Abstract
Background: This retrospective cross-sectional study evaluated the association of the global wall thickness index (GTI), derived from cardiovascular magnetic resonance (CMR), with demographic, clinical, and imaging findings, as well as heart failure history in transfusion-dependent thalassemia (TDT) patients. Methods: We analyzed 1154 TDT patients (52.9% female, 37.46 ± 10.67 years) from the Extension-Myocardial Iron Overload in Thalassemia project and 167 healthy controls (54.5% female, 36.33 ± 15.78 years). The CMR protocol included the T2* technique for the assessment of iron overload, cine imaging for the assessment of left ventricular (LV) function and size, and late gadolinium enhancement (LGE) imaging for the detection of replacement myocardial fibrosis (in the subset of 366 patients who underwent contrast administration). GTI (in mm/m2) was calculated from LV mass and end-diastolic volume. Results: GTI discriminated TDT patients from controls better than the LV end-diastolic volume index. Among TDT patients, GTI was higher in males, in those with diabetes, and in those with severe myocardial iron overload (cardiac T2* < 10 ms), but was unrelated to age, hemoglobin and ferritin levels, splenectomy, hepatic and pancreatic T2* values, LV ejection fraction, and fibrosis. GTI showed a diagnostic performance comparable to global heart T2* and superior to LV ejection fraction in identifying patients with prior heart failure. Conclusions: GTI is elevated in TDT patients compared with healthy controls. Male sex and severe myocardial iron overload are key determinants of GTI in TDT. Increased GTI is linked to a history of heart failure, supporting its role as a complementary tool to conventional CMR indices.
1. Introduction
Transfusion-dependent thalassemia (TDT) represents the most severe clinical phenotype of thalassemia syndromes, caused by genetic defects in hemoglobin synthesis, leading to ineffective erythropoiesis and severe anemia [1,2,3]. Lifelong, regular red blood cell transfusions remain the cornerstone of management, ensuring adequate oxygen delivery and promoting normal growth and development [4,5,6]. These advances, combined with iron chelation therapy, have transformed TDT from a fatal childhood disease into a chronic condition compatible with adulthood [7,8]. Despite these improvements, transfusion therapy inevitably leads to progressive iron accumulation, particularly in the liver, endocrine glands, and heart [9,10,11]. Myocardial iron overload (MIO) is the main determinant of morbidity and mortality in TDT [12,13,14], leading to oxidative stress, cellular damage, and the activation of inflammatory processes [15,16,17]. These processes contribute to the development of a cardiomyopathy, which typically manifests in two principal phenotypes [17,18,19,20]. The more common dilated form is characterized by progressive left ventricular (LV) dilation and systolic dysfunction, whereas the restrictive form is associated with impaired compliance and diastolic dysfunction, often leading to elevated filling pressures. Both pathways may progress to overt heart failure [18,20]. In addition to iron toxicity, chronic anemia contributes to cardiac remodeling [19]. Reduced oxygen-carrying capacity triggers compensatory mechanisms, including increased cardiac output, LV dilation, and eccentric hypertrophy [21,22,23], that can culminate in high-output heart failure [19]. Furthermore, as survival improves, comorbidities such as endocrine dysfunction (e.g., diabetes, hypogonadism, and hypothyroidism) and aging-related changes increasingly contribute to cardiac risk in this population [24,25,26]. The convergence of these stressors produces a heterogeneous spectrum of myocardial remodeling, underscoring the importance of a comprehensive cardiac assessment.
Cardiac magnetic resonance (CMR) imaging is the reference standard for noninvasive assessment of myocardial iron levels [27,28] and of ventricular morphology and function [29,30]. Conventional indices such as LV ejection fraction (EF), mass, and volume are crucial for risk stratification in a wide range of diseases [31,32,33,34,35], including TDT [36], but they often become abnormal only at advanced stages, leaving earlier structural changes in wall thickness, geometry, or myocardial mechanics undetected [37,38]. Novel CMR-derived markers, including strain and myocardial contraction fraction (MCF), have improved early detection of myocardial involvement and prognostic stratification in TDT [39,40] and across diverse disorders [41,42,43,44,45].
Global wall thickness (GT) and its body surface area-indexed counterpart, the global wall thickness index (GTI), have recently emerged as simple, reproducible parameters that integrate LV mass and end-diastolic volume into a single metric, offering a robust global estimate of average myocardial wall thickness [46]. A large CMR study demonstrated that GT independently predicts adverse outcomes, including death and heart failure hospitalization, while GTI can identify at-risk individuals even with preserved LV mass and EF [46]. Their high sensitivity to subtle wall thickness changes, combined with reliance on standard CMR metrics, makes GT and GTI valuable tools for early detection and routine monitoring of myocardial remodeling.
Given the complex and multifactorial nature of cardiac remodeling in TDT, GTI may represent a particularly valuable tool. However, its clinical role in this population remains unexplored.
Against this background, the present multicenter study sought to (1) characterize the distribution of GTI in TDT patients compared with healthy controls; (2) examine cross-sectional associations between GTI and demographic, clinical, and laboratory characteristics in TDT; (3) assess the relationship of GTI with myocardial iron overload, ventricular function, and fibrosis; (4) evaluate the potential clinical utility of GTI as a risk marker for heart failure in TDT.
2. Materials and Methods
2.1. Study Population
We retrospectively selected all 1154 patients with TDT (52.9% females, mean age 37.46 ± 10.67 years) who had been consecutively enrolled in the Extension–Myocardial Iron Overload in Thalassemia (E-MIOT) project in the nine-year interval between 2015 and 2024. The E-MIOT network is a nationwide Italian consortium comprising 66 thalassemia care centers and 15 magnetic resonance (MRI) units adhering to standardized and validated MRI protocols [47]. All centers are linked via a web-based database that records demographic, clinical, and imaging data for all patients.
