Moving Beyond LDL-C and Non-HDL-C: Apolipoprotein B as the Stronger Lipid-Related Predictor of Coronary Artery Disease in Statin-Treated Patients
Abstract
1. Introduction
2. Materials and Methods
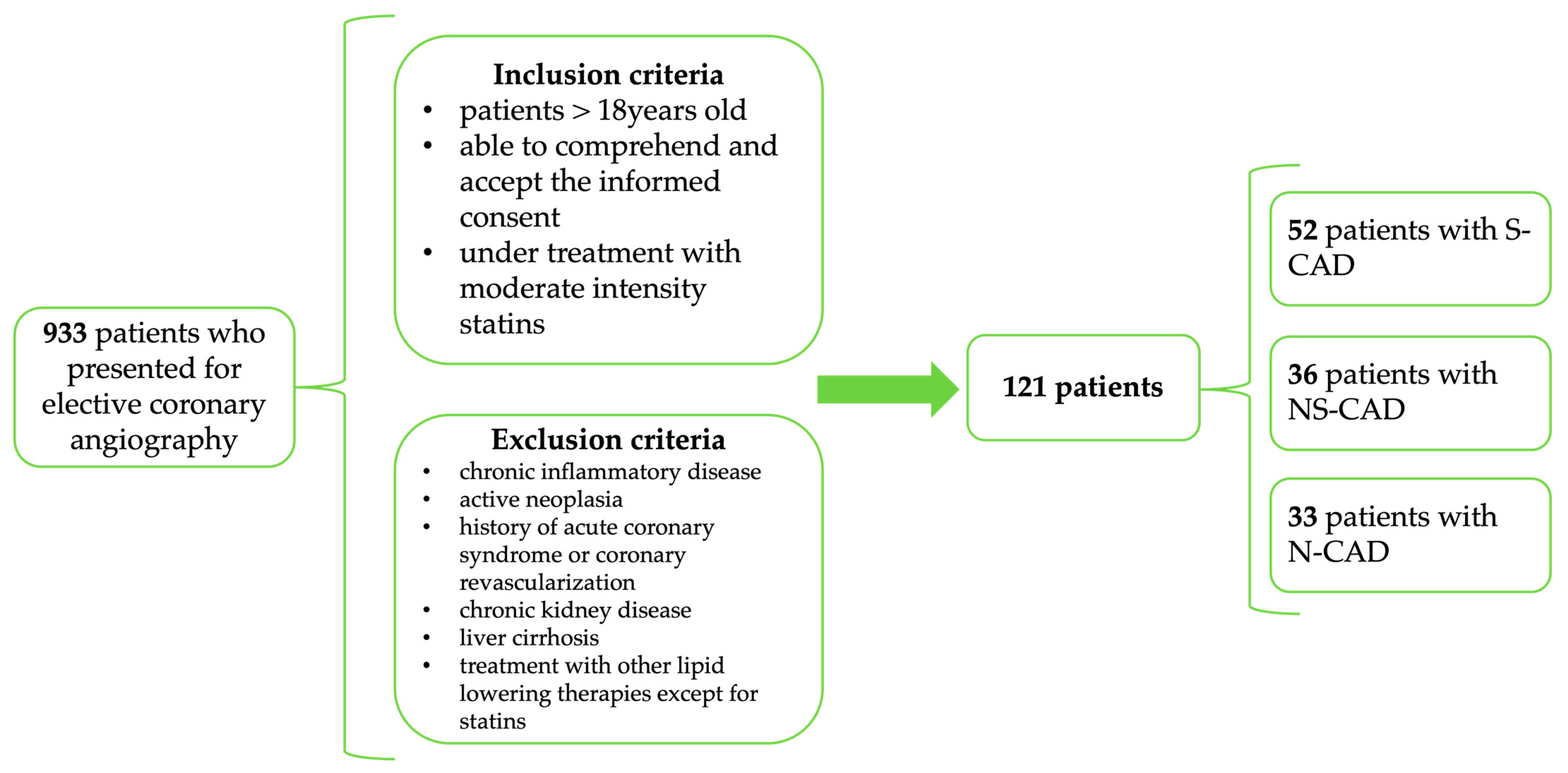
2.1. Study Design, Patient Enrollment, and Investigations
2.2. Statistical Analysis
2.3. Ethics
3. Results
3.1. Baseline Characteristics
3.2. Lipid Parameters
| Parameter | Overall | N-CAD | NS-CAD | S-CAD | p-Value |
|---|---|---|---|---|---|
| TC (mg/dL) | 165.81 ± 41.97 | 149.37 ± 27.45 | 161.12 ± 37.12 | 178.86 ± 48.30 | 0.006 |
| LDL-C (mg/dL) | 102.65 ± 38.38 | 86.40 ± 24.67 | 98.44 ± 34.52 | 115.53 ± 43.64 | 0.003 |
| HDL-C (mg/dL) | 46.82 ± 12.97 | 48.14 ± 14.93 | 46.38 ± 9.50 | 46.36 ± 13.97 | 0.831 |
| Triglycerides (mg/dL) | 107.48 ± 50.23 | 100.37 ± 47.22 | 107.12 ± 46.83 | 112.08 ± 54.58 | 0.606 |
| Non-HDL-C (mg/dL) | 112.49 ± 46.72 | 93.48 ± 38.10 | 108.36 ± 43.33 | 127.40 ± 49.64 | 0.003 |
| ApoB (mg/dL) | 82.34 ± 27.29 | 63.21 ± 18.17 | 82.44 ± 24.31 | 93.17 ± 27.87 | 0.000 |
| LDL/ApoB | 1.27 ± 0.32 | 1.41 ± 0.45 | 1.18 ± 0.23 | 1.25 ± 0.25 | 0.012 |
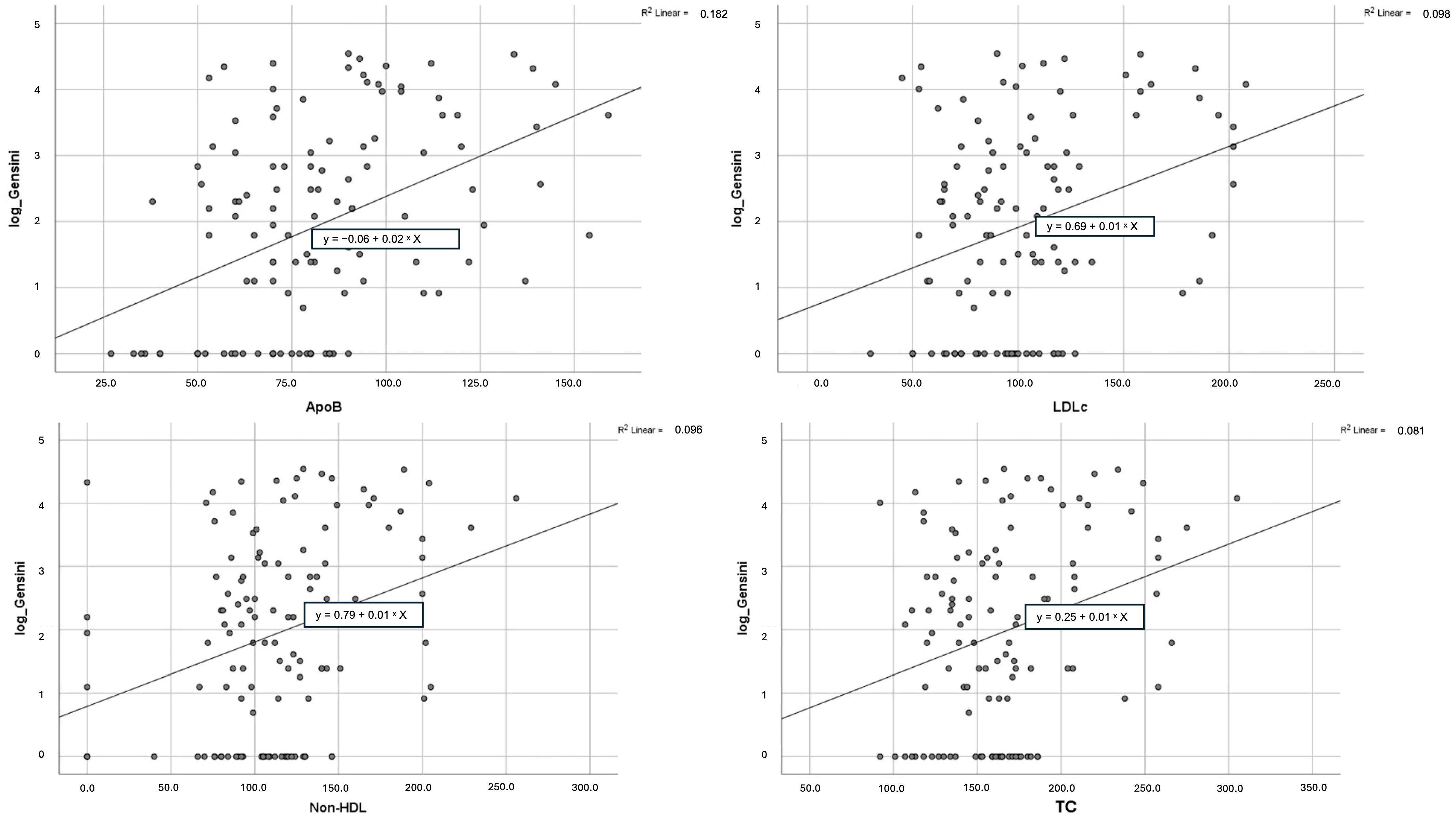
3.3. Correlation with Gensini Score
3.4. Diagnostic Performance
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cenko, E.; Manfrini, O.; Fabin, N.; Dorobantu, M.; Kedev, S.; Milicic, D.; Vasiljevic, Z.; Bugiardini, R. Clinical determinants of ischemic heart disease in Eastern Europe. Lancet Reg. Health Eur. 2023, 33, 100698. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.J.; Thanassoulis, G.; Anderson, T.J.; Barry, A.R.; Couture, P.; Dawes, M.; Francis, G.A.; Genest, J., Jr.; Grover, S.; Gupta, M.; et al. Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can. J. Cardiol. 2021, 37, 1129–1150. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Daly, D.D.; De Ferranti, S.D.; Fernandez, M.L.; Foti, K.; Guzman, C.; Morris, P.B.; et al. ACC Expert Consensus Decision Pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk. J. Am. Coll. Cardiol. 2022, 80, 1366–1418. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira-Gomes, D. Apolipoprotein B: Bridging the gap between evidence and clinical implementation. Circulation 2024, 150, 1952–1962. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Toth, P.P.; Thanassoulis, G.; Furberg, C.D. An evidence-based analysis of the National Lipid Association recommendations concerning non-HDL-C and apoB. J. Clin. Lipidol. 2016, 10, 1248–1258. [Google Scholar] [CrossRef]
