Apremilast Pharmacogenomics in Russian Patients with Moderate-to-Severe and Severe Psoriasis
Abstract
1. Introduction
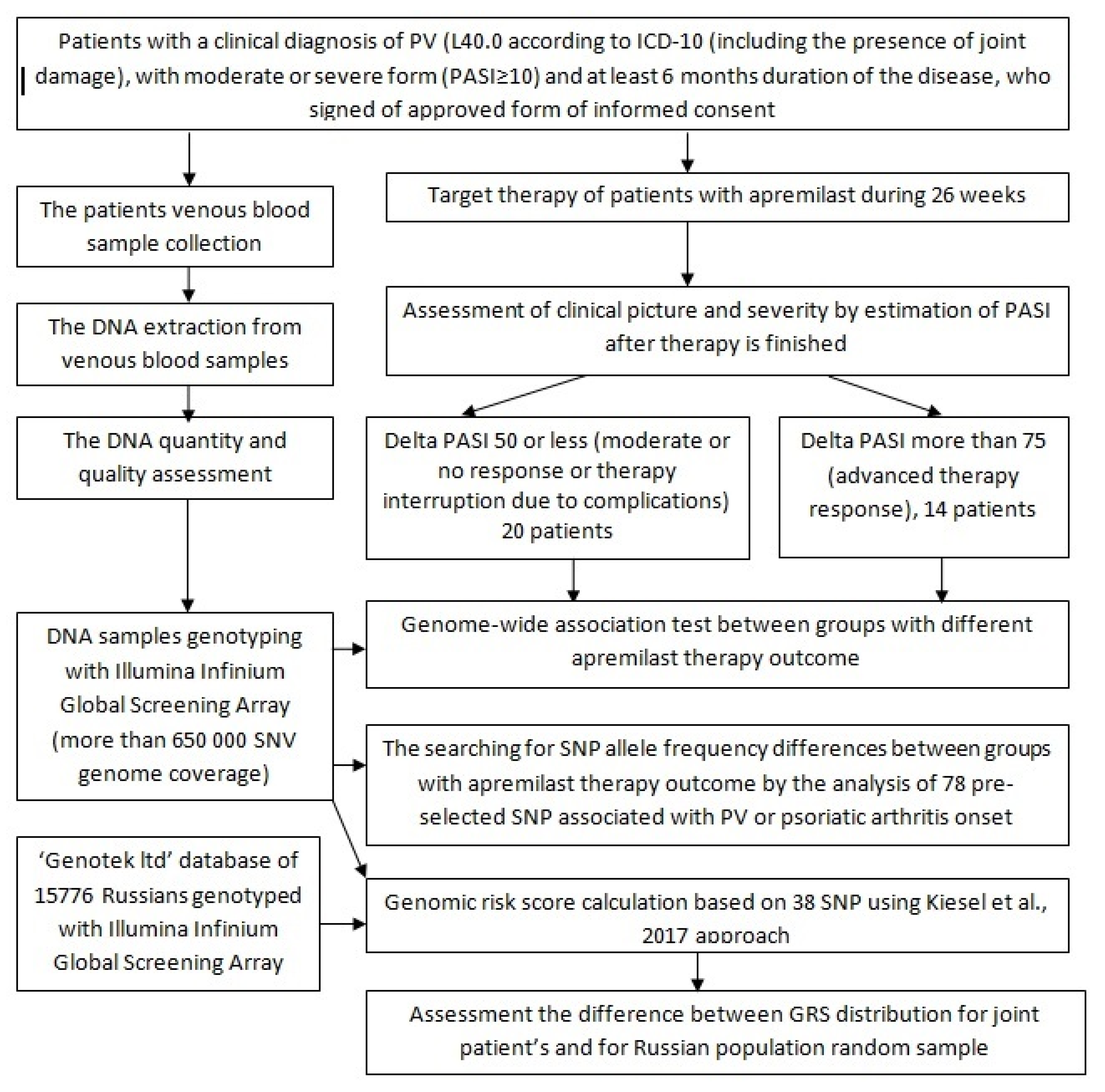
2. Materials and Methods
2.1. Clinical Characteristics of Patients
2.2. Target Psoriasis Therapy
2.3. Sample Collection
2.4. Genotyping
2.5. Data Analyses
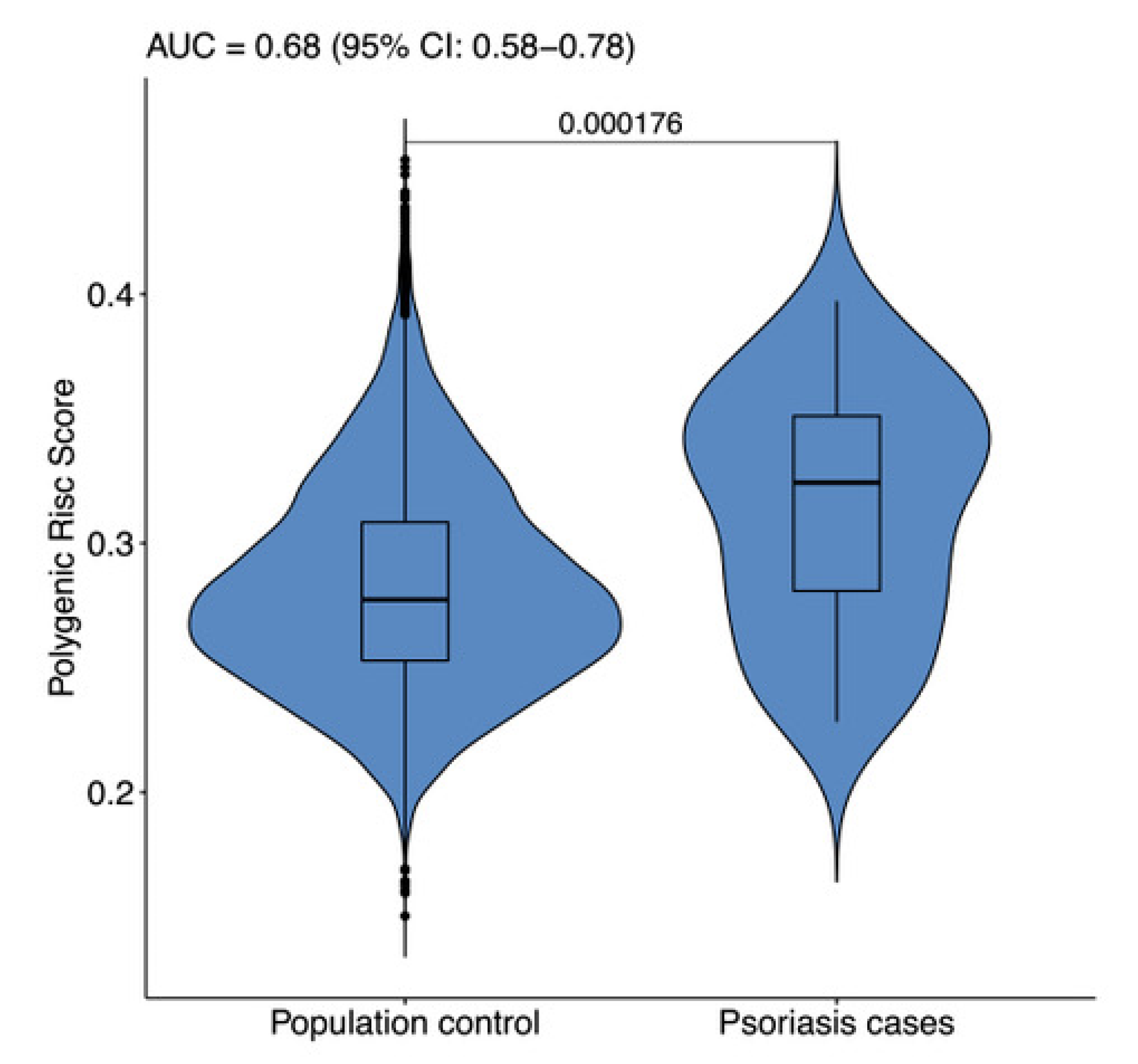
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| cAMP | Cyclic adenosine monophosphate |
| GRC | Global genetic risk score |
| GWAS | Genome-wide association studies |
| PASI | Psoriasis area severity index |
| PDE4 | phosphodiesterase 4 |
| PA | Psoriasis arthritis |
| PV | Psoriasis vulgaris |
| RA | Rheumatoid arthritis |
| STAT | signal transduction and activator of transcription |
References
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation and Treatment of Psoriasis. A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Okada, Y. The current landscape of psoriasis genetics in 2020. J. Dermatol. Sci. 2020, 99, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Kubanov, A.A.; Artamonova, O.G.; Karamova, A.E.; Vasileva, E.L.; Deryabin, D.G. Skin Lesions Cytokines in Moderate and Severe Psoriasis as Predictors for the Type 4 Phosphodiesterase inhibitor (Apremilast) Therapy Effectiveness. Ann. Russ. Acad. Med. Sci. 2020, 75, 500–507. [Google Scholar] [CrossRef]
- Stuart, P.E.; Nair, R.P.; Tsoi, L.C.; Tejasvi, T.; Das, S.; Kang, H.M.; Ellinghaus, E.; Chandran, V.; Callis-Duffin, K.; Ike, R.; et al. Genome-wide Association Analysis of Psoriatic Arthritis and Cutaneous Psoriasis Reveals Differences in Their Genetic Architecture. Am. J. Hum. Genet. 2015, 97, 816–836. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, J.C.; Ilchysyn, A. HLA antigens in psoriasis and psoriatic arthritis. J. Med. Genet. 1985, 22, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.T.; Nair, R.P.; Guo, S.-W.; Henseler, T.; Christophers, E.; Voorhees, J.J. The Genetics of Psoriasis. Arch. Derm. 1994, 130, 216–224. [Google Scholar] [CrossRef]
- Elder, J.T.; Nair, R.P.; Henseler, T.; Jenisch, S.; Stuart, P.; Chia, N.; Christophers, E.; Voorhees, J.J. The Genetics of Psoriasis 2001: The Odyssey Continues. Arch. Derm. 2001, 137, 1447–1454. [Google Scholar] [CrossRef]
- Nair, R.P.; Duffin, K.C.; Helms, C.; Ding, J.; Stuart, P.E.; Goldgar, D.; Gudjonsson, J.E.; Li, Y.; Tejasvi, T.; Feng, B.-J.; et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nat. Genet. 2009, 41, 199–204. [Google Scholar] [CrossRef]
- Nair, R.P.; Ruether, A.; Stuart, P.E.; Jenisch, S.; Tejasvi, T.; Hiremagalore, R.; Schreiber, S.; Kabelitz, D.; Lim, H.W.; Voorhees, J.J.; et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J. Invest. Derm. 2008, 128, 1653–1661. [Google Scholar] [CrossRef]
- Julia, A.; Tortosa, R.; Hernanz, J.M.; Canete, J.D.; Fonseca, E.; Ferrandiz, C.; Unamuno, P.; Puig, L.; Fernandez-Sueiro, J.L.; Sanmarti, R.; et al. Risk variants for psoriasis vulgaris in a large case-control collection and association with clinical subphenotypes. Hum. Mol. Genet. 2012, 21, 4549–4557. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Stuart, P.E.; Tian, C.; Gudjonsson, J.E.; Das, S.; Zawistowski, M.; Ellinghaus, E.; Barker, J.N.; Chandran, V.; Dand, N.; et al. Large scale meta-analysis characterizes genetic architecture for common psoriasis associated variants. Nat. Commun. 2017, 24, 15382. [Google Scholar] [CrossRef]
