. (L.) cavernicola Fain and Elsen, 1987 from Rwanda [30]; L. (L.) droozi Southcott, 1992 from the United States of America [31]; L. (L.) grossi Southcott, 1999 from Australia [29]; L. (L.) killingtoni Turk, 1945 from Portugal (Azores Islands), Spain and the United Kingdom [31]; L. (L.) scutellatus Southcott, 1999 from Papua New Guinea [29]; L. (L.) singhi Saboori and Arbabi, 2003 from India [55]; L. (L.) ubudicus Haitlinger, 2006 from Indonesia (Lesser Sunda Islands) [56].
Saboori et al. [16] placed L. (L.) dolichopodos in the phalangii species group and killingtoni species subgroup. However, the original description there were two solenidia (σ) on Ge I (Seventh line of page 239 and Figures 27–32) [36] suggesting it should be grouped into the torresianus species subgroup.
In this paper, we still compare the new species with L. (L.) dolichopodos.
Leptus (L.) bomiensis sp. nov. differs from L. (L.) albertensis by the number of setae in fD formula (148–150 vs. 103–105); the shape of 3a1 (pointed end vs. bifid); longer L (112–123 vs. 102), W (129–135 vs. 105), PW (114–122 vs. 97), PSE (87–94 vs. 68), Ti I (206–212 vs. 187), and Ti III (251–264 vs. 231).
Leptus (L.) bomiensis sp. nov. differs from L. (L.) brachypodos by the number of setae in fD formula (148–150 vs. 54); longer L (112–123 vs. 98), W (129–135 vs. 111), AL (84–87 vs. 52), PL (84–89 vs. 66), Ti I (206–212 vs. 135), Ti II (176–190 vs. 123), and Ti III (251–264 vs. 162).
Leptus (L.) bomiensis sp. nov. differs from L. (L.) cavernicola by the number of setae in fD formula (148–150 vs. 50); longer L (112–123 vs. 84), W (129–135 vs. 78), AL (84–87 vs. 45), PL (84–89 vs. 55–60), PSE (87–94 vs. 58), Ti I (206–212 vs. 102), Ti III (251–264 vs. 142), and Ta I (168–174 vs. 95).
Leptus (L.) bomiensis sp. nov. differs from L. (L.) dolichopodos by the number of setae in fD formula (148–150 vs. 68), the number of solenidia on Ge I (1 vs. 2); the shorter Ti I (206–212 vs. 250) and Ti III (251–264 vs. 320).
Leptus (L.) bomiensis sp. nov. differs from L. (L.) droozi by the number of setae in fD formula (148–150 vs. 79); the longer L (112–123 vs. 87), W (129–135 vs. 87), Ti I (206–212 vs. 176), Ti II (176–190 vs. 151), Ti III (251–264 vs. 205), Leg I (860–868 vs. 730), and Leg III (922–934 vs. 740).
Leptus (L.) bomiensis sp. nov. differs from L. (L.) grossi by the longer Ti I (206–212 vs. 127), Ti II (176–190 vs. 111), Ti III (251–264 vs. 145), Leg I (860–868 vs. 550), Leg II (758–763 vs. 520), Leg III (922–934 vs. 620), L (112–123 vs. 86), W (129–135 vs. 95), AL (84–87 vs. 62), PL (84–89 vs. 64).
Leptus (L.) bomiensis sp. nov. differs from L. (L.) killingtoni by the longer Ti I (206–212 vs. 134–160), Ti II (176–190 vs. 110–136), Ti III (251–264 vs. 158–182), Ta I (168–174 vs. 112–132), Ta II (141–151 vs. 104–124), Ta III (170–180 vs. 110–129), Leg I (860–868 vs. 604–682), Leg II (758–763 vs. 554–650), Leg III (922–934 vs. 642–735), L (112–123 vs. 80–104), W (129–135 vs. 96–114).
Leptus (L.) bomiensis sp. nov. differs from L. (L.) scutellatus as follows: the dorsal of the cheliceral bases with longitudinal striations (no vs. yes); gnathosoma venter with fine transverse striations (no vs. yes); the number of setae in fD formula (148–150 vs. 46); longer Ti I (206–212 vs. 119), Ti III (251–264 vs. 140), L (112–123 vs. 69), W (129–135 vs. 76), ASE (51–54 vs. 29), and PSE (87–94 vs. 48).
Leptus (L.) bomiensis sp. nov. differs from L. (L.) shimenensis by the number of setae in fD formula (148–150 vs. 54) and fV (42–44 vs. 20); longer L (112–123 vs. 85), W (129–135 vs. 82), AW (99–103 vs. 62), PW (114–122 vs. 75), AL (84–87 vs. 68), and PL (84–89 vs. 65).
Leptus (L.) bomiensis sp. nov. differs from L. (L.) singhi by the number of setae in fD formula (148–150 vs. 60); longer Ti I (206–212 vs. 153), Ti II (176–190 VS. 138), Ti III (251–264 vs. 187), L (112–123 vs. 78), W (129–135 vs. 87), ASE (51–54 vs. 36), PSE (87–94 vs. 51), AL (84–87 vs. 56), and PL (84–89 vs. 68).
Leptus (L.) bomiensis sp. nov. differs from L. (L.) sulciscutus by the number of setae in fD formula (148–150 vs. 59) and fV (42–44 vs. 23); microseta on Ge II (present vs. absent); longer L (112–123 vs. 85), W (129–135 vs. 95), Leg I (860–868 vs. 800), and Leg III (922–934 vs. 840).
Leptus (L.) bomiensis sp. nov. differs from L. (L.) ubudicus by the one half of gnathosoma ventral surface with transverse striations (no vs. yes); the number of setae in fD formula (148–150 vs. 52), fV (42–44 vs. 16); longer Ti I (206–212 vs. 70), Ti II (176–190 vs. 64), and Ti III (251–264 vs. 92).
Leptus (Leptus) longisolenidionus Xu and Jin sp. nov. (Figure 11, Figure 12, Figure 13, Figure 14, Figure 15 and Figure 16)

Figure 11.
Leptus (Leptus) longisolenidionus sp. nov., larva. (A). Dorsal view of idiosoma. (B). Ventral view of idiosoma.
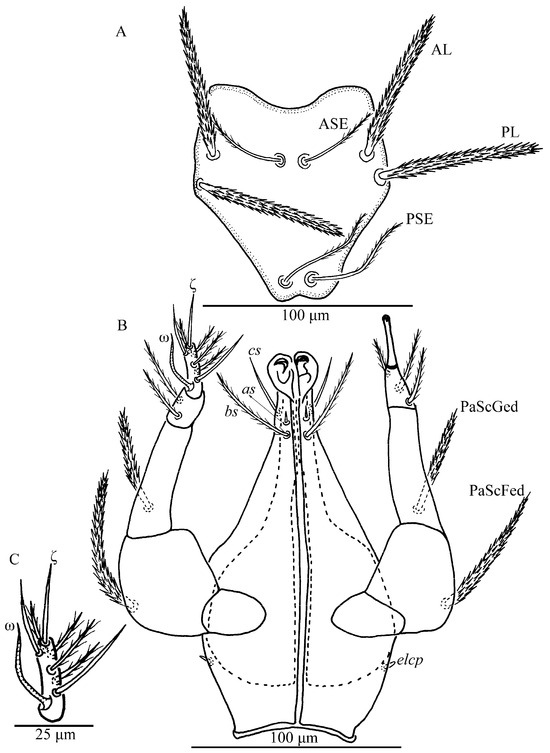
Figure 12.
