Simple Summary
The monogeneric family Vietnamellidae is endemic in the Oriental region and found only in the Indomalayan biogeographic realm. Seven nominal species have previously been described, but three are considered to be junior synonyms. However, some provisional species designations have been established. In this study we described formally Vietnamella sp. C as a new species, V. nanensis sp. n., based on the specimens used for initial DNA data and our expanded set of materials from Nan Province, Thailand. Based on morphology, the larva of the new species can be distinguished from those of other congeners. Additional mitochondrial cytochrome oxidase subunit I gene data for the new species are also provided. A key to larvae of all known species in the genus is provided.
Abstract
The larva, male subimago, female imago, and eggs of V. nanensis sp. n. are described based on specimens from Mae Hong Son and Nan provinces, Thailand. The female subimago is described based on a photograph of a specimen reared to the imago stage. The species previously was distinguished only by DNA barcode data and designated as Vietnamella sp. C. Based on morphology, the larva of the new species can be distinguished with the following combination of characteristics: (i) pattern of serration on the ventral margin of the forefemur, (ii) posterolateral margins of abdominal terga with pairs of acute tubercles, especially terga VI and VII, (iii) a well-developed pair of median ridge projections on tergum X, (iv) the second segment of the maxillary palp being about 1.3× the length of the third segment, and (v) females containing eggs with prominent protuberances on the chorionic surface. A key to larvae of all known species in the genus is provided.
1. Introduction
Mayflies (Ephemeroptera) are one of the most common components of benthic communities and contribute to ecosystem services [1,2,3,4,5]. They have been used as indicators of water quality [6,7,8,9]. Recently, the diversity of Thai mayflies is provided (e.g., Baetidae [10,11,12], Ephemerellidae [13], Heptageniidae [14,15,16], Leptophlebiidae [17,18], Prosopistomatidae [19], Teloganodidae [20], Tricorythidae [21]). Vietnamellidae is a monogeneric family of mayflies found only in the Indomalayan biogeographic realm. It may represent an extant basal lineage of the superfamily Ephemerelloidea, but more data are needed to test this hypothesis [22,23]. Seven nominal species have previously been described, but three are considered to be junior synonyms. All of the named species are known from Vietnam, China, and Thailand [24,25,26]. The species richness of the genus is greater, but lack of sufficient material and stage associations have hampered rapid progress in formally naming and describing this diversity. Until more materials and data are available, some provisional species designations have been established: Vietnamella sp. A from India [27], and Vietnamella sp. B & Vietnamella sp. C from Thailand [26]. Other material has been reported at the genus level from Laos, without provisional species designations [28].
From among the three provisional species, Vietnamella sp. C was only known from a few mitochondrial cytochrome c oxidase subunit I (COI) sequences that had been retrieved by Auychinda et al. [26] from the Barcode of Life Data System (BOLD). No previous attempt had been made to place the larval specimens from which the DNA had been obtained to a species, due to the poor state of systematics of the group (see, e.g., [25,29]). In light of the findings of Auychinda et al. [26], these specimens were investigated again, and new material was discovered, including the male subimago, female subimago, and egg stages. The present study aims to name and describe formally Vietnamella sp. C as a new species based on the specimens used for initial DNA data and our expanded set of materials from Nan Province, Thailand. Additional COI data for the new species are also provided, and these data were analyzed using Bayesian inference methods.
2. Materials and Methods
2.1. Sampling and Morphological Observations
The newly acquired specimens used for description and photography were collected from Nan Province in Northern Thailand. Larvae were collected from cobble in moderate to fast-flowing areas. Mature larvae were reared using earthenware pots connected to an air supply (Figure 8C) until emergence of winged stages. The specimens that have been used to obtain initial COI data were retrieved from L.M.J.’s research collection. The chorionic structure was investigated by drying the eggs, coating them with gold, and observing them with a FEI Quanta 450 Scanning Electron Microscope (SEM). The external structures were prepared on permanent slides using Euparal® as a medium and observed by light microscopy. Final plates were prepared with Adobe Photoshop® CC 2020. Holotype and paratype specimens of the new species are deposited in the collections of the Zoological Museum at Kasetsart University in Bangkok, Thailand [ZMKU] and the Museum of Zoology in Lausanne, Switzerland [MZL]. L.M.J.’s materials are currently stored in trays with other specimens used for DNA barcoding, and these units will be deposited in the Purdue University Entomological Research Collection, West Lafayette, Indiana, United States of America [PERC]. Our species hypotheses are based on the convergence of the morphological species concept and the phylogenetic species concept.