- Young, S.G. Recent progress in understanding apolipoprotein B. Circulation 1990, 82, 1574–1594. [Google Scholar] [CrossRef]
- Barter, P.J.; Ballantyne, C.M.; Carmena, R.; Castro Cabezas, M.; Chapman, M.J.; Couture, P.; de Graaf, J.; Durrington, P.; Faergeman, O.; Frohlich, J.; et al. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: Report of the thirty-person/ten-country panel. J. Intern. Med. 2006, 259, 247–258. [Google Scholar] [CrossRef]
- Borén, J.; Williams, K.J. The central role of arterial retention of cholesterol-rich apolipoprotein B–containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 2016, 27, 473–483. [Google Scholar] [CrossRef]
- Tabas, I.; Williams, K.J.; Borén, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef]
- Soffer, D.E.; Marston, N.A.; Maki, K.C.; Jacobson, T.A.; Bittner, V.A.; Peña, J.M.; Thanassoulis, G.; Martin, S.S.; Kirkpatrick, C.F.; Virani, S.S.; et al. Role of apolipoprotein B in the clinical management of cardiovascular risk in adults: An Expert Clinical Consensus from the National Lipid Association. J. Clin. Lipidol. 2024, 18, e647–e663. [Google Scholar] [CrossRef]
- Sniderman, A.; Shapiro, S.; Marpole, D.; Skinner, B.; Teng, B.; Kwiterovich, P.O. Association of coronary atherosclerosis with hyperapobetalipoproteinemia [increased protein but normal cholesterol levels in human plasma low density (beta) lipoproteins]. Proc. Natl. Acad. Sci. USA 1980, 77, 604–608. [Google Scholar] [CrossRef]
- Deng, K.; Pan, X.F.; Voehler, M.W.; Cai, Q.; Cai, H.; Shu, X.O.; Gupta, D.K.; Lipworth, L.; Zheng, W.; Yu, D. Blood Lipids, Lipoproteins, and Apolipoproteins With Risk of Coronary Heart Disease: A Prospective Study Among Racially Diverse Populations. J. Am. Heart Assoc. 2024, 13, e034364. [Google Scholar] [CrossRef] [PubMed]
- Fujino, M.; Di Giovanni, G.; Butters Bhsc, J.; Kataoka, Y.; Hucko, T.; Nelson, A.J.; Nissen, S.E.; Psaltis, P.J.; Nicholls, S.J. Achieved levels of apolipoprotein B and plaque composition after acute coronary syndromes: Insights from HUYGENS. Atherosclerosis 2025, 403, 119145. [Google Scholar] [CrossRef]
