Abstract
Background: This pilot longitudinal observational study investigated 4-week changes in lower limb muscle quantity and quality in patients with subacute stroke and explored risk factors associated with these changes. Methods: Twenty-six patients with hemiplegia following subacute stroke underwent assessment at baseline and 4-week follow-up. Muscle quantity was evaluated by ultrasound muscle thickness and bioelectrical impedance analysis, while muscle quality was assessed by shear-wave elastography in seven muscles (rectus femoris, vastus intermedius, vastus lateralis [VL], vastus medialis, tibialis anterior, gastrocnemius [GCM], and soleus). Electrophysiological assessments included motor-evoked potential (MEP), somatosensory-evoked potential (SEP), nerve conduction studies (NCSs), and central motor conduction time (CMCT). Results: Muscle thickness and bioimpedance did not significantly change between baseline and follow-up. In contrast, shear modulus increased in the paretic-side VL and GCM muscles (p < 0.001 and p = 0.049), with no differences in the non-paretic side. Greater deterioration in GCM quality was observed in patients with abnormal lower-limb MEP, and increased VL stiffness correlated with prolonged CMCT. Multivariable analyses were performed adjusting for age, sex, National Institutes of Health Stroke Scale, and comorbidity burden; however, due to the small electrophysiology sample (n = 11), these results should be interpreted as exploratory. Conclusions: In subacute stroke, early deterioration in muscle quality can occur despite stable quantity and appears linked to corticospinal integrity. Integrating electrophysiological evaluation with elastography may help identify patients who could benefit from early, targeted neuromuscular rehabilitation. These exploratory findings require validation in larger cohorts.
1. Introduction
Stroke is a leading cause of disability worldwide and the second leading cause of death [1]. The global economic burden of stroke is estimated at USD 721 billion (0.66% of global GDP), and the cost of post-stroke disability continues to rise annually [1]. Accordingly, efforts have been made to improve functional outcomes in stroke survivors through early screening and rehabilitation initiatives. Early mobility and postural control are critical components of stroke rehabilitation, reducing fall risk and facilitating early functional recovery through improved sensorimotor coordination [2].
Following a stroke, muscle abnormalities can develop due to denervation, disuse, remodeling, and spasticity [3]. Age, physical inactivity, and poor nutrition further contribute to muscle wasting [4]. Because muscle wasting is closely linked to reduced gait speed and impaired sit-to-stand performance [5], non-invasive approaches have been actively developed to quantify such changes.
In chronic stroke, consistent evidence shows reductions in muscle quantity and deterioration in quality. Motor unit numbers decline first on the paretic side and later on the non-paretic side within one week, persisting into the chronic stage [6,7]. Cross-sectional and longitudinal studies report lower thigh muscle volume and increased intramuscular fat on the paretic side [7,8].
In contrast, evidence in subacute stroke remains inconsistent. Kim et al. reported decreased thickness of the VL and GCM on the paretic side, with increased echo intensity on both sides [9]. Another longitudinal study found no change in thickness but increased shear-wave modulus in paretic-side VL and GCM [10]. Similarly, higher shear-wave velocity was observed in the paretic-side biceps brachii [11]. These findings suggest that while qualitative changes occur early, quantitative loss in the subacute phase is less evident.
Risk factors for post-stroke sarcopenia include age, dyslipidemia, diabetes, ischemic heart disease, and nutritional status [12]. In subacute stroke, older age, tube feeding, high National Institutes of Health Stroke Scale (NIHSS), and reduced non-paretic calf circumference have been associated with muscle loss [13]. However, most previous studies have focused primarily on muscle quantity, offering limited insight into muscle quality during the subacute stage.
Recent meta-analytic evidence has highlighted that sensorimotor and neuromechanical rehabilitation approaches improve post-stroke motor recovery and gait performance [14], emphasizing the need for integrated assessment of muscle quantity and neural integrity. Recent neural rehabilitation frameworks have also highlighted the role of sensory cueing in shaping neuromotor control and supporting the integration of electrophysiological and biomechanical assessments in stroke rehabilitation [15]. This approach may enhance clinical decision-making by providing a neurophysiological rationale for personalized rehabilitation planning.
This pilot longitudinal study therefore aimed to evaluate 4-week changes in lower limb muscle quantity and quality in patients with subacute stroke. Unlike our previous work focusing solely on morphological parameters [10], the present study integrates electrophysiological markers to link neural integrity with muscle-quality alterations. By combining electrophysiological and elastographic measures, this study provides a mechanistic understanding of how corticospinal integrity relates to peripheral muscle adaptation, offering a neurophysiological basis for individualized rehabilitation planning and early clinical decision-making. This integration provides new insight beyond our previous work, which focused solely on morphological parameters. Furthermore, recent systematic reviews on ultrasound elastography and sarcopenia [16,17] were incorporated to contextualize our findings within the broader literature.
2. Materials and Methods
2.1. Study Design and Participants
The reporting of this study followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines, as recommended by the EQUATOR Network. This pilot observational longitudinal study enrolled patients hospitalized with unilateral ischemic or hemorrhagic stroke between September 2022 and April 2023.
Of 36 patients screened, 26 completed all assessments and were included in the final analysis, while 10 were excluded owing to early discharge (n = 6), or medical complications (n = 4). During their rehabilitation at the Department of Rehabilitation Medicine, all participants received conventional therapy twice daily, five days per week. Conventional rehabilitation consisted of two 30-min sessions of physical and occupational therapy per day, focusing on neurodevelopmental facilitation, task-oriented training, strengthening of paretic limbs, and gait re-education.