2.2. Molecular Analysis
The COI sequence of Vietnamella nanensis sp. n. was newly amplified from the Wa River, Nan Province, Thailand. The 658 bp sequence was included in the Bayesian tree reconstruction with the other vietnamellid mayflies from GenBank and the BOLD system. The preserved specimens were dissected (thorax) for DNA extraction. Total DNA was extracted using a genomic DNA purification kit (NucleoSpin, Macherey-Nagel, Germany) following the manufacturer’s protocol. The COI amplification was performed using LCO1490 and HCO2198 [30]. The polymerase chain reaction (PCR) conditions and procedure were performed as described previously [26]. Purification and sequencing were conducted by Macrogen, Inc. (Seoul, South Korea). The molecular protocols and Bayesian tree construction methods were as previously reported by Auychinda et al. [26]. Nucleotide sequences obtained in this study were deposited in the GenBank database. Other Vietnamella sequences were also obtained from the BOLD system and GenBank, and Teloganella umbrata Ulmer was used as an outgroup (a specimen of Narathiwat Province, Thailand, was amplified and sequenced by the authors); details are presented in Table 1. The Kimura 2-parameter (K2P) genetic distance was analyzed to confirm species delimitation.

Table 1.
The mitochondrial cytochrome c oxidase subunit I (COI) sequences for molecular analysis.
2.3. Ethics Statement
The present study was approved by the ethics committee of Kasetsart University (approval no. ACKU61-SCI-029) for rearing and collecting the mayfly specimens.
3. Results
3.1. Taxonomy
Vietnamella nanensis Auychinda & Boonsoong, sp. n. (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7)
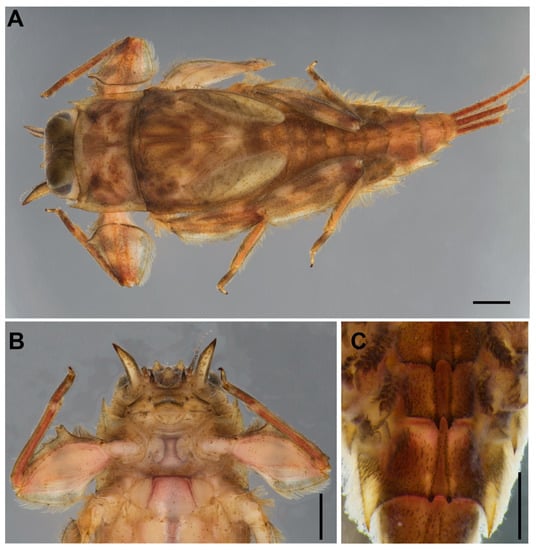
Figure 1.
Vietnamella nanensis sp. n. (paratype) (A) habitus of larva; (B) ventral view of head and thorax; (C) abdominal terga VI–VII. Scale bars: 1 mm.
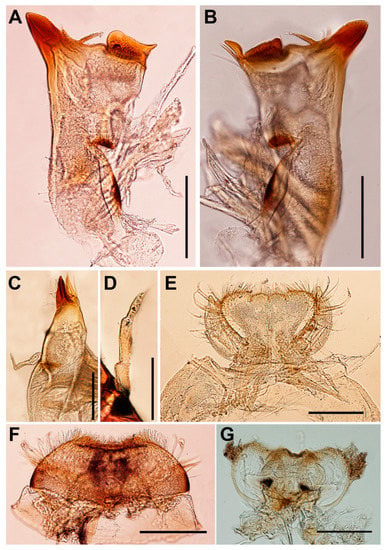
Figure 2.
Vietnamella nanensis sp. n. (A) left mandible; (B) right mandible; (C) maxilla; (D) maxillary palp; (E) labium; (F) labrum; (G) hypopharynx. Scale bars: 0.2 mm.
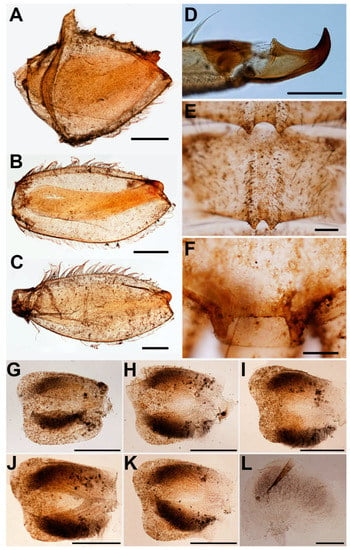
Figure 3.