Leptus (Leptus) longisolenidionus sp. nov., larva. (A). Scutum. (B). Ventral view of gnathosoma. (C). Ventral view of palptarsus.

Figure 13.
Leptus (Leptus) longisolenidionus sp. nov., larva. Showing ASE and PSE shape and outline of scutum.
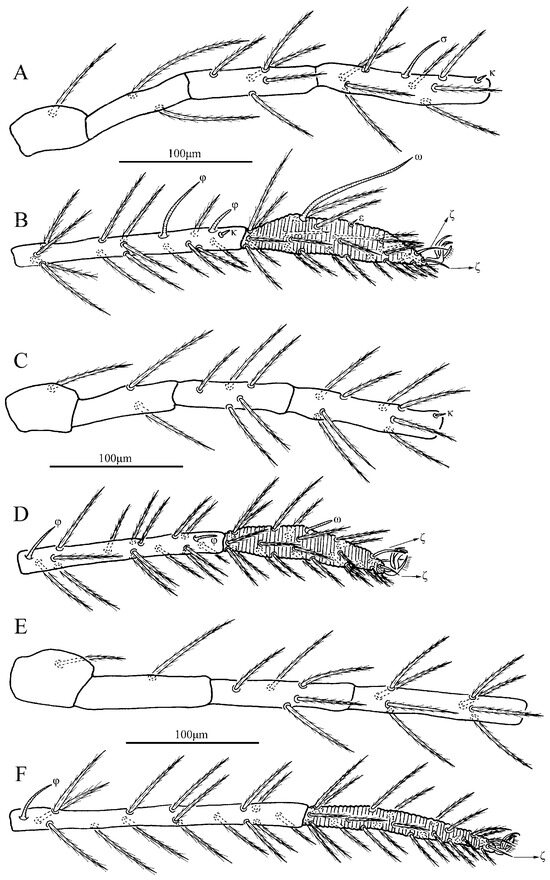
Figure 14.
Leptus (Leptus) longisolenidionus sp. nov., larva. (A). Leg I, trochanter—genu. (B). Leg I, tibia—tarsus. (C). Leg II, trochanter—genu. (D). Leg II, tibia—tarsus. (E). Leg III, trochanter—genu. (F). Leg III, tibia—tarsus.
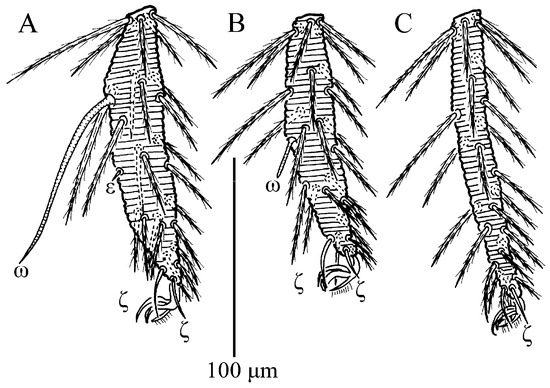
Figure 15.
Leptus (Leptus) longisolenidionus sp. nov., larva. (A). Leg I, tarsus. (B). Leg II, tarsus. (C). Leg III, tarsus.
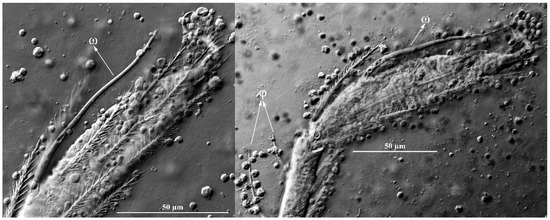
Figure 16.
Leptus (Leptus) longisolenidionus sp. nov., larva. Showing ωI on TaI.
Diagnosis (larva). All normal setae of Ta I shorter than ωI, ωI > 90; ASE and PSE with fine barbs on distal halves; ASE posterior to AL; gnathosoma with four hypostomalae (as and bs); palpfemur and palpgenu each with one barbed seta on the dorsal surface (PaScFed and PaScGed); fD = 64–68; Ti I 163–171; Ti III 209–219; Ti III/AW 2.73–3.03.
Description. Dorsum. Idiosoma with 64 barbed setae (fD = 64–68 in paratypes) (Figure 11A). Scutum with two pairs of sensilla (ASE and PSE), and two pairs of scutalae (AL and PL), anterior margin concave in middle, anterolateral margins almost straight, posterolateral margins slightly concave, posterior margin concave between the bases of PSE (Figure 12A and Figure 13). ASE posterior to the level of AL, PSE near posterior margin of scutum; approximately distal half of ASE and PSE with fine barbs (Figure 12A and Figure 13); PSE longer than ASE; PL slightly longer than AL, both entirely barbed (Table 3).

Table 3.
Measurements of Leptus (Leptus) longisolenidionus sp. nov. (larvae, a–d = paratypes).
Venter. All ventral setae, including coxalae, barbed and acute (Figure 11B). Two pairs of intercoxal setae (1a and 2a), 1a posterior to level of the posterior edge of coxae I, 2a between coxae II. Four intercoxal setae (3a1 and 3a2) present between coxae II and III, 3a2 distinctly longer than 3a1, 1a, 2a and 3a2 almost the same length. Three pairs of coxalae (1b, 2b and 3b), 1b much longer than 2b and 3b, 3b longer than 2b (Table 3). 22 setae behind coxae III (fV = 22–24 in paratypes).
Gnathosoma (Figure 12B). With two nude galealae (cs), four hypostomalae (as and bs), as nude, pointed and minute, bs barbed and pointed; bs much longer than as, bs longer than cs. Palpfemur and palpgenu each with one barbed dorsal seta (PaScFed and PaScGed). Palptibia with three barbed setae, one of them on the ventral surface, odontus simple. Palptarsus with eight setae, of which four barbed, two nude, one solenidion (ω) and one eupathidium (ζ). fPp = 0-B-B-3B-4B2Nωζ. Palpal supracoxal seta (elcp) peg-like.