Among the included patients, 16 (61.5%) had supratentorial and 10 (38.5%) had infratentorial lesions. Twenty (76.9%) had ischemic and six (23.1%) hemorrhagic stroke. Because of the exploratory nature and limited number of eligible inpatients during the study period, the sample size was determined by feasibility rather than a priori power calculation, and the cohort was thus considered a pilot study.
Inclusion criteria were (1) adults aged ≥19 years with first-ever unilateral stroke confirmed by computed tomography or magnetic resonance imaging; (2) ambulatory prior to stroke onset; and (3) provision of written informed consent. Exclusion criteria were (1) pre-existing limitations in walking or balance; (2) neuromuscular, renal, orthopedic, or uncontrolled psychiatric disorders; (3) inability to undergo body-composition or electrophysiological testing due to pacemaker or metallic implants; (4) clinically significant focal entrapment neuropathies (e.g., carpal tunnel or peroneal nerve palsy); (5) cognitive impairment interfering with compliance; (6) severe visual or hearing impairment; and (7) refusal to participate.
All procedures were conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of by Samsung Changwon Hospital, Sungkyunkwan University School of Medicine (protocol code SCMC 2022-07-003; approval date: 10 August 2022). Written informed consent was obtained from all participants.
This pilot observational study was not registered in a public trial registry, because it did not involve interventional procedures.
2.2. Data Collection
Clinical characteristics, including age, sex, education level, comorbidities, height, weight, and dietary habits, were recorded. Laboratory parameters included C-reactive protein (mg/dL) and glycated hemoglobin (HbA1c, %). Stroke severity was assessed using the NIHSS, and cognitive function was evaluated with the Mini-Mental State Examination (MMSE). Comorbidities were quantified using the Charlson Comorbidity Index [18].
Nutritional status was evaluated using the Geriatric Nutritional Risk Index (GNRI), an index for assessing malnutrition, calculated as follows:
where ideal body weight (kg) = height2 (m2) × 22 [19].
GNRI = (1.487 × serum albumin level [g/L]) + (41.7 × body weight/ideal body weight),
Heavy drinking was defined as consumption of >15 drinks per week for men and >8 drinks per week for women, in accordance with the Centers for Disease Control and prevention (CDC) criteria [20]. Calf circumference on the non-paretic side was measured to the nearest 1 cm at the thickest part of the calf, with the knee flexed at 90° in the supine position. The measurement site was standardized at 30% of the distance between the lateral malleolus and the lateral tibial condyle [21].
2.3. Bioelectrical Impedance Analysis
Bioelectrical impedance analysis (BIA) was performed using an InBody S10 device (InBody Co., Ltd., Seoul, Republic of Korea). Measurements were obtained at baseline and at the 4-week follow-up during the same session as the ultrasound and electrophysiological assessments, following a standardized electrode protocol recommended by the manufacturer.
Parameters included total fat mass, fat mass index, appendicular skeletal muscle mass (ASM), body fat percentage, and their derived indices: fat mass index (FMI = fat mass [kg]/height2 [m2]) and fat-free mass index (FFMI = fat-free mass [kg]/height2 [m2]).
All BIA measurements were taken after at least 8 h of fasting, with participants in the supine position and both limbs slightly abducted to minimize impedance error.
2.4. Ultrasonographic Measurements
Ultrasound examinations were performed using a multi-frequency linear transducer (8–12 MHz) on a B-mode ultrasound system (Samsung Medison V8; Samsung Medison, Seoul, Republic of Korea). Muscle thickness and elasticity of the rectus femoris (RF), vastus intermedius (VI), VL, vastus medialis (VM), tibialis anterior (TA), GCM, and soleus were measured bilaterally.
RF and VI were measured midway between the anterior superior iliac spine and the proximal patella; VM at 12.5% medial to 20% of this distance; VL at 10% lateral to the midpoint; TA at 30% proximal between the lateral malleolus and lateral tibial condyle; and GCM/soleus at 30% proximal between the lateral malleolus and lateral tibial condyle [7,10]. Measurements for the GCM and soleus were obtained in the sitting position, and all other muscles were examined in the supine position.
All measurements were performed with the probe placed perpendicular to the skin surface, applying minimal compression and using an ample amount of gel to minimize anisotropy artifacts.
Muscle thickness was recorded to the nearest 0.01 cm using electronic calipers. Shear-wave elastography was used to assess viscoelastic properties, with the probe aligned longitudinally along the muscle fascicles [22]. A circular region of interest (ROI, 1 mm diameter) was placed at the mid-belly of each muscle, and the mean value of eight ROIs was calculated as the elastic shear modulus (kPa) [10,23] (Figure 1). Each measurement was performed twice by the same experienced operator, and the average value was used for analysis to ensure intra-rater reliability.
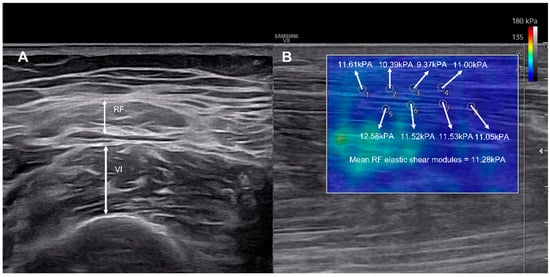
Figure 1.