- Chen, H.; Poon, A.; Yeung, C.; Helms, C.; Pons, J.; Bowcock, A.M.; Kwok, P.Y.; Liao, W. A genetic risk score combining ten psoriasis risk loci improves disease prediction. PLoS ONE 2011, 6, e19454. [Google Scholar] [CrossRef]
- Yin, X.; Cheng, H.; Lin, Y.; Wineinger, N.E.; Zhou, F.; Sheng, Y.; Yang, C.; Li, P.; Li, F.; Shen, C.; et al. A Weighted Polygenic Risk Score Using 14 Known Susceptibility Variants to Estimate Risk and Age Onset of Psoriasis in Han Chinese. PLoS ONE 2015, 10, e0125369. [Google Scholar] [CrossRef]
- Stawczyk-Macieja, M.; Rębała, K.; Szczerkowska-Dobosz, A.; Wysocka, J.; Cybulska, L.; Kapińska, E.; Haraś, A.; Miniszewska, P.; Nowicki, R. Evaluation of psoriasis genetic risk based on five susceptibility markers in a population from northern Poland. PLoS ONE 2016, 11, e0163185. [Google Scholar] [CrossRef]
- Kisiel, B.; Kisiel, K.; Szymański, K.; Mackiewicz, W.; Biało-Wójcicka, E.; Uczniak, S.; Fogtman, A.; Iwanicka-Nowicka, R.; Koblowska, M.; Kossowska, H.; et al. The association between 38 previously reported polymorphisms and psoriasis in a Polish population: High predicative accuracy of a genetic risk score combining 16 loci. PLoS ONE 2017, 12, e0179348. [Google Scholar] [CrossRef] [PubMed]
- Shavit, E.; Shear, N.H. An update on the safety of apremilast for the treatment of plaque psoriasis. Expert Opin. Drug Saf. 2020, 19, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Kubanov, A.A.; Karamova, A.E.; Artamonova, O.G. New opportunities in the treatment of psoriasis and psoriatic arthritis. Rheumatol. Sci. Pract. 2018, 56, 722–726. [Google Scholar] [CrossRef]
- Schett, G.; Sloan, V.S.; Stevens, R.M.; Schafer, P. Apremilast: A novel PDE4 inhibitor in the treatment of autoimmune and inflammatory diseases. Adv. Musculoskelet Dis. 2010, 2, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Morin, S.; Rioux, G.; Séguin, R.; Loing, E.; Pouliot, R.A. Tissue-Engineered Human Psoriatic Skin Model to Investigate the Implication of cAMP in Psoriasis: Differential Impacts of Cholera Toxin and Isoproterenol on cAMP Levels of the Epidermis. Int. J. Mol. Sci. 2020, 21, 5215. [Google Scholar] [CrossRef] [PubMed]
- Kubanov, A.A.; Solomka, V.S.; Karamova, A.E.; Verbenko, D.A.; Vasilieva, E.L.; Artamonova, O.G. The effect of apremilast therapy on skin cytokine levels in patients with psoriasis. Russ. Open Med. J. 2020, 9, 310–316. [Google Scholar] [CrossRef]
- Giacomini, K.M.; Yee, S.W.; Mushiroda, T.; Weinshilboum, R.M.; Ratain, M.J.; Kubo, M. Genome-wide association studies of drug response and toxicity: An opportunity for genome medicine, Nat. Rev. Drug Discov. 2017, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.R.; Johnson, T.; Warren, L.; Hughes, A.R.; Chissoe, S.L.; Xu, C.F.; Waterworth, D.M. The genetics of drug efficacy: Opportunities and challenges. Nat. Rev. Genet. 