All TDT patients had been receiving regular red blood cell transfusions since early childhood to maintain pre-transfusion hemoglobin levels above 9–10 g/dL. MRI assessments were scheduled within one week before a routine transfusion. Chelation therapy was introduced in the mid-to-late 1970s for older patients, while individuals born after this period generally began chelation during early childhood.
The control group consisted of 167 healthy individuals (54.5% females, mean age 36.32 ± 15.78 years) recruited from a multicenter cohort established to determine reference values for cardiac functional parameters and myocardial T1 values [48]. Control subjects were eligible if they had a normal resting electrocardiogram, no history or clinical evidence of cardiovascular disease, no conventional cardiovascular risk factors or systemic disorders, and no absolute contraindications to MRI.
The study adhered to the ethical principles outlined in the Declaration of Helsinki and received approval from the appropriate institutional ethics committee. All participants provided written informed consent.
2.2. MRI
MRI examinations were conducted on standard 1.5T clinical scanners from three vendors (Signa Excite HD or Artist, GE Healthcare, Milwaukee, WI, USA; Ingenia or Achieva, Philips, Best, The Netherlands; MAGNETOM Sola or Aera, Siemens, Erlangen, Germany). All acquisitions were performed during end-expiratory breath-holding with electrocardiographic gating.
T2* gradient-echo multiecho sequences [10 echo times (TEs), echo spacing 2.26 ms] were acquired to assess tissue iron overload. A mid-transverse hepatic slice [49], at least five axial slices covering the entire pancreas [50], and three short-axis LV views (basal, mid, and apical) [51] were obtained. T2* image analysis was performed by experienced (>15 years) operators using HIPPOMIOT® (Version 2.0, Consiglio Nazionale delle Ricerche and Fondazione Toscana Gabriele Monasterio, Pisa, Italy), a custom-written and previously validated software. Hepatic T2* values were measured within a circular region of interest (ROI) [49] and subsequently converted into liver iron concentration (LIC) values [52]. For the pancreas, three small ROIs were manually placed over the head, body, and tail, carefully restricted to parenchymal tissue while avoiding major vessels, ducts, and regions prone to susceptibility artifacts from adjacent structures such as the stomach or colonic lumen [50]. A global pancreatic T2* value was derived as the mean of these three measurements. Myocardial T2* values were analyzed using a 16-segment LV model, following the American Heart Association/American College of Cardiology standardized segmentation guidelines [53]. To account for cardiac and visceral geometric distortions as well as susceptibility artifacts, an internal correction map within the software was applied. The global heart T2* value was then calculated as the average of all segmental values. The reproducibility of T2* measurements across sites, studies, and observers had been previously assessed [47].
To quantify LV function parameters, steady-state free precession (SSFP) cine images were acquired during 8 s breath-holds in the vertical and horizontal long-axis planes, as well as in sequential contiguous 8 mm short-axis slices covering the ventricles from the atrioventricular ring to the apex [54]. Thirty cardiac phases were acquired per heartbeat. Imaging parameters included: flip angle 45°, TE 1.6 ms, repetition time 3.7 ms, and matrix size 192 × 192 pixels. Image analysis was performed by experienced operators, blinded to clinical data, using a commercial clinical workstation (MASS software, Medis, Leiden, The Netherlands, or cmr42, Circle Cardiovascular Imaging Inc., Calgary, AB, Canada). Endocardial and epicardial borders were manually traced in the short-axis stack at end-diastole and end-systole and end-diastolic and end-systolic volumes (EDV and ESV) were calculated without relying on a specific ventricular geometry. LV mass (LVM) was determined by multiplying myocardial volume by the specific myocardial density, assumed to be 1.05 g/cm3. LV volumes and mass were indexed to body surface area (BSA), calculated using a modified Dubois and Dubois formula [55]. Inter-center variability for quantification of LV functional parameters within the E-MIOT network has been previously determined [56]. The mean LV end-diastolic global wall thickness was quantified using the optimized equation [46]:
GT = 0.05 + 1.60 * LVM0.84 * LVEDV−0.49
GTI, expressed in mm/m2, was calculated as the ratio between GT and BSA.
Replacement/focal myocardial fibrosis was assessed by late gadolinium enhancement (LGE) imaging using a T1-weighted gradient-echo inversion-recovery sequence [flip angle 20°, minimum TE, inversion times 250–300 ms, matrix 224 × 192 pixels, slice thickness 8 mm]. Short-axis and long-axis views were acquired 8–18 min after intravenous administration of Gadobutrol (Gadovist®, Bayer Schering Pharma, Berlin, Germany) at a standard dose of 0.2 mmol/kg body weight. LGE imaging was not performed in patients with a glomerular filtration rate (GFR) < 30 mL/min/1.73 m2 or in those who refused the injection of contrast medium. Images were qualitatively assessed for the presence, pattern, and regional distribution of LGE [36]. LGE was confirmed only when observed in at least two imaging planes. Patterns were classified as ischemic when enhancement was subendocardial or transmural within a coronary artery territory; all other patterns were categorized as non-ischemic. The extent of LGE was assessed semi-quantitatively by estimating the number of affected myocardial segments.
2.3. Biochemical Assays
Biochemical tests were performed at the local laboratories of each participating thalassemia center using commercially available, standardized assay kits. For analysis, the average hemoglobin and serum ferritin values over the 12 months prior to the MRI examination were calculated for each patient.