Legs (Figure 14, Figure 15 and Figure 16). With seven segments (femora divided). IP = 2104–2189 (Holotyp and four paratypes). Solenidion of Ta I longer than all normal setae of Ta I, ωI > 90 (Figure 14B, Figure 15A and Figure 16). Dorsum of coxa I with a supracoxal seta (eI) which is peg-like and with a rounded tip. Leg setal formula: leg I: Cx—1n; Tr—1n; Bfe—2n; Tfe—5n; Ge—1σ, 1κ, 8n; Ti—2φ, 1κ, 14n; Ta—1ω, 1ε, 2ζ, 24n. leg II: Cx—1n; Tr—1n; Bfe—2n; Tfe—5n; Ge—1κ, 8n; Ti—2φ, 15n; Ta—1ω, 2ζ, 23n. leg III: Cx—1n; Tr—1n; Bfe—1n; Tfe—5n; Ge—8n; Ti—1φ, 15n; Ta—1ζ, 25n. The lengths of the legs were measured from coxae to tarsus (Table 3).
Etymology. The new species is named after a distinctly long solenidion on Ta I.
Types. Holotype, larva, from an unknown insect host, collected by Xin-feng Zhang on 25 April 2009, from Bawangling National Natural Reserve, Hainan Province, China. Paratypes: four larvae, the same data as the holotype.
The holotype and paratypes are deposited in the Institute of Entomology, Guizhou University, Guiyang, China (GUGC).
Distribution. China: Hainan Province.
Remarks. On the keys by Saboori et al. [16] Leptus (L.) longisolenidionus sp. nov. can be placed in the phalangii group and phalangii subgroup along with 27 other species. The similar species of L. (L.) longisolenidionus sp. nov., sharing the characters Ti III/AW > 2, 200 < Ti III ≤ 235, L > 95, are L. (L.) californicus Southcott, 1992 [31], L. (L.) holgeri Haitlinger, 1999 [57], L. (L.) nearcticus Fain, Gummer and Whitaker, 1987 [58], L. (L.) phalangii (de Geer, 1778) [59], and L. (L.) swani Southcott, 1991 [26].
Leptus (Leptus) longisolenidionus sp. nov. differs from L. (L.) californicus by the number of hypostomalae (four setae vs. two setae), gnathosomal venter without striations (vs. with coarse transverse striations); longer ωI (92–99 vs. 27), DS (41–74 vs. 32–38), PDS (50–74 vs. 32–38), AL (69–74 vs. 42), PL (75–80 vs. 37), shorter W (91–97 vs. 115), PSE (46–54 vs. 80).
Leptus (Leptus) longisolenidionus sp. nov. differs from L. (L.) holgeri by the number of setae in fD formula (64–68 vs. 45), the number of hypostomalae (four vs. two); longer ωI (92–99 vs. 24–28); shorter L (96–104 vs. 118–130), W (91–97 vs. 120–130), PW (82–90 vs. 106–118), PSE (46–54 vs. 70–80), Leg I (689–699 vs. 726–802).
Leptus (Leptus) longisolenidionus sp. nov. differs from L. (L.) nearcticus by the number of setae in fD formula (64–68 vs. 94); longer ωI (92–99 vs. 33), Leg I (689–699 vs. 651), Leg II (631–670 vs. 625), Leg III (775–820 vs. 724); shorter ISD (50–59 vs. 87–100), fn Ti (14-15-15 vs. 14-16-16).
Leptus (Leptus) longisolenidionus sp. nov. differs from L. (L.) phalangii by the number of setae in fD formula (64–68 vs. 98), ωI much longer than all normal setae of Ta I (yes vs. no), shorter W (91–97 vs. 118–123), ASE (33–39 vs. 60), PSE (46–54 vs. 78), ISD (50–59 vs. 70–75), PW (82–90 vs. 103–113), Ti I (163–171 vs. 203–213), Ti II (142–152 vs. 170–185), Ti III (209–219 vs. 233–235), IP (2104–2189 vs. 2633).
Leptus (Leptus) longisolenidionus sp. nov. differs from L. (L.) swani by the number of hypostomalae (4 vs. 2); longer ωI (92–99 vs. 26), PDS (50–74 vs. 33–36), PL (75–80 vs. 50); shorter L (96–104 vs. 118), W (91–97 vs. 121), AW (69–79 vs. 93), PW (82–90 vs. 113), PSE (46–54 vs. 72), Leg I (689–699 vs. 820), Leg II (631–670 vs. 715), Leg III (775–820 vs. 855).
Leptus (Leptus) striatus Xu and Jin sp. nov. (Figure 17, Figure 18, Figure 19, Figure 20 and Figure 21)
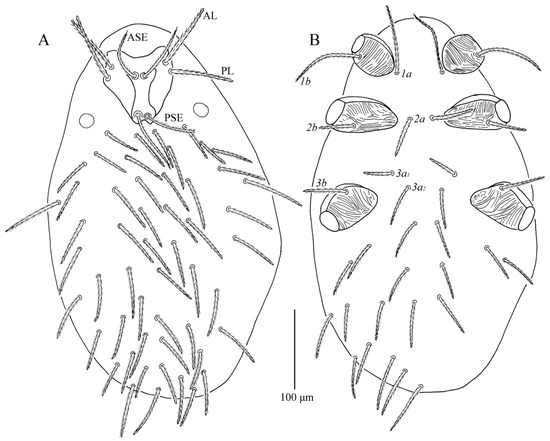
Figure 17.
Leptus (Leptus) striatus sp. nov., larva. (A). Dorsal view of idiosoma. (B). Ventral view of idiosoma.
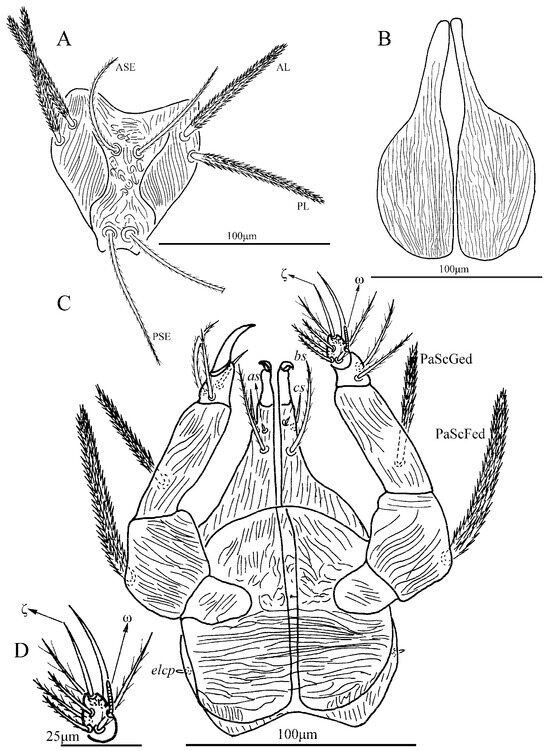
Figure 18.
Leptus (Leptus) striatus sp. nov., larva. (A). Scutum. (B). Dorsal view of the cheliceral base. (C). Ventral view of gnathosoma. (D). Ventral view of palptarsus.