- Raja, V.; Aguiar, C.; Alsayed, N.; Chibber, Y.S.; ElBadawi, H.; Ezhov, M.; Hermans, M.P.; Pandey, R.C.; Ray, K.K.; Tokgözoglu, L.; et al. Non-HDL-cholesterol in dyslipidemia: Review of the state-of-the-art literature and outlook. Atherosclerosis 2023, 383, 117312. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Packard, C.J.; Chapman, M.J.; Borén, J.; A Aguilar-Salinas, C.; Averna, M.; A Ference, B.; Gaudet, D.; A Hegele, R.; Kersten, S.; et al. Triglyceride-Rich Lipoproteins and Their Remnants: Metabolic Insights, Role in Atherosclerotic Cardiovascular Disease, and Emerging Therapeutic Strategies—A Consensus Statement from the European Atherosclerosis Society. Eur. Heart J. 2021, 42, 4791–4806. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Williams, K.; Contois, J.H.; Monroe, H.M.; McQueen, M.J.; de Graaf, J.; Furberg, C.D. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 337–345. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, N.Q.; Li, S.; Zhu, C.G.; Guo, Y.L.; Qing, P.; Xu, R.X.; Li, J.J. Non-HDL-C is a Better Predictor for the Severity of Coronary Atherosclerosis Compared with LDL-C. Heart Lung Circ. 2016, 25, 975–981. [Google Scholar] [CrossRef]
- Nakazato, R.; Gransar, H.; Berman, D.S.; Cheng, V.Y.; Lin, F.Y.; Hayes, S.W.; Thomson, L.E.; Friedman, J.D.; Min, J.K. Relationship of low- and high-density lipoproteins to coronary artery plaque composition by CT angiography. J. Cardiovasc. Comput. Tomogr. 2013, 7, 83–90. [Google Scholar] [CrossRef]
- Ferrannini, G.; Tuomilehto, J.; De Backer, G.; Kotseva, K.; Mellbin, L.; Schnell, O.; Wood, D.; De Bacquer, D.; Rydén, L. Dysglycaemia screening and its prognostic impact in patients with coronary artery disease: Experiences from the EUROASPIRE IV and V cohort studies. Lancet Diabetes Endocrinol. 2024, 12, 790–798. [Google Scholar] [CrossRef]
- Walli-Attaei, M.; Rosengren, A.; Rangarajan, S.; Breet, Y.; Abdul-Razak, S.; Sharief, W.A.; Alhabib, K.F.; Avezum, A.; Chifamba, J.; Diaz, R.; et al. Metabolic, behavioural, and psychosocial risk factors and cardiovascular disease in women compared with men in 21 high-income, middle-income, and low-income countries: An analysis of the PURE study. Lancet 2022, 400, 811–821. [Google Scholar] [CrossRef]
- Oancea, A.F.; Jigoranu, R.A.; Morariu, P.C.; Miftode, R.S.; Trandabat, B.A.; Iov, D.E.; Cojocaru, E.; Costache, I.I.; Baroi, L.G.; Timofte, D.V.; et al. Atrial Fibrillation and Chronic Coronary Ischemia: A Challenging Vicious Circle. Life 2023, 13, 1370. [Google Scholar] [CrossRef]