2.4. Diagnostic Criteria
A threshold of 20 ms was adopted as a conservative cut-off for both segmental and global myocardial T2* values [57]. Patients with MIO were further categorized as having severe MIO (T2* < 10 ms) or moderate-to-mild MIO (T2* between 10 and 20 ms).
Diabetes mellitus was diagnosed in accordance with standard criteria: fasting plasma glucose ≥ 126 mg/dL, 2 h plasma glucose ≥ 200 mg/dL during an oral glucose tolerance test (OGTT), or random plasma glucose ≥ 200 mg/dL in the presence of classic hyperglycemia symptoms or a hyperglycemic crisis [58].
Heart failure was diagnosed based on a combination of clinical symptoms (e.g., fatigue, shortness of breath, ankle swelling), physical signs, biomarker levels, and imaging or other instrumental findings, following current guideline recommendations [59].
2.5. Statistical Analysis
Statistical analysis was conducted using SPSS version 27.0 (IBM Corp, Armonk, NY, USA) and MedCalc version 19.8 (MedCalc Software Ltd., Ostend, Belgium) statistical packages.
Continuous variables are expressed as mean ± standard deviation (SD) and categorical variables as frequencies and percentages.
The Kolmogorov–Smirnov test was used to assess the normality of continuous variables.
For continuous variables with a normal distribution, comparisons between groups were performed using the independent-samples t-test (for two groups) or one-way ANOVA (for more than two groups). For continuous variables with a non-normal distribution, the Mann–Whitney U test or Kruskal–Wallis test was applied. Categorical variables were compared using the χ2 test. The Bonferroni post hoc test was used for multiple comparisons between pairs of groups.
Correlations were assessed using Pearson’s or Spearman’s tests.
Univariable regression analyses were first performed to explore potential determinants of GTI. Variables showing statistical significance (p < 0.05) were subsequently included in a stepwise multivariable regression model to identify independent predictors. Multicollinearity of variables tested in the multivariate model was assessed using the variance inflation factor (inflated if > 5) and its tolerance statistic (inflated if < 0.20).
Receiver operating characteristic (ROC) curve analysis was used to evaluate diagnostic performance and determine optimal cut-off values for predicting a specific condition. The area under the curve (AUC) with 95% confidence intervals (CIs), sensitivity, and specificity were calculated. The DeLong’s test was applied to assess whether differences in AUCs between parameters were statistically significant.
All tests were two-sided, and p < 0.05 was considered statistically significant.
3. Results
3.1. Comparison Between TDT Patients and Healthy Subjects
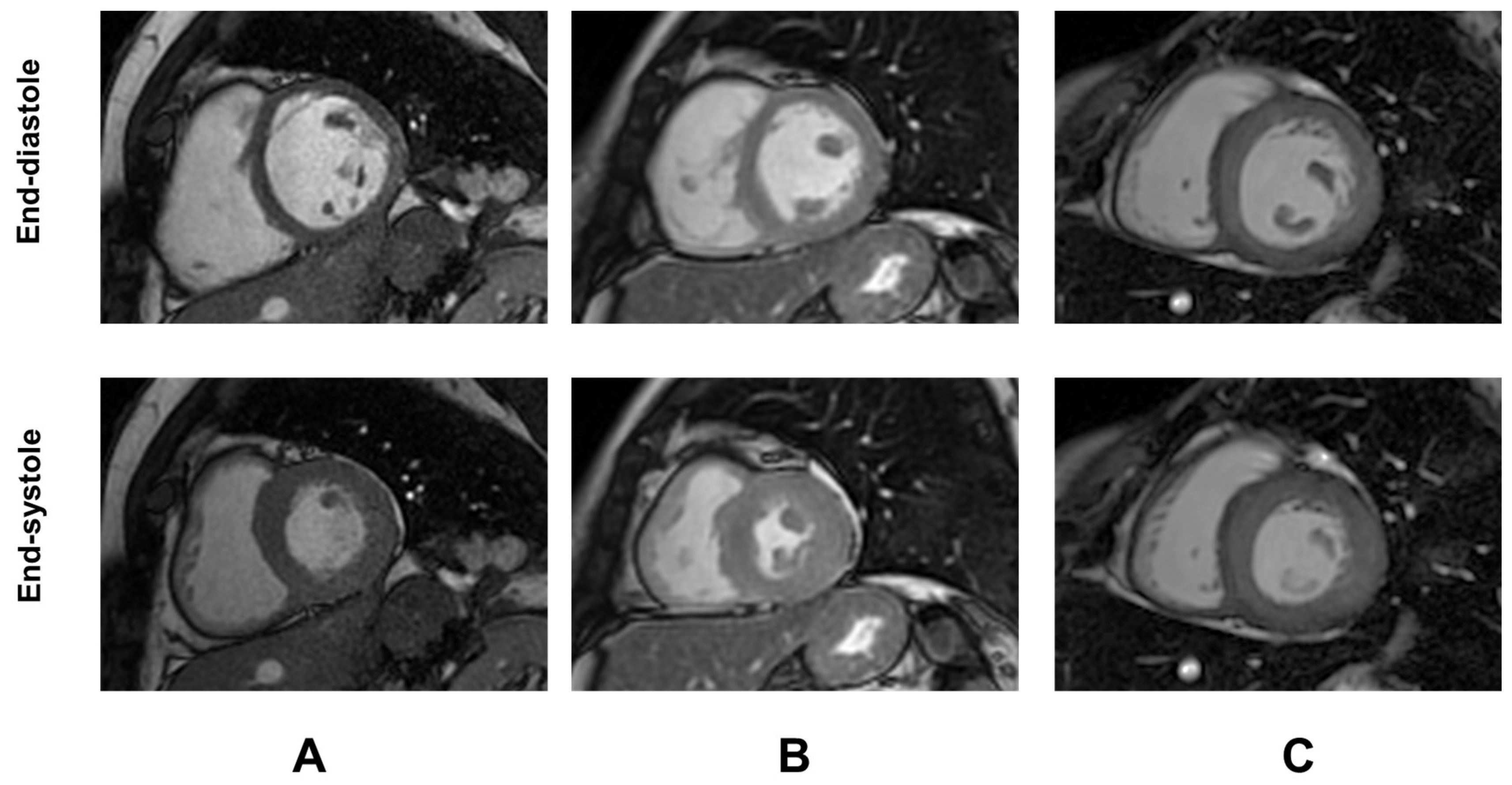
Demographic and clinical characteristics and MRI findings of TDT patients are summarized in Table 1. Representative examples of GTI are shown in Figure 1.