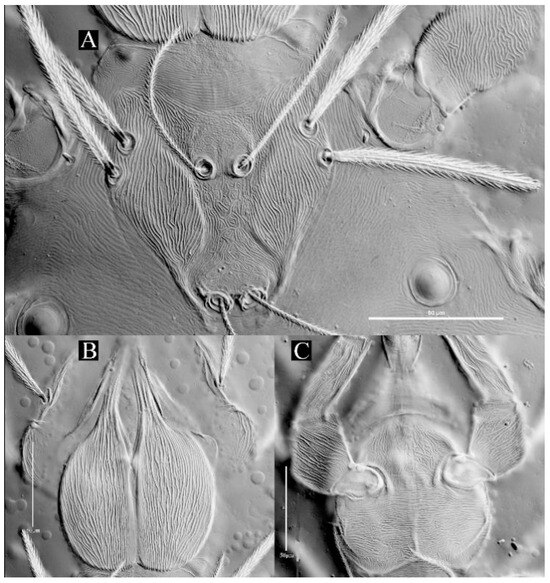
Figure 19.
Leptus (Leptus) striatus sp. nov., larva. (A). Scutum. (B). Dorsal view of the cheliceral base. (C). Ventral view of basis capitula, palpfemur and palpgenu. Scale bar = 50 µm.
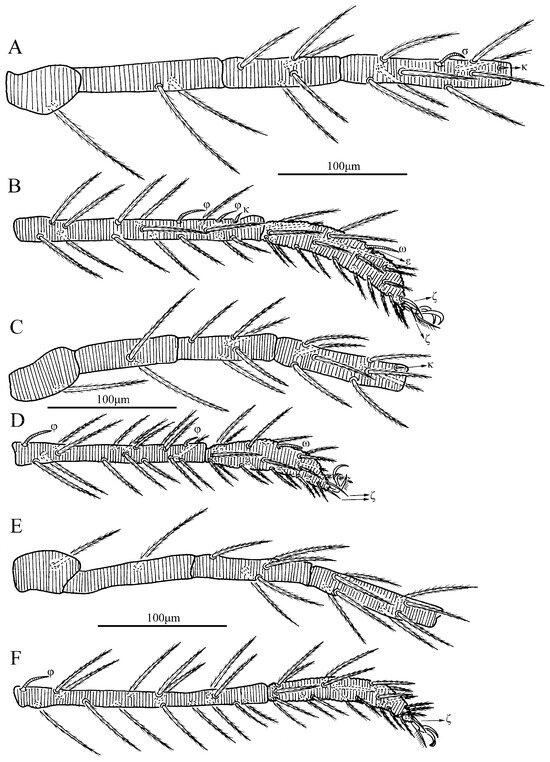
Figure 20.
Leptus (Leptus) striatus sp. nov., larva. (A). Leg I, trochanter—genu. (B). Leg I, tibia—tarsus. (C). Leg II, trochanter—genu. (D). Leg II, tibia—tarsus. (E). Leg III, trochanter—genu. (F). Leg III, tibia—tarsus.
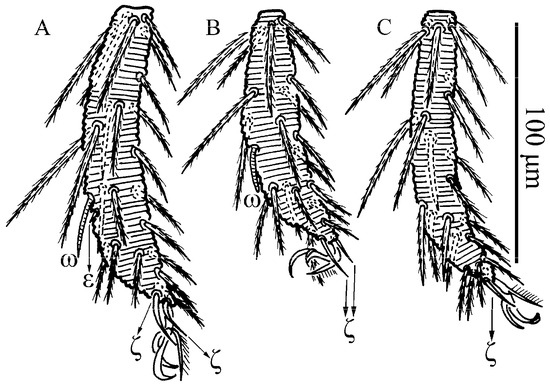
Figure 21.
Leptus (Leptus) striatus sp. nov., larva. (A). Leg I, tarsus. (B). Leg II, tarsus. (C). Leg III, tarsus.
Diagnosis (larva). Cheliceral base dorsally with numerous longitudinal sinuous striations; venter of basis capituli proximally with transverse striations, and distally with numerous fine longitudinal striations; palpfemur and palpgenu with numerous fine striations, and each with one barbed seta on the dorsal surface (PaScFed and PaScGed); scutum with longitudinal striations on both sides, and small disordered striations in the median area; ventral view of coxae I, II and III with numerous fine striations; ASE and PSE entirely with fine barbs; gnathosoma with four hypostomalae; fD = 52–56; Ti I 177–203; Ti III 186–219.
Description. Dorsum. Idiosoma with 52–56 barbed setae (fD = 52 in holotype) (Figure 17A). Scutum length is slightly longer than the width (Table 4), the anterior margin is concave, anterolateral margins and posterolateral margins are slightly sinuous, posterior margin concave (Figure 18A); with longitudinal striations on both sides, and small disordered striations in median region (Figure 18A and Figure 19A); with two pairs of sensilla (ASE and PSE) and two pairs of scutalae (AL and PL); ASE located between AL and PL bases and almost at same level of PL; PSE near posterior margin of scutum; ASE and PSE with fine barbs throughout the length, PSE slightly longer than ASE; PL slightly longer than or subequal to AL, both entirely barbed (Table 4).

Table 4.
Measurements of Leptus (Leptus) striatus sp. nov. (larvae, n = paratypes).
Venter. All ventral setae, including coxalae, barbed and acute (Figure 17B). Coxae I, II and III ventrally with numerous fine striations (Figure 17B). Two barbed intercoxal setae present between coxae I (1a) and between coxae II (2a), respectively. Four intercoxal setae (3a1 and 3a2) between coxae III with 3a1 somewhat anteriorly located; 3a2 distinctly longer than 3a1, 1a longer than 2a, 2a and 3a2 subequal (Table 4). Three pairs of coxalae (1b, 2b and 3b), 1b much longer than 2b and 3b, 2b and 3b subequal, 1b and 1a almost subequal (Table 4). Area behind coxae III with 18–20 setae (fV = 18 in holotype).
Gnathosoma. With one pair of nude galealae (cs), two pairs of hypostomalae (as and bs), as nude and bs barbed (Figure 18); bs much longer than as, bs longer than cs (Table 4). Palpfemur and palpgenu with numerous fine striations, and each with one barbed seta on dorsal surface (PaScFed and PaScGed) (Figure 18C and Figure 19C). Palptibia with two barbed setae and one nude seta, ventral surface with one barbed seta, odontus simple. Palptarsus with eight setae, of which five barbed, one nude, one solenidion (ω) and one eupathidium (ζ) (Figure 18D). fPp = 0-B-B-2BN-5BNωζ. Cheliceral base dorsally with numerous longitudinal sinuous striations; ventral of basis capituli proximally with transverse striations and distally with numerous fine longitudinal striations (Figure 18B,C and Figure 19B,C). Palpal supracoxal seta (elcp) peg-like.