2016, 17, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ovejero-Benito, M.C.; Prieto-Pérez, R.; Llamas-Velasco, M.; Muñoz-Aceituno, E.; Reolid, A.; Saiz-Rodríguez, M.; Belmonte, C.; Román, M.; Ochoa, D.; Talegón, M.; et al. Polymorphisms associated with adalimumab and infliximab response in moderate-to-severe plaque psoriasis. Pharmacogenomics 2018, 19, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kozina, A.A.; Okuneva, E.G.; Baryshnikova, N.V.; Kondakova, O.B.; Nikolaeva, E.A.; Fedoniuk, I.D.; Mikhailova, S.V.; Krasnenko, A.Y.; Stetsenko, I.F.; Plotnikov, N.A.; et al. Neuronal ceroid lipofuscinosis in the Russian population: Two novel mutations and the prevalence of heterozygous carriers. Mol. Genet. Genom. Med. 2020, 8, e1228. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Bidwell, J.; Keen, L.; Gallagher, G.; Kimberly, R.; Huizinga, T.; McDermott, M.F.; Oksenberg, J.; McNicholl, J.; Pociot, F.; Hardt, C.; et al. Cytokine gene polymorphism in human disease: On-line databases. Genes Immun. 1999, 1, 3–19. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Sun, D. Associations between STAT Gene Polymorphisms and Psoriasis in Northeastern China. Dermatology 2017, 233, 30–36. [Google Scholar] [CrossRef]
- Sasayama, D.; Hori, H.; Iijima, Y.; Teraishi, T.; Hattori, K.; Ota, M.; Fujii, T.; Higuchi, T.; Amano, N.; Kunugi, H. Modulation of cortisol responses to the DEX/CRH test by polymorphisms of the interleukin-1beta gene in healthy adults. Behav. Brain Funct. 2011, 7, 23. [Google Scholar] [CrossRef]
- Hebert, H.L.; Bowes, J.; Smith, R.L.; McHugh, N.J.; Barker, J.; Griffiths, C.; Barton, A.; Warren, R. Polymorphisms in IL-1B distinguish between psoriasis of early and late onset. J. Investig. Dermatol. 2014, 134, 1459–1462. [Google Scholar] [CrossRef]
- Prieto-Pérez, R.; Cabaleiro, T.; Daudén, E.; Ochoa, D.; Roman, M.; Abad-Santos, F. Genetics of psoriasis and pharmacogenetics of biological drugs. Autoimmune Dis. 2013, 2013, 613086. [Google Scholar] [CrossRef]
- Bouzid, D.; Fourati, H.; Amouri, A.; Marques, I.; Abida, O.; Tahri, N.; Penha-Gonçalves, C.; Masmoudi, M. Autoimmune diseases association study with the KIAA1109-IL2-IL21 region in a Tunisian population. Mol. Biol. Rep. 2014, 41, 7133–7139. [Google Scholar] [CrossRef] [PubMed]
- Białecka, M.; Ostasz, R.; Kurzawski, M.; Klimowicz, A.; Fabiańczyk, H.; Bojko, P.; Dziedziejko, V.; Safranow, K.; Droździk, M. IL6 -174G>C polymorphism is associated with an increased risk of psoriasis but not response to treatment. Exp Derm. 2015, 24, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Craven, N.; Jackson, C.; Kirby, B.; Perrey, C.; Pravica, V.; Hutchinson, I.; Griffiths, C. Cytokine gene polymorphisms in psoriasis. Br. J. Dermatol. 2001, 144, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Associations between interleukin-10 polymorphisms and susceptibility to psoriasis: A meta-analysis. Inflamm. Res. 2012, 61, 657–663. [Google Scholar] [CrossRef]
- Elder, J.T. Genome-wide association scan yields new insights into the immunopathogenesis of psoriasis. Genes Immun. 2009, 10, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Li, Y.; Yan, C.; Callis-Duffin, K.P.; Matsunami, N.; Garcia, V.E.; Cargill, M.; Civello, D.; Bui, N.; Catanese, J.J.; et al. Variants in the 5q31 cytokine gene cluster are associated with psoriasis. Genes Immun. 2008, 9, 176–181. [Google Scholar] [CrossRef]