Representative ultrasonographic images showing measurement of RF and VI muscle thickness (A), and corresponding shear-wave elastography map (B). The color scale indicates the elastic shear modulus (kPa) ranging from 0 to 180 where blue represents softer tissue and red indicates stiffer tissue. Multiple regions of interest (ROIs) were positioned along the muscle fascicles, and mean shear modulus was automatically calculated by the system.
2.5. Electrophysiological Examination
Electrophysiological testing included MEP, SEP, and NCSs. A Nicolet AT2+6 amplifier (Natus Medical Inc., Middleton, WI, USA) and a MagPro simulator (MagVenture A/S, Farum, Denmark) were used. All studies were performed by a single experienced physician. All electrophysiological and ultrasonographic assessments were conducted by a board-certified physiatrist with over 10 years of clinical experience, and all rehabilitation sessions were administered by licensed physical therapists with more than 5 years of experience in neurorehabilitation. Magnetic cortical stimulation was not performed in patients with metallic devices or those at risk of seizure.
Bilateral MEP recordings were obtained from the abductor pollicis brevis (APB) in the upper limb and the abductor hallucis (AH) in the lower limb using surface electrodes with participants in the supine position. Transcortical stimulation was delivered at 60% of the maximum simulator output using a figure-8 coil. The stimulus duration was 0.5 ms, with a filter of 3–10 Hz and sweep speed of 5 ms for both limbs. MEP latency and amplitude were analyzed. Responses were classified as abnormal if MEPs were absent, latency was prolonged (≥21.7 ms for the upper limb, ≥41.5 ms for the lower limb), or amplitude was reduced more than 50% compared with the contralateral side (n = 11 and n = 9, respectively) [24,25].
SEP responses were elicited by electrical stimulation of the median nerve at the wrist and the posterior tibial nerve at the ankle (7.5 mA for the upper limb, 8.0 mA for the lower limb). The stimulus duration was 0.2 ms and frequency 3.1 Hz. Cortical responses were recorded using surface electrodes placed over contralateral C3’/C4’, referenced to Cpz’, according to the 10–20 system. Recordings were filtered 30–3000 Hz. N17 and P21 latencies were measured for the upper limb, P42 and N50 for the lower limb. SEP abnormalities were defined as latency prolongation beyond normal limits. (≥20.63 ms, ≥28.23 ms, ≥42.6 ms, and ≥51.8 ms, respectively [24].
Motor NCSs were performed on the bilateral median, ulnar, peroneal, and posterior tibial nerves to assess latency, amplitude, and conduction velocity. Sensory NCSs were conducted on the bilateral median, ulnar, superficial peroneal, and sural nerves to measure latency, amplitude, and sensory nerve action potential. Peripheral polyneuropathy was diagnosed and graded using Baba classification(0–4) [26].
Peripheral motor conduction time (PMCT) was calculated from F-wave and M-wave latencies recorded at the APB and AH muscles using the equation (F + M − 1)/2 [27]. CMCT was calculated as:
for the upper limb (n = 10) and lower limb (n = 2) recordings [24]. Because the lesion site (supratentorial vs. infratentorial) can influence conduction latency, results were interpreted in the context of each patient’s lesion location.
CMCT = MEP − (F + M − 1)/2
2.6. Statistical Analysis
Longitudinal changes in muscle thickness, elastic shear modulus, and body composition were analyzed using the Wilcoxon signed-rank test. The normality of continuous variables was examined using the Shapiro–Wilk test, confirming non-normal distribution.
Electrophysiological testing was performed in 21 participants; however, due to absent or unobtainable MEP or F-wave responses, CMCT could not be calculated in 10 patients, leaving 11 participants with complete electrophysiological datasets for regression analysis. Multivariable linear regression adjusting for age, sex, NIHSS, and Charlson Comorbidity Index (CCI) was performed in the full cohort (n = 26). For models including electrophysiological variables (e.g., CMCT, MEP, SEP), the number of complete cases was small (n = 11), yielding fewer than 10 events per variable. Therefore, multivariable adjustment was considered statistically underpowered and was not performed. Univariable analyses were reported as exploratory, and beta coefficients, standard errors, and 95% confidence intervals (CIs) were presented. Given the small electrophysiology subgroup (n = 11), these analyses were statistically underpowered and should be interpreted as hypothesis-generating.
All analyses were conducted using SPSS Statistics, version 21.0 (IBM Corp., Armonk, NY, USA). Effect sizes (r = Z/√N) were calculated for Wilcoxon tests to indicate the magnitude of change. Given the small sample size, Type II errors could not be excluded. Statistical significance was set at p < 0.05. A post hoc power analysis (G*Power v3.1; two-tailed, α = 0.05) for the primary outcome (change in paretic VL shear modulus) was conducted using a paired-sample design. The analysis yielded a Cohen’s dz of 0.76 and a statistical power (1 − β) of 0.959, indicating adequate sensitivity despite the small sample size.
3. Results
3.1. Demographic Characteristics
A total of 36 patients were screened, and 26 completed all assessments and were included in the final analysis, while 10 were excluded due to early discharge (n = 6), or medical complications (n = 4) (Figure 2).