Vietnamella nanensis sp. n. (A) forefemur; (B) midfemur; (C) hindfemur; (D) foretarsal claw; (E) abdominal terga VI–VII; (F) abdominal tergum X; (G) gill II; (H) gill III; (I) gill IV; (J) gill V; (K) gill VI; (L) gill VII. Scale bars: 0.5 mm (A–C, E–K), 0.2 mm (D, L).
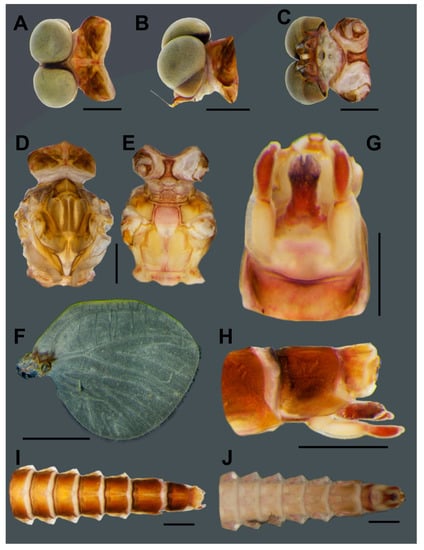
Figure 4.
Male subimago of Vietnamella nanensis sp. n. Dorsal (A), lateral (B) and ventral (C) views of head; dorsal (D) and ventral (E) views of thorax; (F) hindwing; ventral (G) and lateral (H) views of genitalia; dorsal (I) and ventral (J) views of abdomen. Scale bars: 1 mm.
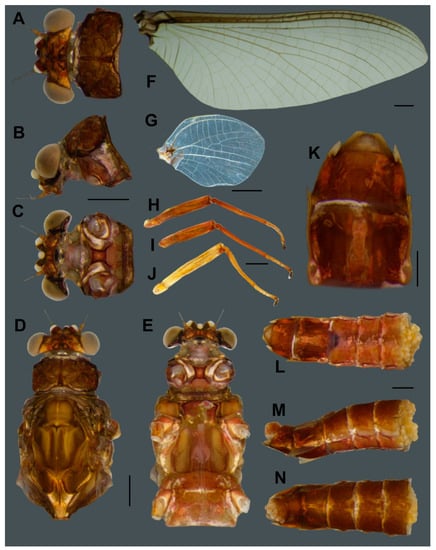
Figure 5.
Female imago of Vietnamella nanensis sp. n. Dorsal (A), lateral (B) and ventral (C) views of head; dorsal (D) and ventral (E) views of thorax; (F) forewing; (G) hindwing; (H) foreleg; (I) middle leg; (J) hindleg; (K) ventral view of genitalia; ventral (L), lateral (M) and dorsal (N) views of abdomen. Scale bars: 1 mm.
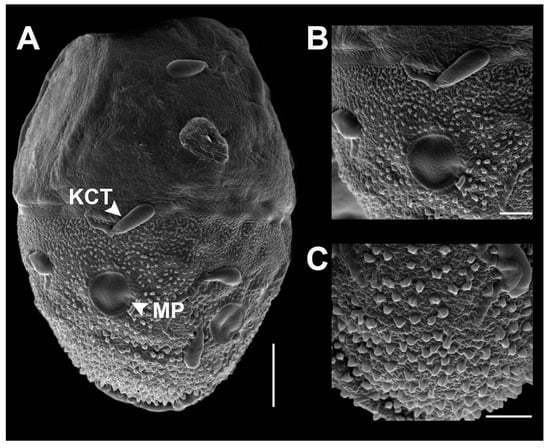
Figure 6.
SEM of egg structure of Vietnamella nanensis sp. n. (A) overview of egg structure; (B) detail of Knob terminated Coiled Thread (KCT) and micropyle; (C) chorionic surface of posterior area. Scale bars 50 µm (A); 20 µm (B); 10 µm (C).
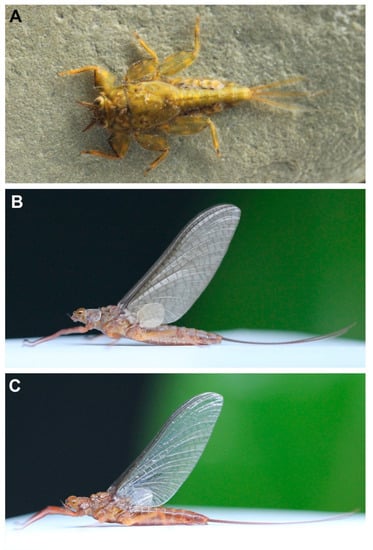
Figure 7.