- Murdaca, G.; Negrini, S.; Magnani, O.; Penza, E.; Pellecchio, M.; Puppo, F. Impact of pharmacogenomics upon the therapeutic response to etanercept in psoriasis and psoriatic arthritis. Expert Opin. Drug Saf. 2017, 16, 1173–1179. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Spain, S.L.; Knight, J.; Ellinghaus, E.; E Stuart, P.; Capon, F.; Ding, J.; Pullinger, C.R.; Tejasvi, T.; Gudjonsson, J.E.; et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat. Genet. 2012, 44, 1341–1348. [Google Scholar] [CrossRef]
- Strange, A.; Capon, F.; Spencer, C.C.A.; Knight, J.; Weale, M.E.; Allen, M.H.; Barton, A.; Band, G.; Bellenguez, C.; Bergboer, J.G.M.; et al. Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium; A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat. Genet. 2010, 42, 985–990. [Google Scholar] [CrossRef]
- Liu, Y.; Helms, C.; Liao, W.; Zaba, L.C.; Duan, S.; Gardner, J.; Wise, C.; Miner, A.; MJ Malloy, M.J.; Pullinger, C.R.; et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008, 4, e1000041. [Google Scholar] [CrossRef]
- Stuart, P.E.; Nair, R.P.; Ellinghaus, E.; Ding, J.; Tejasvi, T.; Gudjonsson, J.E.; Li, Y.; Weidinger, S.; Eberlein, B.; Gieger, C.; et al. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat. Genet. 2010, 42, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zhu, C.-Y.; Shi, G.; Fan, Y. Association of IL23R polymorphisms with psoriasis and psoriatic arthritis: A meta-analysis. Inflamm. Res. 2012, 61, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.-H.; Zou, Y.-F.; Feng, X.-L.; Li, J.; Wang, F.; Pan, F.-M.; Ye, D.-Q. Meta-analysis of TYK2 gene polymorphisms association with susceptibility to autoimmune and inflammatory diseases. Mol. Biol. Rep. 2011, 38, 4663–4672. [Google Scholar] [CrossRef]
- Julia, A.; Rodriguez-Moreno, J.; Fernandez-Sueiro, J.L.; Gratacos, J.; Queiro, R.; Montilla, C.; Torre-Alonso, J.C.; Pérez-Venegas, J.J.; Manrique-Arija, S.; Muñoz-Fernández, S.; et al. PDE3A-SLCO1C1 locus is associated with response to anti-tumor necrosis factor therapy in psoriatic arthritis. Pharmacogenomics 2014, 15, 1763–1769. [Google Scholar] [CrossRef]
- Osmola-Mańkowska, A.; Teresiak-Mikołajczak, E.; Skrzypczak-Zielińska, M.; Adamski, Z. Genetic polymorphism in psoriasis and its meaning for the treatment efficacy in the future. Postepy Derm. Alergol. 2018, 35, 331–337. [Google Scholar] [CrossRef]
- Newcombe, P.J.; Verzilli, C.; Casas, J.P.; Hingorani, A.D.; Smeeth, L.; Whittaker, J.C. Multilocus Bayesian meta-analysis of gene-disease associations. Am. J. Hum. Genet. 2009, 84, 567–580. [Google Scholar] [CrossRef]
- Cabaleiro, T.; Román, M.; Gallo, E.; Ochoa, D.; Tudelilla, F.; Talegón, M.; Prieto-Pérez, R.; García-Díez, A.; Daudén, E.; Abad-Santos, F. Association between psoriasis and polymorphisms in the TNF, IL12B, and IL23R genes in Spanish patients. Eur. J. Derm. 2013, 23, 40–45. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, H.; Qu, L.; Fu, X.; Yu, Y.; Yu, G.; Tian, H.; Yu, Y.; Sun, D.; Peng, J.; et al. Investigation of 20 non-HLA (human leucocyte antigen) psoriasis susceptibility loci in Chinese patients with psoriatic arthritis and psoriasis vulgaris. Br. J. Dermatol. 2013, 168, 1060–1065. [Google Scholar] [CrossRef]
- Bowes, J.; Orozco, G.; Flynn, E.; Ho, P.; Brier, R.; Marzo-Ortega, H.; Coates, L.; McManus, R.; Ryan, A.W.; Kane, D.; et al. Confirmation of TNIP1 and IL23A as susceptibility loci for psoriatic arthritis. Ann. Rheum. Dis. 2011, 70, 1641–1644. [Google Scholar] [CrossRef]