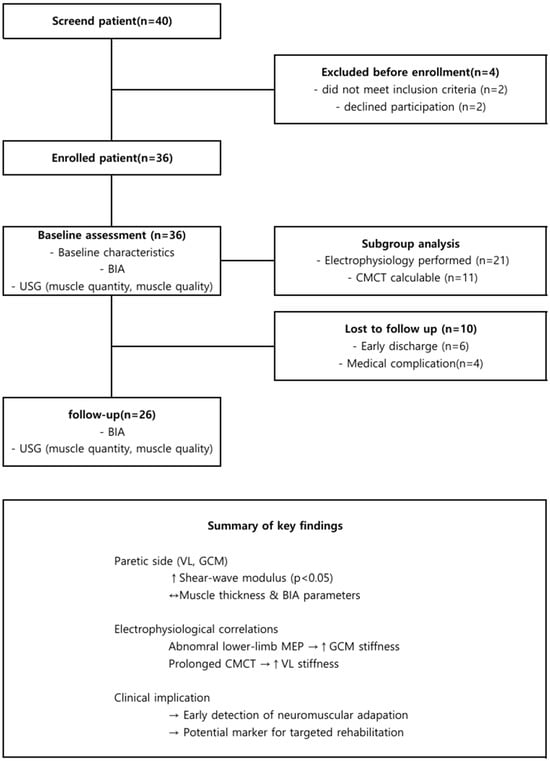
Figure 2.
Participant flow and summary of main findings. (Top): study flow; (Bottom): key quantitative outcomes summarized in a table. ↑, increase; →, causal relationship; ↔, no difference.
The final cohort comprised 9 men and 17 women, with a median age of 72 years (IQR 61–80). The median time since stroke onset was 7 days (range, 4–11). Stroke type distribution included 20 ischemic and 6 hemorrhagic cases. Lesion location consisted of 16 supratentorial and 10 infratentorial cases. No significant between-group differences were observed in 4-week changes in shear modulus according to stroke type (ischemic vs. hemorrhagic), age group or lesion location (supratentorial vs. infratentorial). (all p > 0.05). Education level was recorded as an indicator of cognitive reserve that may influence rehabilitation compliance. Demographic variables are summarized in Table 1.

Table 1.
Baseline characteristics of the study population.
3.2. Bioelectrical Impedance Analysis
BIA showed no significant differences between baseline and 4-week follow-up in any body composition parameter, including fat mass, fat mass index, fat-free mass, fat-free mass index, skeletal muscle mass, body fat percentage, appendicular skeletal muscle mass/height2 (ASM/height2), or body weight (all p > 0.05) (Table 2).

Table 2.
Bioelectrical impedance analysis at baseline and follow-up (n = 26).
3.3. Ultrasonographic Assessment
Muscle thickness across the seven examined muscles showed no significant differences between baseline and 4-week follow-up on either the paretic or non-paretic side (all p > 0.05; Table 3). In contrast, shear-wave elastography revealed significant increases in the elastic shear modulus of the paretic VL and GCM muscles (p < 0.001 and p = 0.049, respectively), with no significant changes observed in non-paretic muscles (Table 4).

Table 3.
Changes in muscle thickness at baseline and follow-up (n = 26).

Table 4.
Changes in elastic shear modules at baseline and follow-up (n = 26).
3.4. Regression Analysis
Univariable regression analysis showed no significant associations between demographic variables and changes in shear-wave modulus of the paretic VL and GCM muscles (Table 5). In multivariable models adjusting for age, sex, NIHSS, and CCI, the direction and statistical significance of the associations remained unchanged (Appendix A Table A1). No additional significant predictors, including lesion location (supratentorial vs. infratentorial) or stroke type (ischemic vs. hemorrhagic), were identified.

Table 5.
Univariable linear regression of changes in shear-wave modulus of the paretic VL and GCM muscles and baseline characteristics.
3.5. Electrophysiologic Correlations
Among the 21 patients who underwent electrophysiological testing, significant differences were observed in shear-wave modulus changes in the paretic GCM muscle between the abnormal and normal lower-limb MEP groups (p = 0.039). Subgroup analysis of 10 upper-limb and 12 lower-limb patients (excluding cases with unobtainable MEP or F-wave measurements) revealed a significant association between increased lower-limb CMCT and shear-wave modulus changes in the paretic VL muscle (p = 0.032).
Because electrophysiology-complete cases were limited (n = 11), these associations between shear-wave modulus changes and CMCT/MEP/SEP were analyzed an univariable estimates only. Multivariable modeling was not performed due to instability of estimates and the risk of model overfitting with such a small sample size.
Clinically, greater muscle stiffness in paretic-side VL and GCM muscles was associated with impaired corticospinal conduction, suggestion that shear-wave elastography may serve as a complementary marker of post-stroke neuromuscular adaption. No significant correlations were found with upper-limb MEP abnormalities, SEP abnormalities, Baba classification, PMCT, or changes in GCM muscle quality (Table 6).

Table 6.
Univariable linear regression of changes in shear-wave modulus of the paretic VL and GCM muscles with electrodiagnostic parameters.
4. Discussion
This longitudinal study of patients with subacute stroke demonstrated that, while lower-limb muscle quantity remained stable over four weeks, muscle quality significantly deteriorated in the paretic VL and GCM. Importantly, these changes were not associated with established risk factors for stroke-related sarcopenia but were instead linked to electrophysiological findings, particularly abnormal lower-limb MEPs and prolonged CMCT. These results highlight the dynamic interplay between peripheral muscle mechanical properties and corticospinal excitability, suggesting that shear-wave elastography may serve as a complementary marker of neuromuscular adaptation after stroke [11,28,29].