Legs (Figure 20 and Figure 21). With seven segments (femora divided). IP = 2038–2291 (holotyp and 17 paratypes). Dorsum of coxa I with a supracoxal seta (eI) which is peg-like with a rounded tip. Leg setal formula: leg I: C x—1n; Tr—1n; Bfe—2n; Tfe—5n; Ge—1σ, 1κ, 8n; Ti—2φ, 1κ, 14n; Ta—1ω, 1ε, 2ζ, 23n. leg II: Cx—1n; Tr—1n; Bfe—2n; Tfe—5n; Ge—1κ, 8n; Ti—2φ, 15n; Ta—1ω, 2ζ, 20n. leg III: Cx—1n; Tr—1n; Bfe—1n; Tfe—5n; Ge—8n; Ti—1φ, 15n; Ta—1ζ, 23n. The lengths of legs were measured from coxae to tarsus (Table 4).
Etymology. The new species is named after exclusively striated gnathosoma, scutum and coxae.
Types. Holotype, larva, an unidentified Opiliones, collected by Si-yuan Xu on 7 May 2016, from Xishuangbanna National Natural Reserve (Altitude: 647 m), Yunnan Province, China. Paratypes: six larvae, the same data as the holotype; three larvae, an unidentified Opiliones, collected by Si-yuan Xu on 7 May 2016, from Xishuangbanna National Natural Reserve (Altitude: 647 m); five larvae, an unidentified Opiliones, collected by Si-yuan Xu on 10 May 2016, from Xishuangbanna National Natural Reserve (Altitude: 672 m); one larva, an unidentified Opiliones, collected by Xue-song Zhang on 25 April 2018, from Xishuangbanna National Natural Reserve (Altitude: 633 m); two larvae, an unidentified Opiliones, collected by Si-yuan Xu on 12 November 2018 from Xishuangbanna National Natural Reserve (Altitude: 1023 m), Yunnan Province, China.
The holotype and paratypes are deposited in the Institute of Entomology, Guizhou University, Guiyang, China (GUGC).
Distribution. China: Yunnan Province.
Remarks. Leptus (Leptus) striatus sp. nov. and L. (L.) bomiensis sp. nov. belongs to the killingtoni subgroup of the phalangii species group.
Leptus (Leptus) striatus sp. nov. can be easily separated from L. (L.) albertensis, L. (L.) grossi, L. (L.) killingtoni, and L. (L.) bomiensis sp. nov., based on fD = 52–56 in L. (L.) striatus sp. nov. (vs. fD > 100 in the later four species); L. (L.) striatus sp. nov. can also be separated from L. (L.) brachypodos, L. (L.) dolichopodos, L. (L.) cavernicola, L. (L.) droozi, L. (L.) shimenensis, L. (L.) singhi, and L. (L.) sulciscutus, based on the strated cheliceral base, palpfemur, palpgenu, scutum and venter of coxae I, II, and III in L. (L.) striatus sp. nov. (vs. without striations according to the original descriptions and illustrations of the seven species above).
In the killingtoni subgroup, there are only two species with striations on gnathosoma, one is L. (L.) scutellatus (dorsum of the chelicera with longitudinal striations and basis capitula with transverse striations), another one is L. (L.) ubudicus (gnathosomal venter half striated).
Leptus (Leptus) striatus sp. nov. differs from L. (L.) scutellatus as follows: proximal venter of basis capitula with transverse striations, and distal venter of basis capitula with longitudinal striations in L. (L.) striatus sp. nov. (vs. proximal basis capitula with transverse striations in L. (L.) scutellatus); scutum, palpfemur, and palpgenu with striations in L. (L.) striatus sp. nov. (vs. absent in L. (L.) scutellatus); longer Ti I (177–203 vs. 119), Ti II (139–160 vs. 102), Ti III (186–219 vs. 140).
Leptus (Leptus) striatus sp. nov. differs from L. (L.) ubudicus by dosum of the cheliceral base with striations (vs. without striations in L. (L.) ubudicus); scutum, palpfemur and palpgenu with striations (vs. absent in L. (L.) ubudicus); longer Ti I (177–203 vs. 70), Ti II (139–160 vs. 64), Ti III (186–219 vs. 92).
3.2. Checklist and Distribution of Charletonia and Leptus from China
Charletonia banksi Southcott, 1966
C. hunanensis Zheng, 1996: 65. Synonymized by Hakmitabar and Saboori [1].
Type locality: nine miles (about 14.48 km) ESE of Capella, Queensland, Australia.
Host. Unidentified Libellulidae (Insecta: Odonata) and unidentified
Megapodagriidae (Insecta: Odonata) in China; Goniaea vocans, Hepalieus gracilis, Nomaducris guttulosa and Oedaleus australis in Australia.
Distribution. Australia, China (Hunan Province).
Charletonia taiwanensis Tsai and Chow, 1988
Type locality. The mountainous area around Taipei (Taibei) city, Taiwan Province, China.
Host. Chondracris rosea (Insecta: Orthoptera: Acrididae).
Distribution. China (Taiwan Province).
Leptus (Leptus) astrubali Haitlinger, 1999
L. (L.) coloanensis Haitlinger, 2006: 91. Synonymized by Saboori et al. [16].
Type locality. Ayutthaya, Thailand.
Host. Unknown. This species was collected only from herbaceous plants.
Distribution. China (Macao Special Administrative Region), India, Myanmar, Nepal, Thailand.
Leptus (Leptus) brachypodos Zheng, 1996
Type locality. Mt. Hupingshan, Shimen County, Hunan Province, China.
Host. Unknown.
Distribution. China (Hunan Province).
Leptus (Leptus) dolichopodos Zheng, 1996
Type locality. Mt. Hupingshan, Shimen County, Hunan Province, China.
Host. Unknown.
Distribution. China (Hunan Province).
Leptus (Leptus) guilinicus Haitlinger, 2006
Type locality. Yangshou County, Guilin City, Guangxi Province, China.
Host. Unknown. This species was collected from herbaceous plants.
Distribution. China (Guangxi Province).
Leptus (Leptus) hupingshanicus Zheng, 1996
Type locality. Mt. Hupingshan, Shimen County, Hunan Province, China.
Host. Unknown.
Distribution. China (Hunan Province).
Leptus (Leptus) shimenensis Zheng, 1996
Type locality. Mt. Hupingshan, Shimen County, Hunan Province, China.
Host. Unknown.
Distribution. China (Hunan Province).
Leptus (Leptus) siemsseni (Oudemans, 1910)
Type locality. Futschou (Fuzhou?), Fokien (Fujian Province), China.
Host. Unknown.
Distribution. China (Fujian Province).
Leptus (Leptus) sulciscutus Zheng, 1996
Type locality. Mt. Hupingshan, Shimen County, Hunan Province, China.
Host. Unknown.
Distribution. China (Hunan Province).
Leptus (Leptus) trisolenidionus Xu and Jin, 2022
Type locality. Datian National Natural Reserve, Hainan Province, China.