Vietnamella nanensis sp. n. (A) habitus of larva; (B) habitus of female subimago; (C) habitus of female imago.
Vietnamella sp. C sensu Auychinda et al., 2020
Material examined. Holotype: Male mature larva, Thailand, Nan Province, Bo Kluea District, Mae Nam Wa, Wa River, 19°16′22.6” N 101°10′48.2” E, 848 m, 26.XI.2019, B. Boonsoong col. [ZMKU]. Paratypes: 24 larvae, 1 male subimago, 2 female subimagoes (incomplete), 2 female imagoes in ethanol, 1 larva on slide, same data as holotype [ZMKU]; one larva in ethanol, same data as holotype [MZL GBIFCH00834909]. Four larvae, 1 female imago, same locality, but 20.III.2020, B. Boonsoong col. [ZMKU]. Additional material: 1 larva, Thailand, Nan Province, Amphur Bo Kluea, Ban Bo Kluea Tai, Nam Mang, 663 m elevation, 19°09.141′ N, 101°09.277′ E, 17.IV.2009, Sites, Vitheepradit, Prommi col. (“L-1044”), BOLD ID: THMAY031-09/Sample ID: 09THMAY-031/Tray ID: L09THMAY-O06 [PERC]. Two larvae, Thailand, Mae Hong Son Province, Amphur Khun Yaum, Tumbon Khun Yaum, Huay Ma Surin at Ban Mae Surin, 408 m elevation, 18°54.588′ N, 97°56.695′ E, 20.IV.2009, Sites, Vitheepradit, Prommi col. (“L-1050”), BOLD ID: THMAY148-12/Sample ID: LTHSIT087/Tray ID: BIOUG00646-E12, BOLD ID: THMAY149-12/Sample ID: LTHSIT089/Tray ID: BIOUG00646-F01 [PERC].
Description.
Mature larva (in alcohol, Figure 1; living, Figure 7A). Body length 10–14 mm (n = 6) without cerci; cerci 8–10 mm; body brown with pale bandings on thorax and femora (Figure 1A).
Head. Brown with pair of occipital tubercles, single sub-occipital tubercle medially; two pairs of projections below eyes; inner pair small, spine-like, and sharp; outer pair large, triangular, cone-shaped, without any serrated spines (Figure 1B). Left mandible slender, outer margin slightly concave at middle; molar block-like shape (Figure 2A). Right mandible slender, outer margin slightly concave at middle; molar block-like shape with tuft of setae below inner molar margin (Figure 2B). Maxillae slender; maxillary palpi three-segmented, with tiny setae, length ratio from basal to apical segments = 1:1.3:1 (Figure 2C,D). Labium: glossae width twice greater than length, glossae with densely short setae anteriorly, outer margins of paraglossae with long setae; labial palpi three-segmented, basal segment broader and longer than second, apical segment small, cone shape; palp with tiny setae most abundant on outer margin (Figure 2E). Labrum: anterior margin with dense short, pectinate setae; anterior half of dorsal surface and margins with relatively long pectinate setae (Figure 2F). Hypopharynx: lingua rounded with anterolateral emargination and superlinguae nearly round, with setae on surfaces (Figure 2G).
Thorax. Pronotum with moderately sharp anterolateral projections and slightly pointed protuberances below anterolateral projection. Mesonotum without projections and tubercles. Forefemora strongly expanded with serrations or tooth-like projections on ventral margin (Figure 3A); transverse ridge serrated with spatulate setae and long, thin setae near inner dorsal margin; dorsal and ventral margins with simple, fine setae. Midfemora moderately expanded, dorsal margin smooth and with row of hair-like setae, ventral margin with small serration apically (Figure 3B). Hindfemora moderately expanded, more slender than midfemora, dorsal margin smooth, with row of hair-like setae; ventral margin with small serration apically (Figure 3C). All claws similar, strongly hooked, each with basal protuberance and 4–5 fine sub-apical setae (Figure 3D).
Abdomen. Terga covered with simple, short setae; Terga I–X each with pair of median ridges or tubercles; posterolateral angles of terga II–X extended into sharp projections with dense simple setae; terga VI–VII each with pair of acute tubercles (Figure 1C and Figure 3E) and tergum X with well-developed pair of blunt tubercles (Figure 3F). Gills I finger-like with setae; gills on segments II–VI similar in structure (Figure 3G–K); gills on segment VII small, with ventral lamella divided into three lobes (Figure 3L). Caudal filaments with long setae on both lateral side at the middle part.