Decreases in skeletal muscle mass are well known to increase the risk of falls, disability, reduced quality of life, and mortality [16]. As the social and clinical burden of muscle wasting continues to rise, early detection is crucial [30]. Beyond muscle mass, recent studies have emphasized the prognostic value of muscle quality, with poorer quality being associated with higher 90-day mortality after stroke [17]. Furthermore, recent meta-analyses have underscored that neuromechanical and sensorimotor retraining interventions can improve post-stroke gait and strength recovery, reinforcing the importance of integrating neural and muscular assessment in rehabilitation [15,17].
Conventional approaches such as DXA or BIA primarily assess muscle mass, whereas CT, although considered the gold standard, is limited by its high cost and radiation exposure [17]. Accordingly, ultrasonography and shear-wave elastography are increasingly used as non-invasive tools to evaluate both muscle quantity and quality.
Previous studies on chronic stroke have consistently demonstrated longitudinal reductions in muscle mass and quality [8,9]. In contrast, findings in subacute stroke have been less consistent, although deterioration in muscle quality tends to be more pronounced than that in quality [10,11,12]. In our cohort, BIA and ultrasound-derived muscle thickness showed no significant changes, whereas the elastic modulus increased in the paretic VL and GCM, confirming early deterioration in muscle quality.
Pathophysiologically, hemiplegic stroke leads to rapid skeletal muscle changes driven by disuse, denervation, and remodeling. Structural adaptations may begin within hours, and limb muscle loss emerges within the first week, accompanied by reductions in motor unit numbers [31]. Prior studies have demonstrated increased echo intensity (EI) in multiple muscles of chronic stroke patients compared with healthy controls [32], and similar increases in paretic VL and GCM in subacute patients [10]. Muscle-specific differences in adaptation are thought to arise from variations in anatomical attachment and immobilization-induced loading, and non-uniform patterns of atrophy have been reported, sparing certain muscles such as the TA and gracilis [33].
Risk factors for post-stroke sarcopenia—such as advanced age, comorbidities, high NIHSS, and reduced non-paretic calf circumference—have been well described [13,14]. However, these studies have largely addressed muscle quantity rather than quality. Our results suggest that such conventional risk factors may not adequately predict early deterioration in muscle quality, which appears to be more directly associated with electrophysiological impairment.
Electrodiagnostic testing is widely used in stroke to assess motor and sensory integrity and detect comorbid neuropathies. In this study, muscle quality deterioration was more pronounced in patients with abnormal lower-limb MEPs, and prolonged CMCT correlated with worsening VL quality. These findings are consistent with previous reports showing reduced MEP amplitudes in association with muscle weakness [34,35]. This link between central motor conduction delay and peripheral stiffness suggest that electrophysiological evaluation could help predict the degree of secondary myogenic adaptation and guide early, targeted rehabilitation strategies [11,28,29]. Recent neurophysiological analyses have shown that post-stroke motor recovery is associated with plastic changes in cortical excitability and sensorimotor network reorganization [29,35], complementing the current findings linking peripheral stiffness with central conduction parameters. These findings align with emerging evidence linking neurophysiological modulation and sensory–motor integration in stroke recovery, including peripheral sensory mechanisms and proprioceptive enhancement via taping [6,36]. Recent advances in SEP pattern analysis, such as the classification approach proposed by Fuseya et al. [6], may further enhance cortical sensory assessment in subacute stroke.
From a clinical perspective, early recognition of muscle quality decline is crucial, as it directly affects functional prognosis, hospital stay, and quality of life. Integrating electrophysiological and shear-wave elastography assessments may enhance individualized rehabilitation planning and monitoring in patients with subacute stroke. Comprehensive rehabilitation outcome frameworks emphasizing sensorimotor performance may facilitate translation of muscle-quality metrics into individualized clinical decision-making [37]. These perspectives reinforce the clinical importance of proprioceptive and neuromechanical interventions for restoring lower-limb function after stroke [38]. This concept is supported by recent frameworks emphasizing integration of neural and muscular assessments for individualized rehabilitation [15,39].
This study has several limitations. First, the overall sample size was small (n = 26), and only 11 participants had complete electrophysiological data, multivariable adjustment including these variables would violate sample-per-variable requirements. Including these variables in regression models would have violated the minimum sample-per-variable requirement and produced unstable estimates. Therefore, the electrophysiological results should be regarded as exploratory and require validation in larger cohorts. Second, all participants underwent standardized rehabilitation, which precluded assessment of natural disease progression. Third, the cohort consisted predominantly of older adults, so the findings may not generalize to younger populations. Additionally, the 4-week interval offers only short-term information, and no healthy control group was included, these pilot findings provide a rationale for larger longitudinal studies incorporating electrophysiological and elastographic markers to track neuromuscular adaptation. Although the cohort was predominantly female and ischemic, sex and etiology-adjusted regression did not reveal significant interaction effects. Future studies could incorporate proprioceptive and motor-retraining approaches shown to enhance neuromuscular activation and stability in lower-limb rehabilitation. In addition, post-stroke fatigue, which can be categorized into distinct subtypes with varying contributing factors, may influence neuromuscular recovery. Recent findings by Etoom et al. [39] highlight the importance of recognizing these subtypes when tailoring rehabilitation strategies. Future multicenter studies with larger and more diverse populations are warranted to validate these results and to explore targeted intervention strategies.