Table 1.
Demographic, clinical, and MRI data of TDT patients.
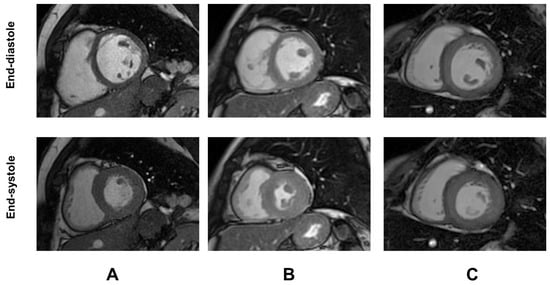
Figure 1.
Representative examples of global wall thickness index (GTI) in three female patients with TDT. (A) Patient with normal GTI. (B) Patient with increased GTI but normal LV mass index [concentric remodeling]. (C) Patient with both increased GTI and LV mass index [concentric hypertrophy].
No significant difference between TDT patients and healthy subjects was found in terms of age and sex (p = 0.234 and p = 0.708, respectively).
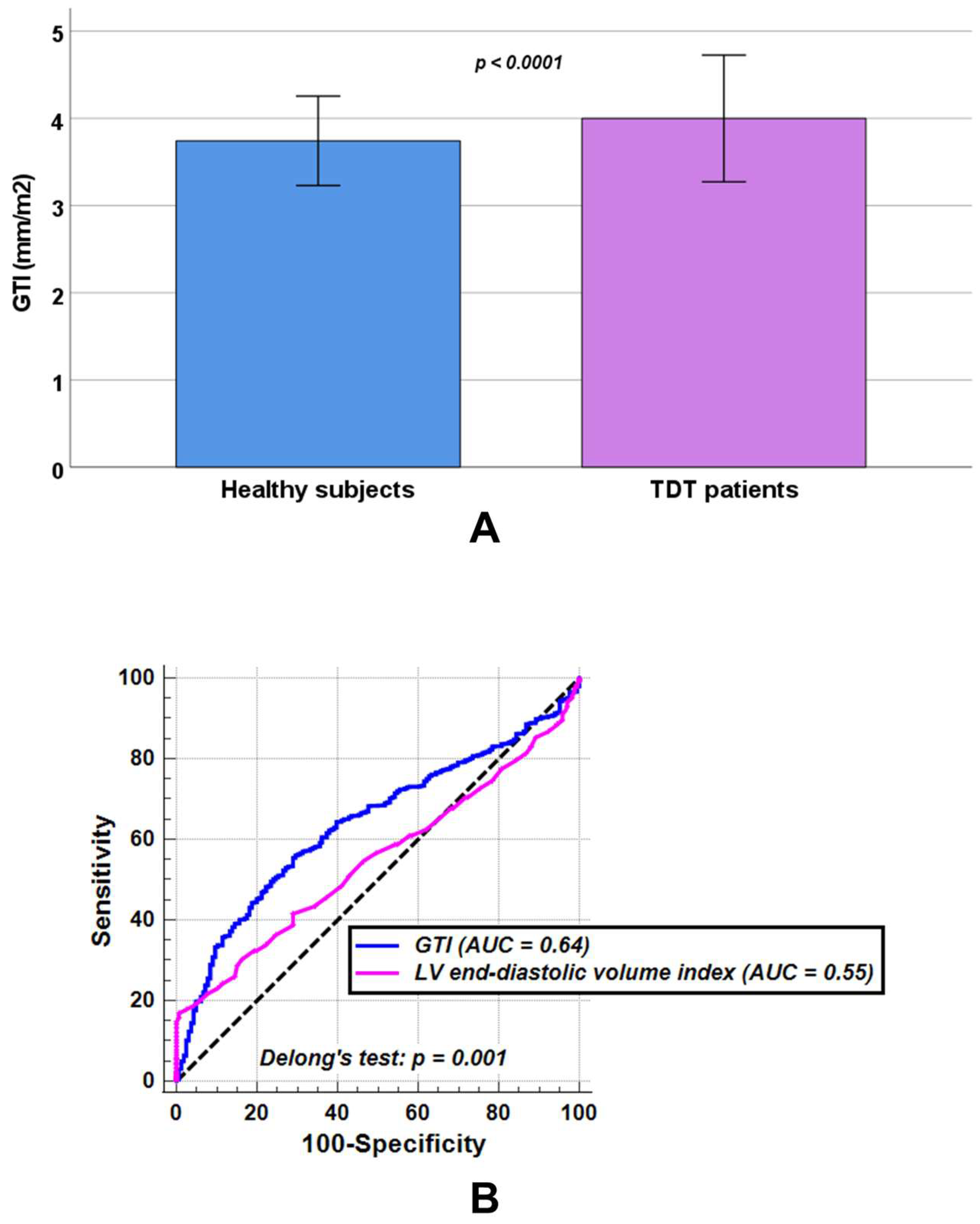
In comparison with healthy individuals, patients with TDT showed significantly increased LV end-diastolic volume index (82.48 ± 16.96 mL/m2 vs. 78.86 ± 10.28 mL/m2, p = 0.041) and LV end-systolic volume index (31.57 ± 10.81 mL/m2 vs. 28.87 ± 6.35 mL/m2, p = 0.011), while no statistically significant differences were observed between the two groups in LV mass index (54.91 ± 13.69 g/m2 vs. 53.62 ± 8.92 g/m2, p = 0.484) or LV ejection fraction (62.32 ± 6.99% vs. 63.35 ± 5.36%, p = 0.097). TDT patients had a significantly higher GTI than healthy subjects (3.99 ± 0.73 mm/m2 vs. 3.74 ± 0.51 mm/m2, p < 0.0001) (Figure 2A).