Male subimago (in alcohol, Figure 4). Body length 12 mm without cerci.
- Head. Eyes with dorsal part yellowish and ventral part pale brown (Figure 4A–C).
- Abdomen. Genitalia with penis and forceps relatively short and broad; penis with apicomedian emargination, length almost equal to second forceps segment; forceps with total length = 0.96 mm, basal:median segment ratio = 1.4:1 (Figure 4G–H). Terga reddish brown; segments VIII–IX with pair of pale median lines (Figure 4I); sterna light brown; segment IX, penis and forceps with dark brown markings (Figure 4J).
- Head. Eyes pale brown (Figure 5A–C).
- Thorax. Mesonotum brown with notable median longitudinal suture (Figure 5D). Mesoternum brown with rectangular basisternum and broad furcasternum (Figure 5E). Forewing stigma area without divided longitudinal vein; C to RA area brown; MA forked at middle of wing; MP forked basally, three intercalaries between MP1 and MP2; CuA and CuP adjacent at base (Figure 5F). Hindwing rounded, leading margin slightly concave, with clear cross-veins; 11 cross-veins between Sc and RA, five cross-veins between MA and MP (Figure 5G). Forelegs (6.31 mm) with length ratio of femur:tibia = 1:1.06 (Figure 5H). Midlegs (6.63 mm) with length ratio of femur:tibia = 1:1.3 (Figure 5I). Hindlegs (6.63 mm) with length ratio of femur:tibia = 1:1.4 (Figure 5J).
- Eggs (dissected from imago, Figure 6). Ovoid, with length approximately 310 µm, width approximately 220 µm; nearly half of egg covered with helmet-shaped polar cap (Figure 6A). Rod-shaped knob terminated coiled thread (KCT) around egg body; 2 or 3 tagenoform-type micropyles at center, without protuberances in micropyle area (Figure 6B). Chorionic surface with protuberances, prominent in posterior area (Figure 6C).
Female subimago (Figure 7B). Body length 12–14 mm without cerci.
- Head. Eyes yellowish.
- Thorax. Brownish veins and crossveins brown.
- Abdomen. Brownish, terga segment VI–IX with pair of median pale line (similar to male subimago).
- Diagnosis. The larva of Vietnamella nanensis sp. n. is most similar to that of V. thani Tshernova, but can be separated from the latter and other Vietnamella species based on the following combination of characteristics: (i) pattern of serration on the ventral margin of the forefemur (Figure 1A,B and Figure 3A), (ii) posterolateral margins of the abdominal terga with pairs of acute tubercles, especially in terga VI and VII (Figure 1A,C and Figure 3E), (iii) well-developed pair of median ridge projections of tergum X (Figure 3F), (iv) second segment of the maxillary palp being slightly longer than the third segment (1.3:1), and (v) chorionic surface with prominent protuberances (imago stage).
- In the alate stages, the anterior margin of forewings is brown and has fewer cross-veins than in the other species. The venation of hind wings shows cross veins between Subcosta (Sc) and Radius sector (RA) similar in number with V. sinensis [31] but more numerous compared to V. thani. The penis of the subimago is most similar to that of V. thani and clearly different from the one of V. ornata (Tshernova) [24].
- Remarks. The morphology of the alate stages was described from male subimago and female imago associated with larvae by rearing method. In addition, the female subimago was photographed before its imago emerged (Figure 7B).
- Etymology. The specific epithet is a reference to the province Nan in Thailand, where the type material was collected and reared.
- Habitat and ecology. The type locality of Vietnamella nanensis sp. n. is Mae Nam Wa, Nan Province, Thailand (Figure 8A). The larvae were found on cobble and pebbles within the moderate to fast-flowing current of run/riffle areas (Figure 8B). Larvae of Vietnamella nanensis sp. n. co-occurred with other Ephemerelloidea larvae, such as Cincticostella insolta (Allen), Notacanthella quadrata (Kluge & Zhou), N. commodema (Allen) (Ephemerellidae), and Dudgeodes sp. (Teloganodidae).
 Figure 8. Vietnamella nanensis sp. n. (A) type locality-Wa river, Nan province, Thailand; (B) microhabitat; (C) rearing chamber.
Figure 8. Vietnamella nanensis sp. n. (A) type locality-Wa river, Nan province, Thailand; (B) microhabitat; (C) rearing chamber.