5. Conclusions
In patients with subacute stroke, muscle quality of the paretic VL and GCM deteriorated significantly compared with the non-paretic side, despite stable muscle quantity. These alterations were not associated with conventional risk factors for stroke-related sarcopenia but were instead linked to electrophysiological abnormalities. Specifically, abnormal lower-limb MEPs were associated with greater deterioration in GCM quality, and prolonged CMCT correlated with worsening VL quality. These findings suggest that electrophysiological assessment, when combined with shear-wave elastography, may serve as a complementary tool for detecting and predicting early neuromuscular alterations in subacute stroke.
Author Contributions
Conceptualization, S.J.K. and J.H.L.; methodology, S.J.K. and J.H.L.; formal analysis, J.Y.K. and D.J.H.; project administration, S.J.K.; visualization, J.H.L.; writing—original draft preparation, S.J.K.; writing—review and editing, J.H.L., Y.S.P., H.J.C., J.G.P. and E.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by Samsung Changwon Hospital, Sungkyunkwan University School of Medicine.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Samsung Changwon Hospital, Sungkyunkwan University School of Medicine (protocol code SCMC 2022-07-003; approval date: 10 August 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Acknowledgments
The authors would like to thank the staff of the Department of Rehabilitation Medicine, Samsung Changwon Hospital, for their administrative and technical support.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| MT | Muscle thickness |
| BIA | Bioelectrical impedance analysis |
| VL | Vastus lateralis |
| GCM | Gastrocnemius |
| MEP | Motor-evoked potential |
| CMCT | Central motor conduction time |
| NIHSS | National institutes of health stroke scale |
| MMSE | Mini-mental state examination |
| GNRI | Geriatric nutritional risk index |
| RF | Rectus femoris |
| VI | Vastus intermedius |
| VM | Vastus medialis |
| TA | Tibialis anterior |
| ROI | Region in interest |
| SEP | Sensory-evoked potential |
| PPN | Peripheral neuropathy |
Appendix A

Table A1.
Multivariable linear regression of changes in shear-wave modulus of the paretic VL and GCM muscles with baseline characteristics.
Table A1.
Multivariable linear regression of changes in shear-wave modulus of the paretic VL and GCM muscles with baseline characteristics.
| Variable | Delta VL (kPa) | Delta GCM (kPa) | ||||
|---|---|---|---|---|---|---|
| β ± Se | 95% CI | p-Value | β ± Se | 95% CI | p-Value | |
| Age | 0.53 ± 0.57 | −0.69 to 1.76 | 0.364 | −0.26 ± 0.37 | −1.06 to 0.54 | 0.491 |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | −16.86 ± 18.19 | −55.88 to 22.16 | 0.370 | 11.33 ± 11.89 | −14.16 to 36.82 | 0.356 |
| Reason for admission | ||||||
| Infarction | Reference | Reference | ||||
| Hemorrhage | 11.56 ± 14.03 | −18.54 to 41.66 | 0.424 | 6.64 ± 9.17 | −13.03 to 26.30 | 0.481 |
| Lesion location | ||||||
| Supratentorial | Reference | Reference | ||||
| Infratentorial | −5.69 ± 12.57 | −2.83 to 21.28 | 0.658 | 4.85 ± 8.21 | −12.77 to 22.47 | 0.564 |
| NIHSS | −0.24 ± 1.21 | −2.83 to 2.35 | 0.847 | 0.75 ± 0.79 | −0.95 to 2.44 | 0.361 |
| Stroke duration | −0.54 ± 0.93 | −2.53 to 1.45 | 0.571 | −0.30 ± 0.61 | −1.60 to 1.00 | 0.631 |
| MMSE | 0.71 ± 0.87 | −1.16 to 2.59 | 0.429 | 0.13 ± 0.57 | −1.10 to 1.35 | 0.830 |
| Education | ||||||
| Un-educated | Reference | Reference | ||||
| Elementary school | −13.93 ± 17.35 | −51.14 to 23.59 | 0.436 | 0.10 ± 11.37 | −24.21 to 24.42 | 0.993 |
| Middle school | −0.33 ± 23.36 | −50.86 to 50.20 | 0.989 | 3.83 ± 15.39 | −29.19 to 36.84 | 0.807 |
| High school | −25.57 ± 14.84 | −57.40 to 6.25 | 0.107 | 2.43 ± 9.70 | −18.36 to 23.23 | 0.806 |
| College | −13.61 ± 21.22 | −59.13 to 31.89 | 0.531 | 7.33 ± 13.86 | −22.40 to 37.07 | 0.605 |
| CCI | −5.53 ± 3.76 | −13.46 to 2.40 | 0.159 | 1.16 ± 2.32 | −3.29 to 6.52 | 0.497 |
| GNRI | −0.07 ± 0.07 | −0.22 to 0.07 | 0.310 | 0.06 ± 0.04 | −0.03 to 0.15 | 0.171 |
| Calf circumference | 0.83 ± 1.04 | −1.36 to 3.03 | 0.435 | 0.797 ± 0.643 | −0.56 to 2.15 | 0.232 |
| Diet | ||||||
| Oral feeding | Reference | Reference | ||||
| L-tube feeding | 8.18 ± 12.39 | −17.93 to 34.29 | 0.518 | 6.63 ± 7.66 | −9.52 to 22.79 | 0.398 |
| Diabetes mellitus | −11.02 ± 14.18 | −40.93 to 18.89 | 0.449 | 10.41 ± 8.78 | −8.09 to 28.91 | 0.252 |
| Heavy drinking | −1.80 ± 12.69 | −28.57 to 24.98 | 0.889 | 2.03 ± 7.85 | −14.53 to 18.59 | 0.799 |
| HbA1c | −0.67 ± 4.85 | −10.90 to 9.57 | 0.892 | −2.88 ± 3.00 | −9.21 to 3.45 | 0.350 |
| CRP | 0.55 ± 0.60 | −0.71 to 1.81 | 0.371 | −0.25 ± 0.37 | −1.04 to 0.53 | 0.501 |
Values are standardized β coefficients (95% confidence interval) from multivariable linear regression analysis after adjustment for age, sex, NIHSS (National Institutes of Health Stroke Scale) score, and Charlson Comorbidity Index (CCI). Models were constructed separately for VL and GCM changes as dependent variables. No additional significant predictors—including lesion location (supratentorial vs. infratentorial) or stroke type (ischemic vs. hemorrhagic)—were identified. Abbreviations: CI = confidence interval.