- Stuart, P.E.; Tejasvi, T.; Shaiq, P.A.; Kullavanijaya, P.; Qamar, R.; Raja, G.K.; Li, Y.; Voorhees, J.J.; Abecasis, G.R.; Elder, J.T.; et al. A Single SNP Surrogate for Genotyping HLA-C*06:02 in Diverse Populations. J. Investig. Dermatol. 2015, 135, 1177–1180. [Google Scholar] [CrossRef]
- Popadic, S.; Savic, E.; Markovic, M.; Ramic, Z.; Medenica, L.; Pravica, V.; Spuran, Z.; Trajkovic, V.; Popadic, D. TNF, IL12B, and IFNG Gene Polymorphisms in Serbian Patients with Psoriasis. Ann. Derm. 2015, 27, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Bowes, J.; Ho, P.; Flynn, E.; Ali, F.; Marzo-Ortega, H.; Coates, L.C.; Warren, R.B.; McManus, R.; Ryan, A.W.; Kane, D.; et al. Comprehensive assessment of rheumatoid arthritis susceptibility loci in a large psoriatic arthritis cohort. Ann. Rheum. Dis. 2012, 71, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Bowes, J.; Budu-Aggrey, A.; Huffmeier, U.; Uebe, S.; Steel, K.; Hebert, H.L.; Wallace, C.; Massey, J.; Bruce, I.N.; Bluett, J.; et al. Dense genotyping of immune-related susceptibility loci reveals new insights into the genetics of psoriatic arthritis. Nat. Commun. 2015, 6, 6046. [Google Scholar] [CrossRef] [PubMed]
- Caldarola, G.; Sgambato, A.; Fanali, C.; Moretta, G.; Farina, M.; Lucchetti, D.; Peris, K.; De Simone, C.D. HLA-Cw6 allele, NFkB1 and NFkBIA polymorphisms play no role in predicting response to etanercept in psoriatic patients. Pharm. Genom. 2016, 26, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, H.; Zuo, X.; Sheng, Y.-J.; Zhou, F.; Tang, X.-F.; Tang, H.-Y.; Gao, J.-P.; Zhang, Z.; He, S.-M.; et al. Association analyses identifying two common susceptibility loci shared by psoriasis and systemic lupus erythematosus in the Chinese Han population. J. Med. Genet. 2013, 50, 812–818. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hur, M.S.; Choi, B.G.; Kim, M.J.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. A preliminary study of new single polymorphisms in the T helper type 17 pathway for psoriasis in the Korean population. Clin. Exp. Immunol. 2017, 187, 251–258. [Google Scholar] [CrossRef]
- Swindell, W.R.; Sarkar, M.K.; Stuart, P.E.; Voorhees, J.J.; Elder, J.T.; Johnston, A.; Gudjonsson, J.E. Psoriasis drug development and GWAS interpretation through in silico analysis of transcription factor binding sites. Clin. Transl. Med. 2015, 4, 13. [Google Scholar] [CrossRef]
- Nikamo, P.; Lysell, J.; Ståhle, M. Association with Genetic Variants in the IL-23 and NF-κB Pathways Discriminates between Mild and Severe Psoriasis Skin Disease. J. Investig. Dermatol. 2015, 135, 1969–1976. [Google Scholar] [CrossRef]
- Smolnikova, M.V.; Freidin, M.B.; Barilo, A.A.; Smirnova, S.V. Analysis of association between cytokine gene polymorphisms and psoriatic disease in Russians of East Siberia. Meta Gene 2019, 19, 60–64. [Google Scholar] [CrossRef]
- Yin, X.; Low, H.Q.; Wang, L.; Li, Y.; Ellinghaus, C.E.E.; Han, J.; Estivill, X.; Sun, L.; Zuo, X.; Shen, C.; et al. Genome-wide meta-analysis identifies multiple novel associations and ethnic heterogeneity of psoriasis susceptibility. Nat. Commun. 2015, 6, 6916. [Google Scholar] [CrossRef]