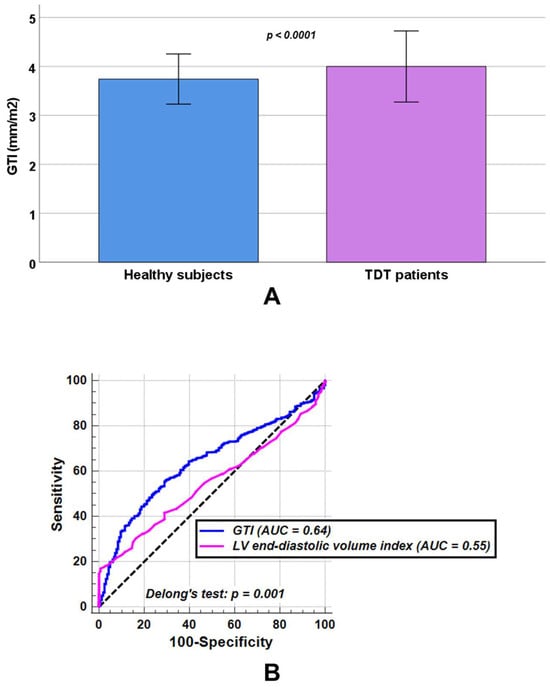
Figure 2.
(A) Comparison of global wall thickness index (GTI) between healthy subjects and patients with transfusion-dependent thalassemia (TDT). (B) Receiver operating characteristic curve (ROC) curve analysis of GTI [blue] and left ventricular (LV) end-diastolic volume index [pink] to discriminate between TDT patients and healthy subjects. AUC = area under the curve.
At ROC curve analysis, a GTI > 3.91 mm/m2 discriminated between healthy subjects and TDT patients with a sensitivity of 55.9% and a specificity of 70.5% (p < 0.0001). The AUC was 0.64 (95% CIs: 0.61 to 0.67). An LV end-diastolic volume index > 98.00 mL/m2 discriminated between healthy subjects and TDT patients with a sensitivity of 16.8% and a specificity of 99.4% (p = 0.014). The AUC was 0.55 (95% CIs: 0.52 to 0.58). According to Delong’s test, GTI performed significantly better in discriminating healthy subjects from TDT patients than LV end-diastolic volume index (p = 0.001) (Figure 2B).
In healthy subjects, the GTI was comparable between males and females (3.75 ± 0.57 mm/m2 vs. 3.73 ± 0.47 mm/m2, p = 0.911) and was independent from age (R = −0.112, p = 0.151).
3.2. Associations Between Demographic/Clinical Characteristics and GTI in TDT
GTI was significantly higher in males than in females (4.06 ± 0.71 mm/m2 vs. 3.95 ± 0.73 mm/m2, p = 0.004), while its correlation with aging showed a trend toward statistical significance (R = −0.057, p = 0.053).
GTI was comparable between non-splenectomized and splenectomized patients (4.01 ± 0.80 mm/m2 vs. 3.99 ± 0.66 mm/m2, p = 0.774), and was not significantly correlated with age at start of regular transfusions (R = −0.031, p = 0.379), chelation starting age (R = −0.098, p = 0.094), mean serum hemoglobin levels (R = −0.016, p = 0.596), and mean serum ferritin levels (R = 0.029, p = 0.350).
Among the 1105 patients evaluated for diabetes mellitus, the condition was identified in 15.7% of cases. The GTI was significantly higher in patients with diabetes than in patients without diabetes (4.08 ± 0.75 mm/m2 vs. 3.97 ± 0.70 mm/m2, p = 0.037).
3.3. Associations Between GTI and Iron Overload in TDT
GTI was not correlated with MRI LIC values (R = 0.056, p = 0.057), pancreatic T2* values (R = −0.026, p = 0.373), global heart T2* values (R = 0.023, p = 0.433), or number of segments with T2* < 20 ms (R = 0.046, p = 0.116).