- Kornej, J.; Henger, S.; Seewöster, T.; Teren, A.; Burkhardt, R.; Thiele, H.; Thiery, J.; Scholz, M. Prevalence of atrial fibrillation dependent on coronary artery status: Insights from the LIFE-Heart Study. Clin. Cardiol. 2020, 43, 1616–1623. [Google Scholar] [CrossRef]
- Jigoranu, R.A.; Roca, M.; Costache, A.D.; Mitu, O.; Oancea, A.F.; Miftode, R.S.; Haba, M.Ș.C.; Botnariu, E.G.; Maștaleru, A.; Gavril, R.S.; et al. Novel Biomarkers for Atherosclerotic Disease: Advances in Cardiovascular Risk Assessment. Life 2023, 13, 1639. [Google Scholar] [CrossRef]
- Kathiresan, S.; Otvos, J.D.; Sullivan, L.M.; Keyes, M.J.; Schaefer, E.J.; Wilson, P.W.; D’Agostino, R.B.; Vasan, R.S.; Robins, S.J. Increased small low-density lipoprotein particle number: A prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation 2006, 113, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Drexel, H.; Larcher, B.; Mader, A.; Vonbank, A.; Heinzle, C.F.; Moser, B.; Zanolin-Purin, D.; Saely, C.H. The LDL-C/ApoB ratio predicts major cardiovascular events in patients with established atherosclerotic cardiovascular disease. Atherosclerosis 2021, 329, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Ra, M.; Bae, H.J.; Hong, S.P. The LDL-C/Apo B predicts coronary atherosclerotic heart disease in non-diabetic patients without high LDL-C. Medicine 2023, 102, e32596. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, R.I.; El-Leboudy, M.H.; El-Deeb, H.M. The relation between ApoB/ApoA-1 ratio and the severity of coronary artery disease in patients with acute coronary syndrome. Egypt. Heart J. 2021, 73, 24. [Google Scholar] [CrossRef]
- Haidari, M.; Moghadam, M.; Chinicar, M.; Ahmadieh, A.; Doosti, M. Apolipoprotein B as the best predictor of coronary artery disease in Iranian normolipidemic patients. Clin. Biochem. 2001, 34, 149–155. [Google Scholar] [CrossRef]
- Huynh, T.B.; Doan, C.T.; Ho, A.B.; Mai, X.A. Studying Variable in Serum Apolipoprotein B Levels in Damaged Coronary Artery Patients. J. Clin. Med.-Hue Central Hosp. 2023, 86, 90–97. [Google Scholar] [CrossRef]
- Hermans, M.P.; Sacks, F.M.; Ahn, S.A.; Rousseau, M.F. Non-HDL-cholesterol as valid surrogate to apolipoprotein B100 measurement in diabetes: Discriminant Ratio and unbiased equivalence. Cardiovasc. Diabetol. 2011, 10, 20. [Google Scholar] [CrossRef]