Distribution. Northern Thailand (Nan and Mae Hon Son provinces).
3.2. Identification Key to Known Mature Larvae of Vietnamella Species
1 Head with serration of outer projections···············································································································2
Head without serration of outer projections·········································································································3
2 Abdominal tergum VII with a pair of tubercles on posterior margin·································Vietnamella sp. A
Abdominal tergum VII with a single tubercle on posterior margin·····································Vietnamella sp. B
3 Second segment of maxillary palp greater than half the length of other segments························V. sinensis
Second segment of maxillary palp nearly equal in length to the other segments···········································4
4 Transverse ridge of forefemur with small rounded setae·······························································V. maculosa
Transverse ridge of forefemur with spatulate setae·····························································································5
5 Abdominal tergum VII with a pair of acute tubercles on posterior margin and Posterolateral projection of segment X well developed······································································································V. nanensis sp. n.
Abdominal tergum VII with a pair of blunt tubercles on posterior margin and posterolateral projection of segment X less developed·······················································································································V. thani
3.3. Molecular Analysis
The COI analysis revealed three major clades, namely Vietnamella sp. B, V. thani, and a group of Vietnamella species with distribution in Northern Thailand and China. The last major clade was distinguished into four clusters and referred to four different species: V. maculosa Auychinda, Sartori & Boonsoong, V. cf. ornata, V. sinensis, and V. nanensis sp. n. The V. nanensis sp. n. cluster is divided into two branches based on geography including Mae Hon Son and Nan provinces (Figure 9).
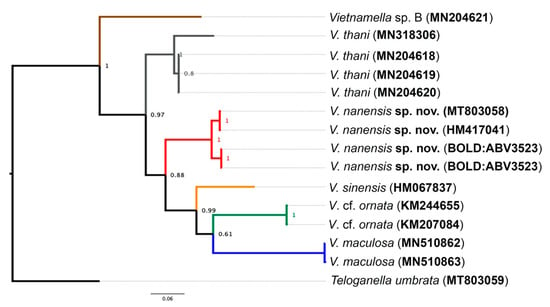
Figure 9.
Bayesian interference of Vietnamellidae. The COI phylogenetic reconstruction of six different species of Vietnamella with the branch probability support. The accession number of GenBank or Barcode Index Number (BIN) of Barcode of Life Data System (BOLD) in brackets. Teloganella umbrata was used as the outgroup.
The intraspecific genetic distances varied between 0% and 3.4%, whereas interspecific distances were very high, ranging from 16.1% to 31.2% (Table 2).

Table 2.
Pairwise genetic distances (COI) between species of Vietnamella using the K2P.
4. Discussion and Conclusions
The currently known larvae of Vietnamella species can be clearly distinguished from one another by their morphology, and their character comparison is shown in Table 3. Vietnamella ornata is not included, as it has been reported only from the subimaginal stage [24]. Vietnamella sp. A from India might represent the larva of V. ornata [27]; more work is needed in this area. Both morphological and molecular evidence shows similar preliminary results for species numbers and delimitation.

Table 3.
Comparisons of known larvae and eggs of Vietnamellidae.
We found that the venation of the stigma area of the forewing can be used to separate Vietnamella species into two groups as follows: i) with divided longitudinal vein, including species that have been found only in China (V. sinensis and V. ornata) and ii) without divided longitudinal vein, including three species (V. nanensis sp. n., V. maculosa and V. thani). The MA fork position of forewings of V. nanensis sp. n. shows intraspecific variation, with one specimen having the MA fork situated medially and another having it submedially. The penis of the male subimago of V. nanensis sp. n. is most similar to V. thani, whereas it differs completely from V. ornata. Therefore, the combination of wing and male genitalia characters can be considered for species diagnosis of the adult stages. The details of the adult characters are presented in Table 4.

Table 4.
Comparison of adult characteristics of known Vietnamella species.
The chorionic structure can be used for distinguishing between the four species V. sinensis [25], V. maculosa, V. thani [26], and V. nanensis sp. n. The eggs of Vietnamella show a unique body shape and the helmet-like shape of the polar cap. Differences exist in the details of the chorionic surface and the KCT shape (Table 3). Vietnamella nanensis sp. n. shows prominent protuberances on the posterior surface, and the egg size is the largest of the four species. The COI reconstruction showed the existence of six species of Vietnamella, in agreement with a previous study [26].