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Shahid, J.; Kashif, A.; Shahid, M.K. A Comprehensive Review of Physical Therapy Interventions for Stroke Rehabilitation: Impairment-Based Approaches and Functional Goals. Brain Sci. 2023, 13, 717. [Google Scholar] [CrossRef]
- Scherbakov, N.; von Haehling, S.; Anker, S.D.; Dirnagl, U.; Doehner, W. Stroke-Induced Sarcopenia: Muscle Wasting and Disability After Stroke. Int. J. Cardiol. 2013, 170, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, N.; Sandek, A.; Doehner, W. Stroke-Related Sarcopenia: Specific Characteristics. J. Am. Med. Dir. Assoc. 2015, 16, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wan, C.-S.; Ktoris, K.; Reijnierse, E.M.; Maier, A.B. Sarcopenia Is Associated With Mortality in Adults: A Systematic Review and Meta-Analysis. Gerontology 2022, 68, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Fuseya, H.; Tashiro, S.; Takahashi, O.; Kobayashi, Y.; Tsuji, T.; Mizuno, K. Somatosensory-Evoked Potentials and Clinical Assessments of Sensory Function Over Time in Patients with Subacute Stroke. Neural Plast. 2025, 2025, 7939662. [Google Scholar] [CrossRef]
- Monjo, H.; Fukumoto, Y.; Asai, T.; Kubo, H.; Ohshima, K.; Tajitsu, H.; Koyama, S. Changes in Muscle Thickness and Echo Intensity in Chronic Stroke Survivors: A 2-Year Longitudinal Study. J. Clin. Neurol. 2022, 18, 308–314. [Google Scholar] [CrossRef]
- Akazawa, N.; Harada, K.; Okawa, N.; Kishi, M.; Tamura, K.; Moriyama, H. Changes in Quadriceps Thickness and Echo Intensity in Chronic Stroke Survivors: A 3-Year Longitudinal Study. J. Stroke Cerebrovasc. Dis. 2021, 30, 105543. [Google Scholar] [CrossRef]
- Kim, J.M.; Tay, M.R.J.; Rajeswaran, D.K.; Tham, S.-L.; Lui, W.L.; Kong, K.H. Changes in Muscle Architecture on Ultrasound in Patients Early After Stroke. NeuroRehabilitation 2021, 49, 565–572. [Google Scholar] [CrossRef]
- Kim, D.H.; Cho, E.S.; Park, Y.S.; Chang, H.J.; Park, J.G.; Kim, J.Y.; Lee, J.H. Changes in Lower Extremity Muscle Quantity and Quality in Patients with Subacute Stroke. Ann. Rehabil. Med. 2023, 47, 493–501. [Google Scholar] [CrossRef]
- Wu, C.-H.; Ho, Y.-C.; Hsiao, M.-Y.; Chen, W.-S.; Wang, T.-G. Evaluation of Post-Stroke Spastic Muscle Stiffness Using Shear Wave Ultrasound Elastography. Ultrasound Med. Biol. 2017, 43, 1105–1111. [Google Scholar] [CrossRef]
- Su, Y.; Yuki, M.; Otsuki, M. Prevalence of Stroke-Related Sarcopenia: A Systematic Review and Meta-analysis. J. Stroke Cerebrovasc. Dis. 2020, 29, 105092. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, Y.-A. Prevalence and Risk Factors of Possible Sarcopenia in Patients with Subacute Stroke. PLoS ONE 2023, 18, e0291452. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual Reality for Stroke Rehabilitation. Cochrane Database Syst. Rev. 2021, 11, CD008349. [Google Scholar] [CrossRef]
- Bonanno, M.; De Pasquale, P.; Fonti, B.; Gjonaj, E.; De Salvo, S.; Quartarone, A.; Calabrò, R.S. Neural Control Meets Biomechanics in the Motor Assessment of Neurological Disorders: A Narrative Review. Front. Neural Circuits 2025, 19, 1608328. [Google Scholar] [CrossRef]
- Bastijns, S.; De Cock, A.-M.; Vandewoude, M.; Perkisas, S. Usability and Pitfalls of Shear-Wave Elastography for Evaluation of Muscle Quality and Its Potential in Assessing Sarcopenia: A Review. Ultrasound Med. Biol. 2020, 46, 2891–2907. [Google Scholar] [CrossRef]
- Fu, H.; Wang, L.; Zhang, W.; Lu, J.; Yang, M. Diagnostic Test Accuracy of Ultrasound for Sarcopenia Diagnosis: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 57–70. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.-P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A New Index for Evaluating At-Risk Elderly Medical Patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Definitions of Heavy Drinking: Men = ≥15 Drinks/Week, Women = ≥8 Drinks/Week. CDC Website. Available online: https://www.cdc.gov/drink-less-be-your-best/facts-about-excessive-drinking/index.html (accessed on 10 September 2025).