- Johannesen, C.D.L.; Mortensen, M.B.; Langsted, A.; Nordestgaard, B.G. Apolipoprotein B and Non-HDL Cholesterol Better Reflect Residual Risk Than LDL Cholesterol in Statin-Treated Patients. J. Am. Coll. Cardiol. 2021, 77, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.J.; Cleveland, J.C.; Welt, F.G.; Anwaruddin, S.; Bonow, R.O.; Firstenberg, M.S.; Gaudino, M.F.; Gersh, B.J.; Grubb, K.J.; Kirtane, A.J.; et al. A Practical Approach to Left Main Coronary Artery Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 2119–2134. [Google Scholar] [CrossRef] [PubMed]
- Zarepour, Z.; Parsa Mahjoob, M.; Taherpour, N.; Haji Aghajani, M. Assessing Serum Apolipoproteins A-I and b100 and the Apo A-I/Apo b100 Ratio in Relation to Premature Coronary Artery Disease and Its Severity. Catheter. Cardiovasc. Interv. 2025, 106, 153–162. [Google Scholar] [CrossRef]
- Ryoo, J.H.; Ha, E.H.; Kim, S.G.; Ryu, S.; Lee, D.W. Apolipoprotein B is highly associated with the risk of coronary heart disease as estimated by the Framingham risk score in healthy Korean men. J. Korean Med. Sci. 2011, 26, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.K.; Takeuchi, F.; Thao, L.T.P.; Nicholls, S.J.; Chew, D.P.; Peter, K. Integration of apolipoprotein B into the SCORE2 framework: Implications for cardiovascular risk prediction. Eur. J. Prev. Cardiol. 2025, 32, 575–584. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef]
- Choi, C.U.; Seo, H.S.; Lee, E.M.; Shin, S.Y.; Choi, U.J.; Na, J.O.; Lim, H.E.; Kim, J.W.; Kim, E.J.; Rha, S.W.; et al. Statins do not decrease small, dense low-density lipoprotein. Tex. Heart Inst. J. 2010, 37, 421–428. [Google Scholar]
- Sniderman, A.D.; Dufresne, L.; Pencina, K.M.; Bilgic, S.; Thanassoulis, G.; Pencina, M.J. Discordance among apoB, non-High-Density Lipoprotein Cholesterol, and Triglycerides: Implications for Cardiovascular Prevention. Eur. Heart J. 2024, 45, 2410–2418. [Google Scholar] [CrossRef]
- Sayed, A.; Peterson, E.D.; Virani, S.S.; Sniderman, A.D.; Navar, A.M. Individual Variation in the Distribution of Apolipoprotein B Levels across the Spectrum of LDL-C or Non–HDL-C Levels. JAMA Cardiol. 2024, 9, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Nomura, S.O.; Steffen, B.T.; Guan, W.; Remaley, A.T.; Karger, A.B.; Ouyang, P.; Michos, E.D.; Tsai, M.Y. Apolipoprotein B Discordance with Low-Density Lipoprotein Cholesterol and Non-High-Density Lipoprotein Cholesterol in Relation to Coronary Artery Calcification in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Clin. Lipidol. 2020, 14, 109–121. [Google Scholar] [CrossRef]