Host. Unidentified Cicadellinae (Insecta: Hemiptera: Cicadellidae).
Distribution. China (Guizhou and Hainan Province).
Leptus (Leptus) zhejiangensis Zheng, 2003
Type locality. Mt. Tianmushan, Linan District, Hangzhou City, Zhejiang Province, China.
Host. Siobla ferox (Insecta: Hymenoptera: Tenthredinidae)
Distribution. China (Zhejiang Province).
Leptus (Leptus) zhutingensis Zheng, 1996
Type locality. Zhuting Town, Zhuzhou County, Hunan Province, China.
Host. Colaspoides opaca (Insecta: Coleoptera: Chrysomelidae) and unidentified Cicadidae (Insecta: Hemiptera: Cicadidae)
Distribution. China (Hunan Province).
Distribution data and host information were obtained from Haitlinger [38,60], Tasi and Chow [14], Xu et al. [40], Zhang [39], and Zheng [13,36,37].
4. Discussion
The genera Charletonia and Leptus are distributed worldwide (except Antarctica) with 86 and more than 240 species described with larvae, respectively. However, only three species of Charletonia and 14 species of Leptus were reported from China (Figure 22). Charletonia and Leptus in China represent less than 4% and 6% of the known world species, respectively. It suggests an urgent need to collect and study these genera and even the Erythraeidae in China.
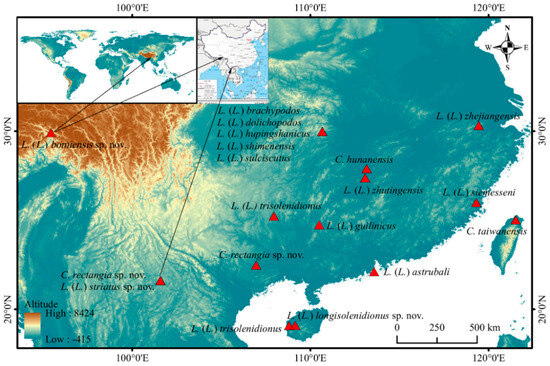
Figure 22.
Distribution map of known and new species of the genera Charletonia and Leptus from China.
Host records for Chinese Erythraeidae are mostly unknown. The literature and the current study recorded seven families in four insect orders and only one arachnid family for Leptus. For Charletonia hosts, only eight families in six insect orders are known in China, while there are 35 families in 11 insect orders and four arachnid orders reported worldwide.
Being ectoparasitic mites, the host information of larval Erythraeidae could be helpful for researchers to collect the mite specimens according to the host habits. The information of the hosts is quite important and should be recorded as detailed as possible when investigating the diversity of Erythraeid mites. In general, the comprehensive host information could provide valuable biological information for the study of erythraeid fauna, a phylogeny of the family, and co-evolution with the hosts.
Author Contributions
Conceptualization, all authors; methodology, all authors; software, S.-Y.X.; data curation, T.-C.Y. and J.-J.G.; writing—original draft preparation, S.-Y.X.; writing—review and editing, all authors; supervision, D.-C.J.; project administration, D.-C.J.; funding acquisition, D.-C.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 31872275 and 31272357 and the Guizhou Provincial Science and Technology Projects (Qiankehe Pingtai Rencai-GCC [2022]029-1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in this paper.
Acknowledgments
We would like to thank Xin-Feng Zhang (General Station of Forest and Grassland Pest Management, State Forestry and Grassland Administration, China) for collecting the ectoparasitic mites described in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the reference 16. This change does not affect the scientific content of the article.
References
- Hakimitabar, M.; Saboori, A. A review of Charletonia Oudemans (Trombidiformes: Erythraeidae) based on the larval stage. Syst. Appl. Acarol. 2022, 27, 1035–1056. [Google Scholar] [CrossRef]
- Beron, P. Acarorum Catalogus I. Acariformes: Calyptostomatoidea (Calyptostomatidae), Erythraeoidea (Smarididae, Erythraeidae); Edition of Pensoft Publishers and the National Museum of Natural History; Bulgarian Academy of Sciences: Sofia, Bulgaria, 2008; ISBN 9789546423085. [Google Scholar]
- Haitlinger, R. The Genus Leptus Latreille, 1796 and Charletonia Oudemans, 1910 (Acari, Prostigmata, Erythraeidae) in Poland (Larvae). Pol. Pismo Entomol. 1987, 57, 339–349. [Google Scholar]
- Beron, P. Acarorum Catalogus I—First Supplement (2008–2016). Hist. Nat. Bulg. 2017, 24, 131–154. [Google Scholar]
- Southcott, R.V. Revision of the Genus Charletonia Oudemans (Acarina: Erythraeidae). Aust. J. Zool. 1966, 14, 687–819. [Google Scholar] [CrossRef]
- Southcott, R.V. A further revision of Charletonia (Acarina: Erythraeidae) based on larvae, protonymphs and deutonymphs. Invertebr. Syst. 1991, 5, 61–131. [Google Scholar] [CrossRef]
- Treat, A.E. Nymphal Sphaerolophus reared from larval Charletonia (Acarina: Erythraeidae). Int. J. Acarol. 1980, 6, 205–214. [Google Scholar] [CrossRef]
- Stroiński, A.; Felska, M.; Ma̧kol, J. A Review of Host-Parasite Associations between Terrestrial Parasitengona (Actinotrichida: Prostigmata) and Bugs (Hemiptera). Ann. Zool. 2013, 63, 195–221. [Google Scholar] [CrossRef]
- Haitlinger, R. Larval Erythraeidae (Acari, Prostigmata) from Madagascar. Pol. J. Entomol./Pol. Pismo Entomol. 1987, 57, 701–723. [Google Scholar]
- Fain, A.; Jocqué, R. A new larva of the genus Leptus latreille, 1796 (Acari: Erythraeidae) parasitic on a spider from Rwanda. Int. J. Acarol. 1996, 22, 101–108. [Google Scholar] [CrossRef]
- Key, K.H.L. Host Relations and Distribution of Australian Species of Charletonia (Acarina, Erythraeidae) Parasitizing Grasshoppers. Aust. J. Zool. 1991, 39, 31–43. [Google Scholar] [CrossRef]
- Costa, S.G.D.S.; Klompen, H.; Bernardi, L.F.D.O.; Gonçalves, L.C.; Ribeiro, D.B.; Pepato, A.R. Multi-instar descriptions of cave dwelling Erythraeidae (Trombidiformes: Parasitengona) employing an integrative approach. Zootaxa 2019, 4717, 137–184. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.-Y. Two New Larval Mites of The Erythraeidae from China (Acari: Prostigmata). Acta Zootaxonomica Sin. 1996, 21, 62–69. [Google Scholar]