- Inoue, T.; Maeda, K.; Shimizu, A.; Nagano, A.; Ueshima, J.; Sato, K.; Murotani, K. Calf Circumference Value for Sarcopenia Screening Among Older Adults with Stroke. Arch. Gerontol. Geriatr. 2021, 93, 104290. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.M.; Spear, S.; Rymer, W.Z. Quantifying Changes in Material Properties of Stroke-Impaired Muscle. Clin. Biomech. 2015, 30, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; He, W.; Du, L.-J.; Chen, J.; Park, D.; Wells, M.; Fowlkes, B.; O’Dell, M. Quantitative Ultrasound Imaging to Assess the Biceps Brachii Muscle in Chronic Post-Stroke Spasticity: Preliminary Observation. Ultrasound Med. Biol. 2018, 44, 1931–1940. [Google Scholar] [CrossRef]
- DeLisa, J.A. Manual of Nerve Conduction Velocity and Clinical Neurophysiology, 3rd ed.; Raven Press: New York, NY, USA, 1994; pp. 214–215, 248–249, 314, 344. [Google Scholar]
- Abboud, T.; Schwarz, C.; Westphal, M.; Martens, T. A Comparison Between Threshold Criterion and Amplitude Criterion in Transcranial Motor Evoked Potentials During Surgery for Supratentorial Lesions. J. Neurosurg. 2018, 131, 740–749. [Google Scholar] [CrossRef]
- Baba, M.; Suzuki, C. Electrophysiological Grading of Diabetic Polyneuropathy by Nerve Conduction Study. Jpn. J. Clin. Neurophysiol. 2013, 41, 143–150. [Google Scholar] [CrossRef]
- Kimura, J. F-Wave Velocity in the Central Segment of the Median and Ulnar Nerves: A Study in Normal Subjects and in Patients With Charcot-Marie-Tooth Disease. Neurology 1974, 24, 539–546. [Google Scholar] [CrossRef]
- Veldema, J.; Bösl, K.; Nowak, D.A. Corticospinal Excitability and Hand Motor Recovery in Stroke: A Longitudinal Study. J. Neurol. 2018, 265, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.-Y.; Bruyère, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef]
- Bonatti, M.; Lombardo, F.; Valletta, R.; Comai, A.; Petralia, B.; Avesani, G.; Franchini, E.; Rossi, A.; De Santis, N.; Guerriero, M.; et al. Myosteatosis as an Independent Predictor of Short-Term Mortality in Successfully Reperfused Acute Ischemic Stroke. Neuroradiol. J. 2023, 36, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Hunnicutt, J.L.; Gregory, C.M. Skeletal Muscle Changes Following Stroke: A Systematic Review and Comparison to Healthy Individuals. Top. Stroke Rehabil. 2017, 24, 463–471. [Google Scholar] [CrossRef]
- Monjo, H.; Fukumoto, Y.; Asai, T.; Kubo, H.; Ohshima, K.; Tajitsu, H.; Koyama, S. Differences in Muscle Thickness and Echo Intensity Between Stroke Survivors and Age- and Sex-Matched Healthy Older Adults. Phys. Ther. Res. 2020, 23, 188–194. [Google Scholar] [CrossRef]
- Jakubowski, K.L.; Terman, A.; Santana, R.V.; Lee, S.S.M. Passive Material Properties of Stroke-Impaired Plantarflexor and Dorsiflexor Muscles. Clin. Biomech. 2017, 49, 48–55. [Google Scholar] [CrossRef]
- Clark, L.A.; Manini, T.M.; Wages, N.P.; Simon, J.E.; Russ, D.W.; Clark, B.C. Reduced Neural Excitability and Activation Contribute to Clinically Meaningful Weakness in Older Adults. J. Gerontol. Ser. A 2021, 76, 692–702. [Google Scholar] [CrossRef]
- Maitland, S.; Baker, S.N. Ipsilateral Motor Evoked Potentials as a Measure of the Reticulospinal Tract in Age-Related Strength Changes. Front. Aging Neurosci. 2021, 13, 612352. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I.; Narciss, S. Influence of taping on joint proprioception: A systematic review with meta-analysis. BMC Musculoskelet. Disord. 2024, 25, 480. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Gao, Z.; Yang, H.; Song, C. Influence of Proprioceptive Training Based on Ankle–Foot Robot on Improving LowerLimb Function in Patients after a Stroke. Front. Neurorobot. 2022, 16, 969671. [Google Scholar] [CrossRef]
- Etoom, M.; Al Battat, M.; Hanafi, I.; Manocchio, N.; Foti, C.; Alghwiri, A. Post-stroke fatigue subtypes and associated factors: Insights for targeted rehabilitation. J. Clin. Neurosci. 2025, 131, 111418. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).