- Su, X.; Cai, X.; Pan, Y.; Sun, J.; Jing, J.; Wang, M.; Meng, X.; Wang, Y.; Wei, T.; He, Y. Discordance of Apolipoprotein B with Low-Density Lipoprotein Cholesterol or Non-High-Density Lipoprotein Cholesterol and Coronary Atherosclerosis. Eur. J. Prev. Cardiol. 2022, 29, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Hong, S.; Chang, Y.; Lee, J.A.; Shin, H.; Ryu, S. Discordance between Apolipoprotein B and Low-Density Lipoprotein Cholesterol and Progression of Coronary Artery Calcification in Middle Age. Circ. J. 2021, 85, 900–907. [Google Scholar] [CrossRef]
- Owczarek, J.; Rychlik-Sych, M.; Barańska, M.; Dudarewicz, M. The Importance of APOB Gene Expression as a Marker of Atherosclerosis Severity in Coronary Vessels. Pol. Arch. Intern. Med. 2023, 133, 16540. [Google Scholar] [CrossRef] [PubMed]
- Melnychuk, I.O.; Sharaieva, M.L.; Lyzogub, V.H. Lipid Exchange and Inflammatory Markers in Patients with Coronary Artery Disease and Atrial Fibrillation. Mod. Med. Technol. 2023, 3, 25–30. [Google Scholar] [CrossRef]
- Dorobanțu, M.; Halațiu, V.B.; Gheorghe-Fronea, O.; Bala, C.G.; Moldovan, H.; Irinel-Parepa, R.; Rodean, I.P.; Benedek, I.; Benedek, T. The Association between Apolipoprotein B, Cardiovascular Risk Factors and Subclinical Atherosclerosis—Findings from the SEPHAR National Registry on Hypertension in Romania. Int. J. Mol. Sci. 2023, 24, 2813. [Google Scholar] [CrossRef]
| Parameter | Overall | N-CAD | NS-CAD | S-CAD | p-Value |
|---|---|---|---|---|---|
| Age (years) | 64.2 ± 9.3 | 61.9 ± 10.2 | 63.8 ± 8.3 | 66 ± 9.33 | 0.192 |
| Male gender (%) | 63.6% | 54.5% | 63.9% | 69.2% | 0.390 |
| Smoking (%) | 56.2% | 51.5% | 61.1% | 55.8% | 0.722 |
| Pack-years | 18.19 ± 22.68 | 14.30 ± 17.85 | 21.25 ± 28.14 | 18.54 ± 21.22 | 0.445 |
| BMI (kg/m2) | 30.17 ± 5.70 | 30.53 ± 7.35 | 30.94 ± 4.37 | 29.42 ± 5.34 | 0.436 |
| AF (%) | 28.9% | 18.1% | 30.5% | 30.7% | 0.388 |
| DM (%) | 24.8% | 24.2% | 15.7% | 30.7% | 0.481 |
| CKD (%) | 21% | 15.2% | 25% | 22% | 0.589 |
| PAD (%) | 10.7% | 6.1% | 5.6% | 17.3% | 0.129 |
| COPD (%) | 9.1% | 0.3% | 11.1% | 13.5% | 0.096 |