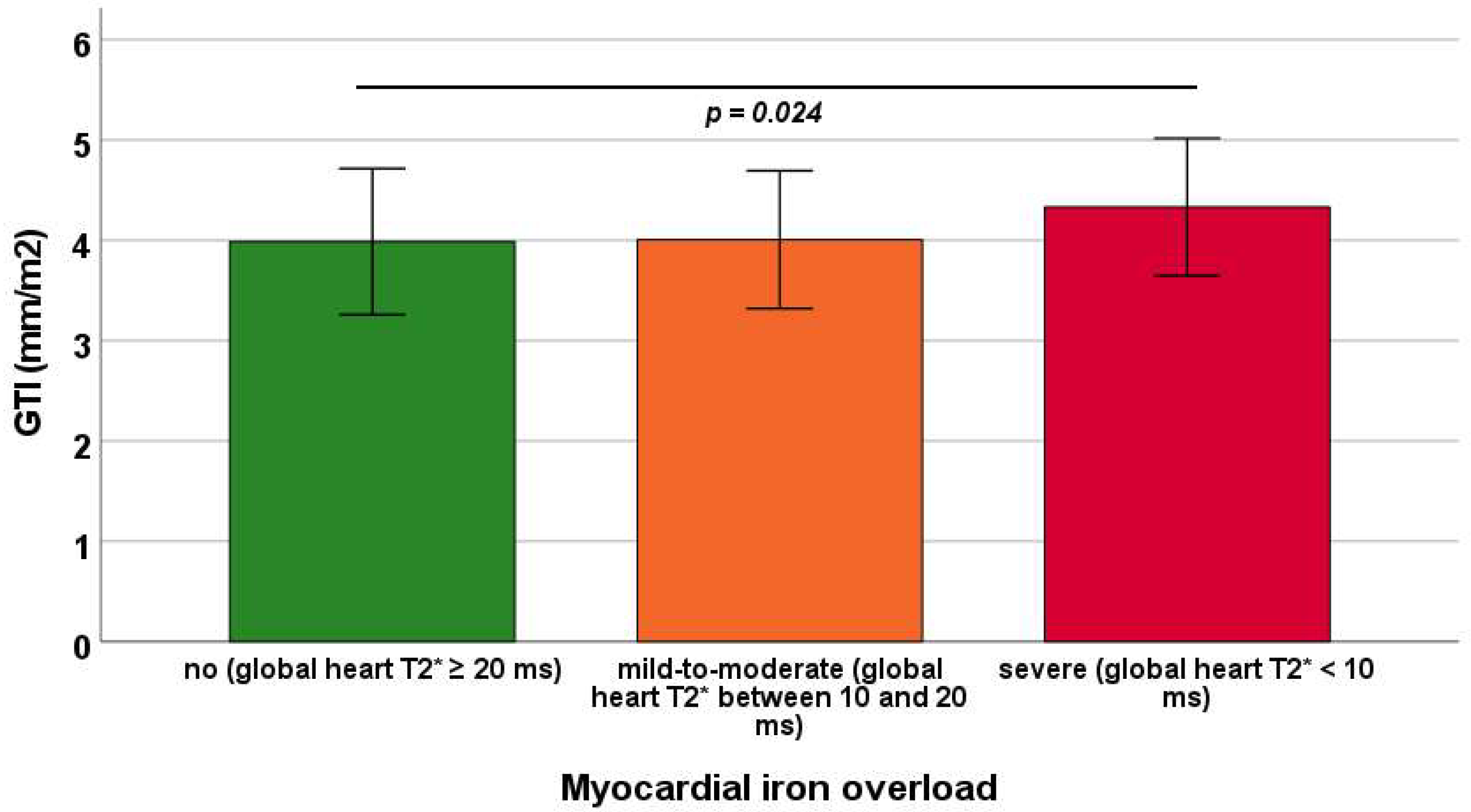
Mean GTI was 3.99 ± 0.73 mm/m2 in patients without MIO, 4.01 ± 0.69 mm/m2 in those with mild-to-moderate MIO, and 4.33 ± 0.68 mm/m2 in those with severe MIO. GTI was significantly increased in patients with severe MIO compared with those without MIO (p = 0.024), whereas other between-group comparisons did not reach statistical significance (Figure 3).

Figure 3.
Mean GTI (global wall thickness index) in the three groups of transfusion-dependent thalassemia patients identified based on global heart T2* values. The horizontal line indicates a significant difference between two groups.
3.4. Predictors of GTI
Table 2 summarizes the results of the univariable and multivariable regression analyses for predictors of GTI. In the univariable models, male sex and severe MIO (compared with patients with moderate-to-mild or no MIO grouped together) were significantly associated with GTI, and both remained independent predictors in the multivariable analysis (F = 7.77, p < 0.0001). No evidence of collinearity was found between the two variables (tolerance = 0.99 and variance inflation factor = 1.01).

Table 2.
Determinants of GTI identified by univariable and multivariable linear regression analyses.
3.5. Association of GTI with LV Function and Fibrosis
GTI was not associated with LV ejection fraction (R = 0.021, p = 0.472).
Of the 366 (31.7%) patients who underwent contrast administration, 95 (26.3%) demonstrated replacement myocardial fibrosis in at least one myocardial segment. The majority exhibited a non-ischemic pattern of LGE (N = 91, 95.8%), with septal involvement observed in 81.1% of cases. GTI was comparable between patients without and with LGE (4.12 ± 0.61 mm/m2 vs. 4.17 ± 0.69 mm/m2, p = 0.899). Among LGE-positive patients, the mean number of affected myocardial segments was 2.6 ± 1.8 (range: 1–9), and 63 (66.3%) patients displayed multifocal involvement, defined as two or more affected segments. GTI was not correlated with the number of LGE-positive segments (R = 0.137, p = 0.186).
3.6. Association Between GTI and History of Heart Failure
Sixty-six patients had a history of heart failure.