- Tsai, R.; Chow, Y. Charletonia taiwanensis, n. sp. (Acari, Prostigmata, Erythraeidae), an ectoparasite of the grasshopper Condracris rosea De Geer (Orthoptera, acridiidae) in Taiwan. Int. J. Acarol. 1988, 14, 171–181. [Google Scholar] [CrossRef]
- Mąkol, J.; Wohltmann, A. An Annotated Checklist of Terrestrial Parasitengona (Actinotrichida: Prostigmata) of the World, Excluding Trombiculidae and Walchiidae. Ann. Zool. 2012, 62, 359–562. [Google Scholar] [CrossRef]
- Saboori, A.; Hakimitabar, M.; Khademi, N.; Masoumi, H.; Katouzian, A.R. Leptus Latreille (Trombidiformes: Erythraeidae) of the World: Revised Classification and Keys. Persian J. Acarol. 2020, 9, 1–57, Corrections and Additions in Persian J. Acarol. 2020, 9, 209–212. [Google Scholar]
- Haitlinger, R.; Šundiċ, M.; Ázara, L.; Bernardi, L.F.O. A new species of larval Leptus (Leptus) (Trombidiformes: Erythraeidae) from Brazil with list of host-parasite associations between Leptus and arthropods in America. Biologia 2020, 75, 1921–1930. [Google Scholar] [CrossRef]
- Bassini-Silva, R.; Jacinavicius, F.D.C.; Bouzan, R.S.; Iniesta, L.F.M.; Campos-De-Oliveira, E.; Welbourn, C.; Šundić, M.; Ochoa, R.; Brescovit, A.D.; Barros-Battesti, D.M. A new species of Leptus (Leptus) (Trombidiformes: Erythraeidae) and new records of Leptus (Leptus) haitlingeri Jacinavicius, Bassini-Silva & Welbourn, 2019 for Brazil. Int. J. Acarol. 2020, 46, 213–221. [Google Scholar] [CrossRef]
- Haitlinger, R.; Šundić, M. Two new species of Leptus Latreille, 1796 (Trombidiformes: Erythraeidae) from the Canary Islands, parasitising Curculionidae (Insecta: Coleoptera), with new metrical data for some Leptus spp. Syst. Parasitol. 2020, 97, 835–846. [Google Scholar] [CrossRef]
- Haitlinger, R.; Šundić, M.; Nkwala, A.L.D. Description of Leptus (Leptus) cameroonicus sp. nov. and first record of Charletonia braunsi (Oudemans, 1910) from Cameroon (Trombidiformes: Erythraeidae), with new metric and meristic data for some African Leptus. Syst. Appl. Acarol. 2020, 25, 607–621. [Google Scholar] [CrossRef]
- Hakimitabar, M.; Joharchi, O.; Jung, C. A new species of Leptus, the first erythraeid mite (Acari: Trombidiformes) from South Korea. Int. J. Acarol. 2020, 46, 155–159. [Google Scholar] [CrossRef]
- Hakimitabar, M.; Saboori, A.; Fadaei, E. A New Species of Leptus (Acari: Erythraeidae) from Iran. Persian J. Acarol. 2021, 10, 137–143. [Google Scholar] [CrossRef]
- Moradian, H.; Nazarpoor, F.; Ostovan, H. The First Report of Leptus N. Sp. (Acari: Erythraeidae) as Ectoparasite of Cassida Persica Spaeth (Coleoptera: Chrysomelidae) from Oil and Gas Company of Gachsaran. Int. J. Adv. Biol. Biomed. Res. 2015, 3, 35–37. [Google Scholar]
- Arillo, A.; Blagoderov, V.; Peñalver, E. Early Cretaceous parasitism in amber: A new species of Burmazelmira fly (Diptera: Archizelmiridae) parasitized by a Leptus sp. mite (Acari, Erythraeidae). Cretac. Res. 2018, 86, 24–32. [Google Scholar] [CrossRef]
- Souza, U.A.; Gabana, A.M.; Farezin, L.D.C.; Vaz, D.B.; Girotto-Soares, A.; Nunes, P.; Soares, J.F. First record of Leptus spp. (Acari: Erythraeidae) parasitizing Scaptia (Lepnia) spp. (Diptera: Tabanidae). Int. J. Acarol. 2019, 45, 509–511. [Google Scholar] [CrossRef]
- Southcott, R. Descriptions of larval Leptus (Acarina: Erythraeidae) ectoparasitic on Australian diptera, and two earlier described Australian larvae. Invertebr. Syst. 1991, 5, 717–763. [Google Scholar] [CrossRef]
- Gabryś, G.; Felska, M.; Kłosińska, A.; Staręga, W.; Mąkol, J. Harvestmen (Opiliones) as hosts of Parasitengona (Acari: Actinotrichida, Prostigmata) larvae. J. Arachnol. 2011, 39, 349–351. [Google Scholar] [CrossRef]
- Southcott, R. Larvae of Leptus (Acarina: Erythraeidae) ectoparasitic on higher insects of Australia and New Guinea. Invertebr. Syst. 1993, 7, 1473–1550. [Google Scholar] [CrossRef]
- Southcott, R.V. Larvae of Leptus (Acarina: Erythraeidae), free-living or ectoparasitic on arachnids and lower insects of Australia and Papua New Guinea, with descriptions of reared post-larval instars. Zool. J. Linn. Soc. 1999, 127, 113–276. [Google Scholar] [CrossRef]
- Fain, A.; Elsen, P. Observations Sur Les Larves Du Genre Leptus Latreille 1795 (Acari, Erythraeidae) d’Afrique Centrale. Rev. Zool. Afr. 1987, 101, 103–123. [Google Scholar]
- Southcott, R.V. Revision of the larvae of Leptus Latreille (Acarina: Erythraeidae) of Europe and North America, with descriptions of post-larval instars. Zool. J. Linn. Soc. 1992, 105, 1–153. [Google Scholar] [CrossRef]
- Haitlinger, R. Four New Species of Leptus Latreille, 1796 (Acari, Prostigmata, Erythraeidae) from the Canary Islands. Syst. Acarol. Acarol. 2009, 14, 140–152. [Google Scholar] [CrossRef]
- Haitlinger, R. Arthropods (Acari, Anoplura, Siphonaptera) of Small Mammals of the Warmińsko-Mazurskie Province. Zesz. Nauk. Uniw. Przyr. Wroc. Biol. Hod. Zwierzat 2015, 78, 35–60. [Google Scholar]
- Salvatierra, L.; Almeida, M.Q. First record of a Leptus Latreille mite (Trombidiformes, Erythraeidae) associated with a Neotropical trapdoor spider (Araneae: Mygalomorphae: Actinopodidae). Cogent Biol. 2017, 3, 1295823. [Google Scholar] [CrossRef]
- Pereira, M.C.S.D.A.; Bernardi, L.F.D.O.; Hermes, M.G. First record of a Leptus Latreille mite (Trombidiformes, Erythraeidae) associated with a Neotropical solitary wasp (Hymenoptera, Vespidae, Eumeninae). Int. J. Acarol. 2016, 42, 391–393. [Google Scholar] [CrossRef]