| Stroke/TIA (%) | 9.9% | 6.1% | 11.1% | 11.5% | 0.682 |
| Biological parameters | |||||
| Glycaemia (mg/dL) | 110.15 ± 29.83 | 115.04 ± 37.81 | 105.62 ± 21.42 | 110.71 ± 30.25 | 0.477 |
| Ly/neut | 0.49 ± 0.24 | 0.47 ± 0.17 | 0.5 ± 0.26 | 0.5 ± 0.26 | 0.778 |
| AST (U/L) | 24.45 ± 10.79 | 23.29 ± 5.85 | 26.81 ± 16.58 | 23.41 ± 6.92 | 0.331 |
| ALT (U/L) | 24.74 ± 12.97 | 23.14 ± 8.09 | 26.82 ± 17.89 | 24.06 ± 10.69 | 0.522 |
| GGT (U/L) | 49.07 ± 57.57 | 40.38 ± 26.68 | 69.95 ± 87.15 | 40.17 ± 40.74 | 0.127 |
| Creatinine (mg/dL) | 0.88 ± 0.29 | 0.88 ± 0.31 | 0.89 ± 0.26 | 0.87 ± 0.30 | 0.952 |
| eGFR (mL/min/1.73 m2) | 86.43 ± 18.35 | 86.51 ± 20.56 | 85.80 ± 18.85 | 86.86 ± 16.81 | 0.968 |
| Gensini Score | LDL-C | HDL-C | Non-HDL-C | LDL/ApoB | ||
|---|---|---|---|---|---|---|
| Gensini Score | r | 1.000 | 0.268 ** | 0.084 | 0.285 ** | 0.073 |
| p-value (95% CI) | 0.004 (0.074–0.441) | 0.378 (−0.253–0.131) | 0.002 (−0.242–0.158) | 0.440 (−0.242–0.158) | ||
| N | 121 | 121 | 121 | 121 | 121 | |
| ApoB | r | 0.430 ** | 0.768 ** | 0.152 | 0.734 ** | 0.087 |
| p-value (95% CI) | 0.000 (0.202–0.543) | 0.000 (0.658–0.847) | 0.109 (−0.011–0.350) | 0.000 (0.711–0.877) | 0.360 (−0.263–0.150) | |
| N | 121 | 121 | 121 | 121 | 121 | |
| Biomarker | B | Std. Error | Standardized β | 95% CI for B | R2 | p-Value |
|---|---|---|---|---|---|---|
| LDL-C | 0.012 | 0.004 | 0.312 | 0.005–0.019 | 0.098 | 0.001 |
| Non-HDL-C | 0.010 | 0.003 | 0.310 | 0.004–0.016 | 0.096 | 0.001 |
| ApoB | 0.024 | 0.005 | 0.427 | 0.015–0.034 | 0.182 | <0.001 |
| TC | 0.010 | 0.003 | 0.285 | 0.004–0.017 | 0.081 | 0.002 |
| LDL/ApoB | −0.752 | 0.440 | −0.160 | −1.624–0.120 | 0.026 | 0.090 |
| Biomarker | B | Std. Error | Standardized β | 95% CI for B | Model R2 | p-Value |
|---|---|---|---|---|---|---|
| ApoB | 0.024 | 0.005 | 0.421 | 0.015–0.034 | 0.313 | <0.001 |
| Constant | −4.671 | 2.110 | - | −8.859–−0.484 | 0.029 |
| Biomarker | OR (95% CI) | p-Value | Nagelkerke R2 |
|---|---|---|---|
| ApoB (SD) | 2.386 (1.52–3.75) | 0.000 | 0.185 |
| LDL-C (SD) | 1.905 (1.24–2.92) | 0.001 | 0.115 |
| LDL/ApoB (SD) | 0.865 (0.588–1.271) | 0.460 | 0.007 |
| Non-HDL-C (SD) | 1.884 (1.23–2.9) | 0.004 | 0.106 |
| TC (SD) | 1.819 (1.2–2.76) | 0.003 | 0.101 |
| Outcome | OR (95% CI) | p-Value | Nagelkerke R2 |
|---|---|---|---|
| (A) Simple bivariate analysis | |||