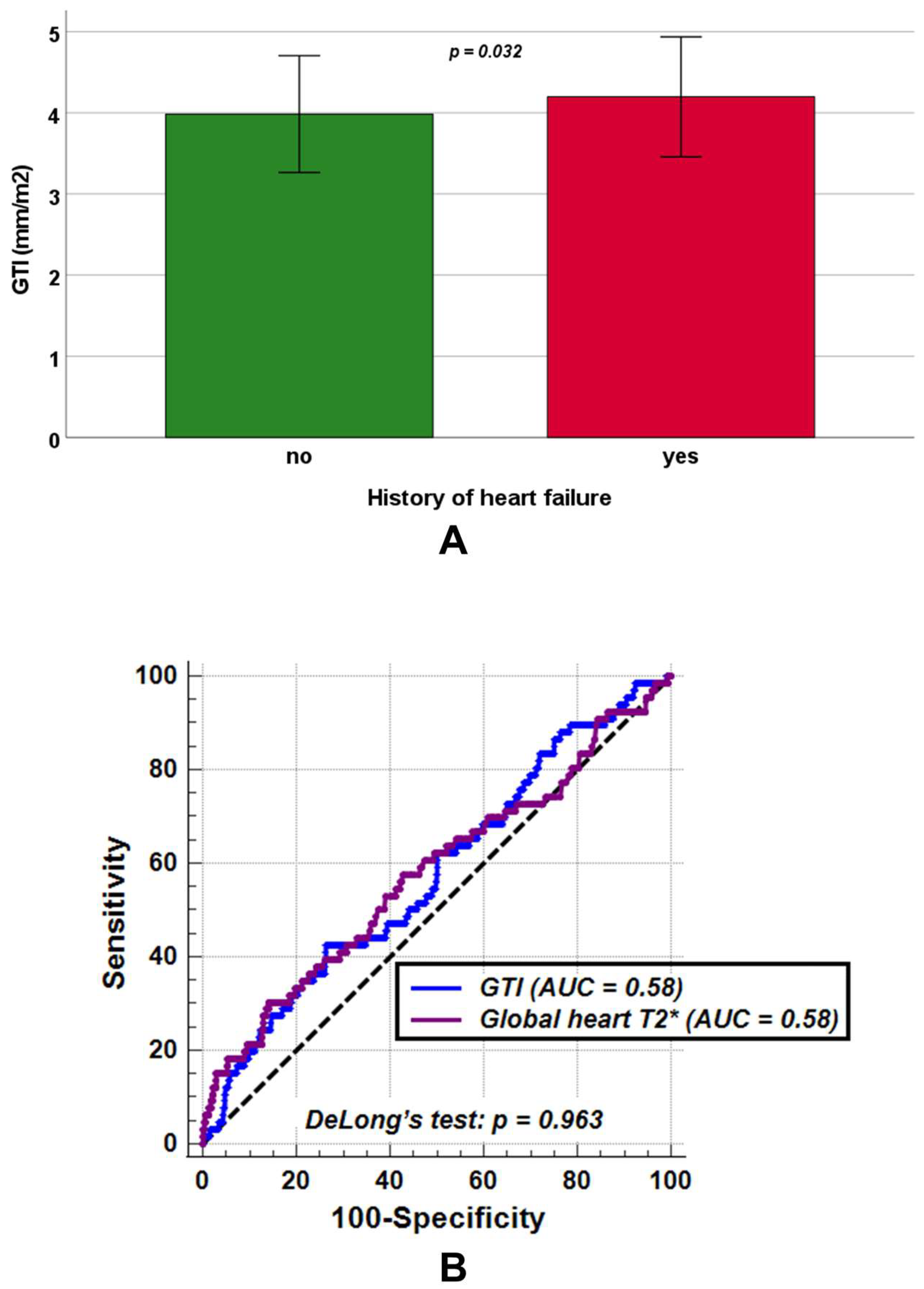
No significant differences between patients without and with a history of heart failure were found in LV end-diastolic volume index (82.48 ± 16.90 mL/m2 vs. 81.58 ± 17.99 mL/m2, p = 0.761) and LV mass index (54.72 ± 13.76 g/m2 vs. 56.76 ± 13.12 g/m2, p = 0.081), while GTI was significantly increased among patients with a history of heart failure (4.19 ± 0.74 mm/m2 vs. 3.98 ± 0.72 mm/m2, p = 0.038) (Figure 4A).Moreover, patients with a history of heart failure exhibited significantly lower LV ejection fraction (59.77 ± 9.88% vs. 62.49 ± 6.76%, p = 0.030) and global heart T2* values (33.16 ± 13.17 ms vs. 37.28 ± 9.30 ms, p = 0.032).
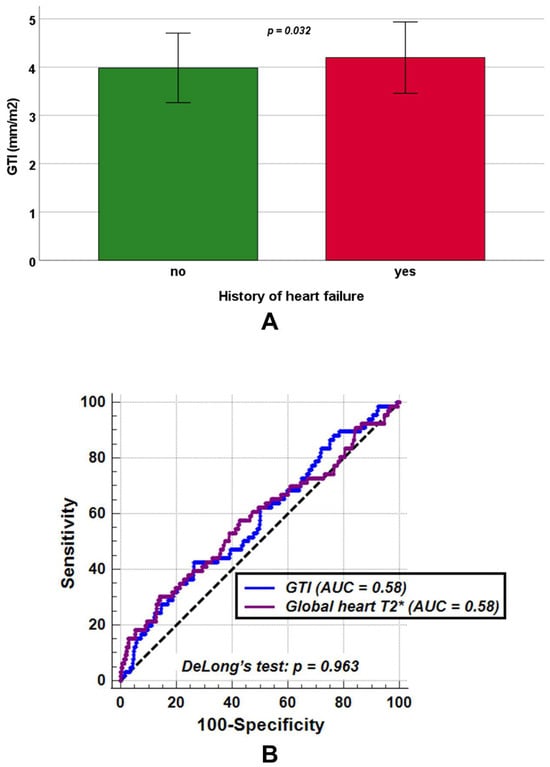
Figure 4.