- Papp, K.; Reich, K.; Leonardi, C.L.; Kircik, L.; Chimenti, S.; Langley, R.G.; Korman, N.J. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J. Am. Acad. Dermatol. 2015, 73, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Cather, J.; Gooderham, M.; Poulin, Y.; Mrowietz, U.; Ferrandiz, C.; Day, R.M. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: A phase III, randomized controlled trial (ESTEEM 2). Br. J. Dermatol. 2015, 173, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Vujic, I.; Herman, R.; Sanlorenzo, M.; Posch, C.; Monshi, B.; Rappersberger, K.; Richter, L. Apremilast in psoriasis—A prospective real-world study. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Kleyn, C.E.; Conrad, C.; Sator, P.G.; Ståhle, M.; Eyerich, K.; Cordey, M. Characteristics and Outcomes of Patients Treated With Apremilast in the Real World: Results From the APPRECIATE Study. J. Eur. Acad. Dermatol. Venereol. 2020. [CrossRef]
- De, A.; Das, S.; Dhoot, D.; Sarda, A. Real-World Insight on Apremilast Therapy in Patients with Plaque Psoriasis: Indian Experience. Indian J. Derm. 2020, 65, 396–400. [Google Scholar] [CrossRef]
- Calautti, E.; Avalle, L.; Poli, V. Psoriasis: A STAT3-Centric View. Int. J. Mol. Sci. 2018, 19, 171. [Google Scholar] [CrossRef]
- Reich, K.; Mossner, R.; Konig, I.R.; Westphal, G.; Ziegler, A.; Neumann, C. Promoter polymorphisms of the genes encoding tumor necrosis factor-α and interleukin-1β are associated with different subtypes of psoriasis characterized by early and late disease onset. J. Investig. Dermatol. 2002, 118, 155–163. [Google Scholar] [CrossRef]
- Johansson, Å.; Rask-Andersen, M.; Karlsson, T.; Ek, W.E. Genome-wide association analysis of 350,000 Caucasians from the UK Biobank identifies novel loci for asthma, hay fever and eczema. Hum. Mol. Genet. 2019, 28, 4022–4041. [Google Scholar] [CrossRef]
- Black, S.; Teixeira, A.S.; Loh, A.X.W.; Vinall, L.; Holloway, J.W.; Hardy, R.; Swallow, D.M. Contribution of functional variation in the IL13 gene to allergy, hay fever and asthma in the NSHD longitudinal 1946 birth cohort. Allergy 2009, 64, 1172–1178. [Google Scholar] [CrossRef]
- Safrany, E.; Szabo, M.; Szell, M.; Kemeny, L.; Sumegi, K.; Melegh, B.I.; Magyari, L.; Matyas, P.; Figler, M.; Weber, A.; et al. Difference of interleukin-23 receptor gene haplotype variants in ulcerative colitis compared to Crohn’s disease and psoriasis. Inflamm. Res. 2013, 62, 195–200. [Google Scholar] [CrossRef]
- Kara, S.; Pirela-Morillo, G.A.; Gilliam, C.T.; Wilson, G.D. Identification of novel susceptibility genes associated with seven autoimmune disorders using whole genome molecular interaction networks. J. Autoimmun. 2019, 97, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.G.; O’Neill, L.A.G. Krebs cycle rewired for macrophage and dendritic cell effector functions. FEBS Lett. 2017, 591, 2992–3006. [Google Scholar] [CrossRef] [PubMed]
- Zasłona, Z.; O’Neill, L.A.J. Cytokine-like Roles for Metabolites in Immunity. Mol. Cell 2020, 78, 814–823. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef]
- Sands, W.A.; Palmer, T.M. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal. 2008, 20, 460–466. [Google Scholar] [CrossRef]