- Zheng, B. Five New Species of The Genus Leptus Latreille from Mt. Hupingshan of Hunan China (Acari: Erythraeidae). Entomol. Sin. 1996, 3, 229–242. [Google Scholar] [CrossRef]
- Zheng, B.-Y. A New Species of Leptus Latreille (Acari, Erythraeidae) Ecto-Parasitic on an Adult Sawfly (Hymenoptera, Tenthredinidae). Acta Zootaxonomica Sin. 2003, 28, 56–58. [Google Scholar]
- Haitlinger, R. Eight new species and new records of mites (Acari: Prostigmata: Erythraeidae, Trombidiidae, Johnstonianidae) from China including Macao. Syst. Appl. Acarol. 2006, 11, 83–105. [Google Scholar] [CrossRef]
- Zhang, Z.-Q. On Leptus siemsseni Oudemans from Fujian, China (Acari: Erythraeidae). Syst. Acarol. Acarol. Spéc. Publ. 2001, 9, 23–24. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Jin, D.-C.; Guo, J.-J.; Yi, T.-C. Four new species of larval Erythraeoidea (Acari: Trombidiformes: Prostigmata) and three higher taxa new to China: Genus Hirstiosoma and subfamily Hirstiosomatinae (Smarididae), and genus Grandjeanella (Erythraeidae: Abrolophinae). Syst. Appl. Acarol. 2022, 27, 1813–1840. [Google Scholar] [CrossRef]
- Myers, N. Threatened biotas: “Hot spots” in tropical forests. Environmentalist 1988, 8, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, G.-S. An Introduction and Analysis of Biodiversity Conservation in Indo-Burma Biodiversity Hotspot. Landsc. Archit. 2015, 22, 16–24. [Google Scholar]
- Lei, F.; Qu, Y.; Song, G.; Alström, P.; Fjeldså, J. The potential drivers in forming avian biodiversity hotspots in the East Himalaya Mountains of Southwest China. Integr. Zool. 2015, 10, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Bassini-Silva, R.; Scopel, W.; Lima, E.F.B.; Silva-Neto, A.M.; Flechtmann, C.H.W.; Welbourn, C.; Ochoa, R.; Brescovit, A.D.; Barros-Battesti, D.M.; Jacinavicius, F.C. Charletonia rocciai Treat and Flechtmann, 1979 (Trombidiformes: Erythraeidae): Larval redescription and new records for Brazil. Int. J. Acarol. 2022, 48, 433–441. [Google Scholar] [CrossRef]
- De Castro Jacinavicius, F.; Bassini-Silva, R.; Soares, J.F.; Virginio, F.; Welbourn, C.; Barros-Battesti, D.M. Description of Leptus (Leptus) haitlingeri n. sp. (Trombidiformes: Erythraeidae), Parasitising Horse Flies (Diptera: Tabanidae), and a Key to the Larvae of Leptus spp. in Brazil. Syst. Parasitol. 2019, 4, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Haitlinger, R.; Saboori, A. Seven New Larval Mites (Acari, Prostigmata, Erythraeidae) from Iran. Misc. Zool. 1996, 19, 117–131. [Google Scholar]
- Šundić, M.; Haitlinger, R.; Milošević, D. Charletonia elbasani, a new species from Albania (Acari: Erythraeidae), with notes on C. kalithensis Haitlinger, 2006. Acarologia 2017, 57, 563–569. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Yi, T.-C.; Guo, J.-J.; Jin, D.-C. A new species of larval Caeculisoma (Acari: Erythraeidae: Callidosomatinae) ectoparasitic on insects from China and a revised generic diagnosis. Zootaxa 2019, 4604, 511–524. [Google Scholar] [CrossRef]
- Haitlinger, R. New larval mites (Acari: Prostigmata: Erythraeidae, Microtrombidiidae, Trombidiidae) from Turkey, Peru and Poland. Wiadomosci Parazytol. 2000, 46, 379–396. [Google Scholar]
- Southcott, R.V. Two New Larval Ectoparasitic Mites (Acarina: Erythraeidae) from Sri Lankan Tetrigid Grasshoppers. Entomol. Scand. 1988, 30, 151–159. [Google Scholar]
- Haitlinger, R. Three New Species of Larval Charletonia Oudemans, 1910 (Acari: Prostigmata: Erythraeidae) and the New Records of Charletonia lankensis Southcott, 1988, C. shiroyama Yaita, Kato & Toriyama, 1961 and C. Volzi (Oudemans, 1910) from Asia. Zesz. Nauk. Uniw. Przyr. Wroc Biol. Hod. Zwierząt 2007, 55, 71–84. [Google Scholar]
- Haitlinger, R. New Records of Mites (Acari: Prostigmata, Erythraeidae) from Africa with Descriptions of Four New Species. Zesz. Nauk. Uniw. Przyr. Wroc Biol. Hod. Zwierząt 2007, 55, 55–69. [Google Scholar]
- Hakimitabar, M.; Saboori, A. Charletonia stekolnikovi sp. n. (Acari, Erythraeidae) from Iran. Vestnik Zool. 2011, 45, 40–46. [Google Scholar] [CrossRef]
- Hakimitabar, M.; Saboori, A.; Seiedy, M. A new species of larval Charletonia (Acari: Erythraeidae) parasitic on Arachnida from Iran. Syst. Appl. Acarol. 2013, 18, 163–176. [Google Scholar] [CrossRef]
- Saboori, A.; Arbabi, M. A new species of Leptus larva (Acari: Erythraeidae) from India. Syst. Appl. Acarol. 2003, 8, 175–182. [Google Scholar] [CrossRef]
- Haitlinger, R. New species and new records of mites (Acari: Prostigmata: Erythraeidae) from Bali, Lombok and Linnga Islands, Indonesia. Syst. Appl. Acarol. 2006, 12, 219–230. [Google Scholar] [CrossRef]
- Haitlinger, R. Six New Species of Leptus Latreille, 1796 (Acari, Prostigmata, Erythraeidae) from South-East Asia. Miscellània Zoològica 1999, 22, 51–68. [Google Scholar]
- Fain, A.; Gummer, S.L.; Whitaker, J.O., Jr. Two new species of Leptus Latreille, 1796 (Acari, Erythraeidae) from the U.S.A. Int. J. Acarol. 1987, 13, 135–140. [Google Scholar] [CrossRef]
- Mąkol, J.; Gabryś, G.; Łaydanowicz, J. Leptus phalangiid (De Geer, 1778) (Acari: Actinotrichida: Prostigmata)—Redescription, Ecology and Taxonomic Notes on Its Relatives. Ann. Zool. 2011, 61, 535–546. [Google Scholar] [CrossRef]
- Haitlinger, R. New records of mites (Acari: Prostigmata: Erythraeidae) from Nepal, with a description of Leptus (Leptus) kattikus sp. nov. Syst. Appl. Acarol. 2009, 14, 60–64. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).