Author Contributions
Conceptualization, B.B. and C.A.; methodology, C.A., B.B. and L.M.J.; validation, C.A., M.S., L.M.J. and B.B.; investigation, C.A., M.S., L.M.J. and B.B.; resources, B.B., C.A. and L.M.J.; specimen curation, B.B., C.A., M.S. and L.M.J; data curation, C.A. and B.B.; writing—original draft preparation, C.A. and B.B.; writing—review and editing, M.S. and L.M.J.; visualization, M.S.; funding acquisition, B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Centre of Excellence on Biodiversity (BDC) Office of Higher Education Commission, grant number BDC-PG2-161004.
Acknowledgments
We are most grateful to our colleagues for assistance during field trips. We would like to thank the Department of Zoology for their assistance and use of their facilities. Bob Sites (University of Missouri) provided the early material of this species, and Jeff Webb & Xin Zhou (formerly of Canadian Center for DNA Barcoding) facilitated DNA sequencing for this material. B.B. was supported by Department of Zoology and International SciKU Branding (ISB), Faculty of Science at Kasetsart University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Soldán, T. Mayflies (Ephemeroptera): One of the earliest insect groups known to man. In Ephemeroptera & Plecoptera Biology-Ecology-Systematics; Landolt, P., Sartori, M., Eds.; Mauron+Tinguely & Lachat SA: Fribourg, Switzerland, 1997; pp. 511–513. [Google Scholar]
- Sartori, M.; Brittain, J.E. Order Ephemeroptera. In Ecology and General Biology, Vol I: Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J.H., Rogers, D.C., Eds.; Academic Press: New York, NY, USA, 2015; pp. 873–891. [Google Scholar]
- Barber-James, H.M.; Gattolliat, J.-L.; Sartori, M.; Hubbard, M.D. Global diversity of mayflies (Ephemeroptera, Insecta) in freshwater. Hydrobiologia 2008, 595, 339–350. [Google Scholar] [CrossRef]
- Macadam, C.R.; Stockan, J.A. More than just fish food: Ecosystem services provided by freshwater insects. Ecol. Entomol. 2015, 40, 113–123. [Google Scholar] [CrossRef]
- Jacobus, L.M.; Macadam, C.R.; Sartori, M. Mayflies (Ephemeroptera) and their contributions to ecosystem services. Insects 2019, 10, 170. [Google Scholar] [CrossRef]
- Beattie, A.; Ehrlich, P.R. Wild Solutions: How Biodiversity Is Money in the Bank; Yale University Press: New Haven, CT, USA, 2001; p. 239. [Google Scholar]
- Boonsoong, B.; Sangpradub, N.; Barbour, M.T. Development of rapid bioassessment approaches using benthic macroinvertebrates for Thai streams. Environ. Monit. Assess. 2009, 155, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Arimoro, F.O.; Muller, W.J. Mayfly (Insecta: Ephemeroptera) community structure as an indicator of the ecological status of a stream in the Niger Delta area of Nigeria. Environ. Monit. Assess. 2010, 166, 581–594. [Google Scholar] [CrossRef]
- Stepanian, P.M.; Entrekin, S.A.; Wainwright, C.E.; Mirkovic, D.; Tank, J.L.; Kelly, F. Declines in an abundant aquatic insect, the burrowing mayfly, across major North American waterways. PNAS. 2020, 117, 2987–2992. [Google Scholar] [CrossRef]
- Kluge, N.J.; Novikova, E.A. Occurrence of Anafroptilum Kluge 2012 (Ephemeroptera: Baetidae) in Oriental Region. Zootaxa 2017, 4282, 453–472. [Google Scholar] [CrossRef]
- Sutthinun, C.; Gattolliat, J.-L.; Boonsoong, B. A new species of Platybaetis Müller-Liebenau, 1980 (Ephemeroptera: Baetidae) from Thailand, with description of the imago of Platybaetis bishopi Müller-Liebenau, 1980. Zootaxa 2018, 4378, 85–97. [Google Scholar] [CrossRef]
- Kluge, N.J.; Suttinun, C. Review of the Oriental genus Indocloeon Müller-Liebenau 1982 (Ephemeroptera: Baetidae) with descriptions of two new species. Zootaxa 2020, 4779, 451–484. [Google Scholar] [CrossRef]