| Left main disease | 2.43 (1.38–4.30) | 0.002 | 0.160 |
| Three vessel disease | 1.52 (0.98–2.37) | 0.063 | 0.045 |
| (B) Multivariate logistic regression | |||
| Significant CAD | 2.51 (1.46–4.31) | 0.000 | 0.299 |
| Left main disease | 1.46 (0.85–2.5) | 0.045 | 0.221 |
| Three vessel disease | 4.79 (1.57–14.59) | 0.006 | 0.416 |
| Biomarker | OR (95% CI) | p-Value | Nagelkerke R2 |
|---|---|---|---|
| ApoB (SD) | 4.64 (2.31–9.31) | 0.000 | 0.311 |
| LDL/ApoB ratio (SD) | 0.55 (0.35–0.87) | 0.010 | 0.096 |
| Outcome | OR (95% CI) | p-Value | Nagelkerke R2 |
|---|---|---|---|
| Coronary atherosclerosis | 5.22 (1.93–14.12) | 0.001 | 0.105 |
| Significant CAD | 1.92 (0.91–4.08) | 0.089 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jigoranu, R.-A.; Mitu, O.; Costache, A.-D.; Oancea, A.; Miftode, R.-S.; Buburuz, A.M.; Bazyani, A.; Simion, P.; Gavril, R.S.; Cianga, P.; et al. Moving Beyond LDL-C and Non-HDL-C: Apolipoprotein B as the Stronger Lipid-Related Predictor of Coronary Artery Disease in Statin-Treated Patients. Diagnostics 2025, 15, 3002. https://doi.org/10.3390/diagnostics15233002
Jigoranu R-A, Mitu O, Costache A-D, Oancea A, Miftode R-S, Buburuz AM, Bazyani A, Simion P, Gavril RS, Cianga P, et al. Moving Beyond LDL-C and Non-HDL-C: Apolipoprotein B as the Stronger Lipid-Related Predictor of Coronary Artery Disease in Statin-Treated Patients. Diagnostics. 2025; 15(23):3002. https://doi.org/10.3390/diagnostics15233002
Chicago/Turabian StyleJigoranu, Raul-Alexandru, Ovidiu Mitu, Alexandru-Dan Costache, Alexandru Oancea, Radu-Stefan Miftode, Ana Maria Buburuz, Amin Bazyani, Paul Simion, Radu Sebastian Gavril, Petru Cianga, and et al. 2025. "Moving Beyond LDL-C and Non-HDL-C: Apolipoprotein B as the Stronger Lipid-Related Predictor of Coronary Artery Disease in Statin-Treated Patients" Diagnostics 15, no. 23: 3002. https://doi.org/10.3390/diagnostics15233002
APA StyleJigoranu, R.-A., Mitu, O., Costache, A.-D., Oancea, A., Miftode, R.-S., Buburuz, A. M., Bazyani, A., Simion, P., Gavril, R. S., Cianga, P., Harnau, M. S., Onofrei, V., Petris, A. O., Costache Enache, I.-I., & Mitu, F. (2025). Moving Beyond LDL-C and Non-HDL-C: Apolipoprotein B as the Stronger Lipid-Related Predictor of Coronary Artery Disease in Statin-Treated Patients. Diagnostics, 15(23), 3002. https://doi.org/10.3390/diagnostics15233002