| Gene or Nearby Genomic Region | SNP Reference Sequence | Most Frequent Allele Variant | Minimal Frequency Allele Variants | Allele Frequency in Efficient Target Therapy Response Group | Allele Frequency in Lowered Target Therapy Response Group | Allele Frequency in the Population of European Origin | References |
|---|---|---|---|---|---|---|---|
| IL1A, IL1B | rs12469600 | T | C | 0.79 | 0.70 | 0.752 | [25] |
| IL1B | rs1143633 * | C | T | 0.46 | 0.70 | 0.666 | [29] |
| IL1B | rs11676014 | G | A | 0.68 | 0.60 | 0.551 | [26] |
| IL1B 2KB upstream | rs16944 | G | A | 0.75 | 0.55 | 0.65 | [28] |
| IL1B 500B downstream | rs2853550 | G | A | 0.96 | 0.87 | 0.954 | [28] |
| IL2 2KB upstream | rs2069762 | A | C | 0.75 | 0.62 | 0.708 | [31] |
| IL2 | rs2069778 | G | A | 0.64 | 0.77 | 0.849 | [31] |
| IL-6 | rs1800795 | C | G | 0.46 | 0.43 | 0.444 | [32] |
| IL10 2KB upstream | rs1800871 | G | A | 0.79 | 0.62 | 0.76 | [34] |
| IL10 2KB upstream | rs1800872 | G | T | 0.79 | 0.62 | 0.76 | [34] |
| IL10 2KB upstream | rs1800896 | T | C | 0.50 | 0.62 | 0.547 | [33] |
| IL10RA | rs947889 | T | C | 0.46 | 0.62 | 0.54 | Live Ref SNPs |
| noncoding (IL-12B) | rs2082412 | G | A | 0.89 | 0.85 | 0.774 | [35] |
| IL13, IL4 | rs1800925 | C | T | 0.71 | 0.75 | 0.822 | [36] |
| IL13, IL4 | rs20541 * | G | A | 0.57 | 0.80 | 0.793 | [36] |
| IL4 2KB upstream | rs2243250 | C | T | 0.57 | 0.75 | 0.832 | [59] |
| IL13 3′UTR | rs848 | C | A | 0.57 | 0.77 | 0.787 | [36] |
| IL17A | rs10484879 | G | T | 0.93 | 0.82 | 0.75 | [37] |
| IL17A 2KB upstream | rs2275913 | G | A | 0.75 | 0.55 | 0.62 | [37] |
| IL-17F | rs763780 | T | (C) | 0.89 | 1 | 0.942 | [37] |
| IL17RA: 2KB upstream | rs4819554 | A | G | 0.79 | 0.90 | 0.792 | [37] |
| IL22 | rs12307915 | T | C | 0.86 | 0.77 | 0.808 | [58] |
| IL22 2kb upstream | rs2227473 | C | T | 0.86 | 0.77 | 0.81 | [58] |
| IL22 2kb upstream | rs2227483 | A | T | 0.46 | 0.60 | 0.547 | [58] |
| IL23R 500B downstream | rs9988642 | T | C | 0.96 | 0.95 | 0.928 | [38] |
| IL23R | rs12564022 | C | T | 0.61 | 0.72 | 0.702 | [60] |
| IL-23R | rs11209026 | G | A | 0.96 | 0.95 | 0.938 | [42] |
| IL23R | rs2201841 * | A | G (T) | 0.5 | 0.75 | 0.7 | [35] |
| IL23R | rs2295359 | G | A | 0.64 | 0.55 | 0.686 | [60] |
| IL23R | rs11209032 | G | A | 0.57 | 0.65 | 0.666 | [42] |
| IL28RA | rs4649203 | A | G | 0.82 | 0.77 | 0.725 | [48] |
| TYK2 | rs12720356 | A | C(G) | 0.96 | 0.97 | 0.908 | [43] |
| TYK2 | rs280519 | A | G(C) | 0.50 | 0.55 | 0.505 | [43] |
| TYK2 | rs2304256 | C | A | 0.79 | 0.80 | 0.738 | [43] |
| TYK2 | rs34536443 | G | C | 1 | 1 | 0.971 | [43] |
| PDE3A-SLC01C1 | rs3794271 | A | G | 0.54 | 0.60 | 0.635 | [44,45] |
| PDE4D (PART1) | rs152312 | G | (A, T) | 0.96 | 0.92 | 0.9 | [46] |
| PDE4D | rs2910829 | A | G | 0.71 | 0.52 | 0.56 | [46] |
| TNFa | rs1799724 | C | T | 0.79 | 0.82 | 0.906 | [37] |
| TNFa | rs1799964 | T | C | 0.82 | 0.72 | 0.79 | [47] |
| TNFa | rs1800629 * | G | A | 0.82 | 0.97 | 0.866 | [47] |
| TNF 2KB upstream | rs361525 | G | A | 0.93 | 0.92 | 0.936 | [47] |
| TNFAIP3 | rs610604 | T | G | 0.64 | 0.67 | 0.662 | [37] |
| STAT1 | rs2083482 | T | C | 0.71 | 0.62 | 0.496 | [57] |
| STAT2 | rs2020854 | T | C | 0.93 | 0.90 | 0.931 | [53] |
| STAT2 | rs2066807 | C | G | 0.93 | 0.90 | 0.933 | [8] |
| STAT2 | rs2066808 | A | G | 0.93 | 0.90 | 0.93 | [58] |