- Auychinda, C.; Sartori, M.; Boonsoong, B. Review of Notacanthella Jacobus & McCafferty, 2008 (Ephemeroptera: Ephemerellidae) in Thailand, with the redescription of Notacanthella commodema (Allen, 1971). Zootaxa 2020, 4731, 414–424. [Google Scholar]
- Boonsoong, B.; Braasch, D. Heptageniidae (Insecta, Ephemeroptera) of Thailand. ZooKeys 2013, 272, 61–93. [Google Scholar] [CrossRef] [PubMed]
- Boonsoong, B.; Sartori, M. A new species of Compsoneuriella Ulmer, 1939 (Ephemeroptera: Heptageniidae) from Thailand. Zootaxa 2015, 3936, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Sutthacharoenthad, W.; Sartori, M.; Boonsoong, B. Integrative taxonomy of Thalerosphyrus Eaton, 1881 (Ephemeroptera, Heptageniidae) in Thailand. J. Nat. Hist. 2019, 53, 1491–1514. [Google Scholar] [CrossRef]
- Boonsoong, B.; Sartori, M. The nymph of Gilliesia Peters & Edmunds, 1970 (Ephemeroptera: Leptophlebiidae), with description of a new species from Thailand. Zootaxa 2015, 3981, 253–263. [Google Scholar] [PubMed]
- Boonsoong, B.; Sartori, B. Sangpradubina, An astonishing new mayfly genus from Thailand (Ephemeroptera: Leptophlebiidae: Atalophlebiinae). Zootaxa 2016, 4169, 587–599. [Google Scholar] [CrossRef]
- Boonsoong, B.; Sartori, M. Review and integrative taxonomy of the genus Prosopistoma Latreille, 1833 (Ephemeroptera, Prosopistomatidae) in Thailand, with description of a new species. ZooKeys 2019, 825, 123–144. [Google Scholar] [CrossRef]
- Martynov, A.V.; Palatov, D.M.; Boonsoong, B. A new species of Dudgeodes Sartori, 2008 (Ephemeroptera: Teloganodidae) from Thailand. Zootaxa 2016, 4121, 545–554. [Google Scholar] [CrossRef]
- Piraonapicha, K.; Sangpradub, N. Description of nymphs and female subimago of Sparsorythus multilabeculatus Sroka & Soldán, 2008 (Ephemeroptera: Tricorythidae) associated with male imago based on DNA sequence data. Zootaxa 2019, 4695, 501–515. [Google Scholar]
- Jacobus, L.M.; McCafferty, W.P. Reevaluation of the phylogeny of the Ephemeroptera infraorder Pannota (Furcatergalia), with adjustments to higher classification. Trans. Am. Entomol. Soc. 2006, 132, 81–90. [Google Scholar]
- Ogden, T.H.; Breinholt, J.W.; Bybee, S.M.; Miller, D.B.; Sartori, M.; Shiozawa, D.; Whiting, M.F. Mayfly phylogenomics: Initial evaluation of anchored hybrid enrichment data for the order Ephemeroptera. Zoosymposia 2019, 16, 167–181. [Google Scholar]
- Tshernova, O.A. Some new species of mayflies from Asia (Ephemeroptera, Heptageniidae, Ephemerellidae) (in Russian). Rev. Entomol. URSS 1972, 51, 604–614. [Google Scholar]
- Hu, Z.; Ma, Z.X.; Luo, J.Y.; Zhou, C.F. Redescription and commentary on the Chinese mayfly Vietnamella sinensis (Ephemeroptera: Vietnamellidae). Zootaxa 2017, 4286, 381–390. [Google Scholar] [CrossRef]
- Auychinda, C.; Sartori, M.; Boonsoong, B. Vietnamellidae (Insecta, Ephemeroptera) of Thailand. ZooKeys 2020, 902, 17–36. [Google Scholar] [CrossRef]
- Selvakumar, C.; Sinha, B.; Vasanth, M.; Subramanian, K.A.; Sivaramakrishnan, K.G. A new record of monogeneric family Vietnamellidae (Insecta: Ephemeroptera) from India. J. Asia-Pac. Entomol. 2018, 21, 994–998. [Google Scholar] [CrossRef]
- Hwang, J.M.; Seateun, S.; Nammanivong, M.; Bae, Y.J. Ephemeroptera fauna of Nam Et National Biodiversity Conservation Area in Laos. Bull. Entomol. Res. 2010, 26, 77–80. [Google Scholar]
- Jacobus, L.M.; McCafferty, W.P.; Sites, R.W. Significant range extensions for Kangella and Vietnamella (Ephemeroptera: Ephemerellidae, Vietnamellidae). Entomol. News. 2005, 116, 268–270. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Hsu, Y.C. New Chinese mayflies from Kiangsi Province (Ephemeroptera). Peking Nat. Hist. Bull. 1936, 10, 319–326. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).