| STAT3 | rs2293152 | C | G | 0.50 | 0.60 | 0.596 | [27] |
| STAT3 | rs8074524 | C | T | 0.86 | 0.77 | 0.8 | Live Ref SNPs |
| STAT3 | rs744166 | A | G | 0.68 | 0.55 | 0.585 | [56] |
| STAT4 | rs7574865 | G | T | 0.71 | 0.85 | 0.77 | [52] |
| STAT4 | rs10181656 | C | G | 0.71 | 0.85 | 0.766 | [52] |
| NFkB1 | rs28362491 | INS | DEL | 0.54 | 0.60 | 0.595 | [54] |
| NF-kB1A | rs2145623 | G | C | 0.82 | 0.70 | 0.724 | [30] |
| NF-kB1A | rs8016947 | T | G | 0.50 | 0.47 | 0.471 | [55] |
| NFkB1A | rs12586317 | T | C | 0.61 | 0.65 | 0.737 | [41] |
| REL | rs62149416 | T | C | 0.61 | 0.80 | 0.636 | [53] |
| REL | rs702873 | C | T | 0.50 | 0.70 | 0.553 | [52] |
| RNF114 | rs495337 | G | A | 0.61 | 0.62 | 0.602 | [41] |
| ZNF313 | rs2235617 | C | G | 0.61 | 0.65 | 0.602 | [39] |
| REV3L | rs240993 | C | A | 0.71 | 0.55 | 0.712 | [30] |
| IFNG | rs2430561 | A | T | 0.54 | 0.40 | 0.462 | [51] |
| CAST, ERAP1 | rs27524 | G | A | 0.54 | 0.65 | 0.634 | [48] |
| HLA-C | rs4406273 | G | A | 0.79 | 0.72 | 0.902 | [50] |
| NOS2 | rs4795067 | A | G | 0.50 | 0.57 | 0.641 | [41] |
| CYP3A5 | rs776746 | C | T | 1 | 0.92 | 0.943 | PharmGKB |
| TSC1 | rs1076160 | C | T | 0.71 | 0.52 | 0.489 | [55] |
| FBXL19 | rs10782001 | A | G | 0.54 | 0.62 | 0.641 | [41] |
| KCNH7 | rs17716942 | T | C | 0.96 | 0.90 | 0.839 | [39] |
| ANXA6 | rs17728338 | G | A | 0.96 | 0.87 | 0.93 | [35] |
| HCP5 | rs2395029 | T | G | 0.93 | 0.92 | 0.956 | [40] |
| COG6 | rs7993214 | C | T(G) | 0.57 | 0.62 | 0.657 | [40] |
| SDC4 | rs1008953 | C | T | 0.86 | 0.72 | 0.775 | [41] |
| RPS26 | rs12580100 | A | G | 0.93 | 0.85 | 0.867 | [41] |
| NONE | rs1975974 | A | G | 0.82 | 0.77 | 0.763 | [41] |
| LCE3D | rs4112788 | G | A | 0.68 | 0.62 | 0.644 | [8] |
| LOC105376976 | rs6809854 | A | G | 0.64 | 0.77 | 0.802 | [41] |
| LOC107984144 | rs10484554 | C | T | 0.68 | 0.60 | 0.857 | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbenko, D.A.; Karamova, A.E.; Artamonova, O.G.; Deryabin, D.G.; Rakitko, A.; Chernitsov, A.; Krasnenko, A.; Elmuratov, A.; Solomka, V.S.; Kubanov, A.A. Apremilast Pharmacogenomics in Russian Patients with Moderate-to-Severe and Severe Psoriasis. J. Pers. Med. 2021, 11, 20. https://doi.org/10.3390/jpm11010020
Verbenko DA, Karamova AE, Artamonova OG, Deryabin DG, Rakitko A, Chernitsov A, Krasnenko A, Elmuratov A, Solomka VS, Kubanov AA. Apremilast Pharmacogenomics in Russian Patients with Moderate-to-Severe and Severe Psoriasis. Journal of Personalized Medicine. 2021; 11(1):20. https://doi.org/10.3390/jpm11010020
Chicago/Turabian StyleVerbenko, Dmitry A., Arfenya E. Karamova, Olga G. Artamonova, Dmitry G. Deryabin, Alexander Rakitko, Alexandr Chernitsov, Anna Krasnenko, Artem Elmuratov, Victoria S. Solomka, and Alexey A. Kubanov. 2021. "Apremilast Pharmacogenomics in Russian Patients with Moderate-to-Severe and Severe Psoriasis" Journal of Personalized Medicine 11, no. 1: 20. https://doi.org/10.3390/jpm11010020
APA StyleVerbenko, D. A., Karamova, A. E., Artamonova, O. G., Deryabin, D. G., Rakitko, A., Chernitsov, A., Krasnenko, A., Elmuratov, A., Solomka, V. S., & Kubanov, A. A. (2021). Apremilast Pharmacogenomics in Russian Patients with Moderate-to-Severe and Severe Psoriasis. Journal of Personalized Medicine, 11(1), 20. https://doi.org/10.3390/jpm11010020