(A) Mean GTI (global wall thickness index) in transfusion-dependent thalassemia patients without and with a history of heart failure. (B) Comparison of receiver operating characteristic curve (ROC) curves for GTI [blue] and global heart T2* values [purple] to detect a positive history of heart failure. AUC = area under the curve.
ROC curve analysis demonstrated that a GTI > 4.34 mm/m2 predicted a positive history of heart failure with a sensitivity of 42.4% and a specificity of 73.7% (p = 0.041), yielding an AUC of 0.58 (95% CIs: 0.55 to 0.61). Similarly, a global heart T2* ≤ 29.56 ms predicted a positive heart failure history with a sensitivity of 30.3% and a specificity of 85.9% (p = 0.049), also with an AUC of 0.58 (95% CIs: 0.55 to 0.61). DeLong’s test revealed no significant difference between the two AUCs (p = 0.963) (Figure 4B). The ROC curve analysis did not identify an LV ejection fraction threshold that significantly improved the detection of a positive heart failure history with acceptable sensitivity and specificity (AUC = 0.55, 95% CI: 0.52 to 0.58, p = 0.223).
4. Discussion
In this large cohort of well-treated TDT patients, we explored the clinical significance of the GTI in comparison with healthy subjects, as well as its associations with demographic and clinical variables, iron overload, myocardial function, and heart failure.
GTI emerged as a more sensitive marker of LV remodeling in TDT than conventional volumetric or functional indices. While LV mass index and ejection fraction did not differ significantly between patients and healthy subjects, both LV end-diastolic volume index and GTI were consistently elevated in TDT. These findings support the concept that myocardial remodeling in TDT follows a stepwise course, with early geometric alterations detectable by CMR preceding overt systolic dysfunction or increases in LV mass [18,60,61]. ROC analysis demonstrated that GTI had superior discriminatory ability compared with LV end-diastolic volume index, highlighting its potential value in distinguishing TDT patients from healthy individuals. Although its diagnostic accuracy was modest (AUC 0.64), limiting its use as a standalone parameter, GTI appears to capture subtle myocardial changes not reflected by conventional indices, underscoring that standard measures of systolic function may underestimate early cardiac involvement in this population.
In TDT, male sex emerged as a consistent predictor of higher GTI, both in univariable and multivariable analyses. In contrast, GTI was comparable between sexes in our healthy controls. A previous CMR study reported significantly higher absolute GT values in healthy males compared to females (7.2 ± 0.7 mm vs. 5.9 ± 0.6 mm) [46]. However, indexing for BSA reduced this difference, making GTI less sex-dependent (females: 3.4 ± 0.4 mm/m2 vs. males: 3.6 ± 0.4 mm/m2), suggesting that BSA adjustment compensates for sex-related differences in cardiac morphology and provides a standardized measure suitable for clinical practice. Several factors may explain the association between male sex and higher GTI in TDT. One possibility is the relative under-transfusion in males, as current transfusion protocols do not adjust for gender-related differences in blood volume [62]. This may result in a more chronically anemic state, contributing to volume overload and ventricular remodeling. Another potential mechanism is the differential susceptibility to oxidative stress: despite similar cardiac iron levels, females may tolerate chronic oxidative stress more effectively [63,64], reducing the impact of iron toxicity on myocardial structure over time.
Although GTI was not significantly correlated with global heart T2* values, severe MIO emerged as an independent predictor of higher GTI, indicating that extreme iron deposition can drive maladaptive left ventricular remodeling. The lack of a direct association with current T2* values is likely influenced by prior CMR-guided adjustments in chelation therapy, as most patients were not naïve to CMR imaging. While intensive chelation can effectively reduce myocardial iron overload [65,66,67,68,69,70], structural changes induced by previous iron accumulation may be only partially reversible. Consequently, GTI may reflect the cumulative or residual effects of past iron burden on LV geometry rather than the contemporaneous iron load alone. The observation that GTI increases primarily in patients with severe MIO further underscores the progressive nature of iron-related cardiac remodeling, suggesting a threshold effect in which only substantial iron overload produces measurable structural alterations. These findings highlight the importance of early detection and aggressive iron management to prevent irreversible myocardial remodeling and potential progression to cardiomyopathy.
The lack of an association between GTI and LV ejection fraction suggests that early geometric remodeling captured by GTI can occur independently of global systolic function. Similarly, the absence of a relationship between GTI and replacement myocardial fibrosis indicates that GTI primarily reflects diffuse or early structural remodeling rather than an established focal scar. However, this result should be interpreted with caution, as fibrosis assessment by LGE was available for only a third of the cohort, which may have affected the statistical power of the analysis.
Our findings show that GTI was significantly higher in TDT patients with a history of heart failure, accompanied by reduced LVEF and impaired myocardial iron status. The relatively modest sensitivity of GTI in predicting heart failure history likely reflects both the limited number of positive cases and the multifactorial pathogenesis of heart failure in thalassemia. Importantly, GTI demonstrated a diagnostic performance comparable to global myocardial T2* and showed higher sensitivity and specificity than LV ejection fraction. From a pathophysiological perspective, our results are clinically meaningful. LV ejection fraction is a conventional measure of systolic performance [38,71], but in thalassemia it is heavily influenced by chronic anemia and a compensatory hyperdynamic circulation, which maintains elevated stroke volumes and ventricular dimensions [18,21]. As a result, LV ejection fraction often remains within normal or near-normal ranges, even in the presence of impaired myocardial shortening and evolving systolic dysfunction [72,73]. This limitation reduces its utility for early risk stratification. In contrast, GTI captures changes in myocardial geometry and deformation that are more closely linked to subtle alterations in ventricular structure. Thus, elevations in GTI may reflect the onset of adverse remodeling well before declines in LV ejection fraction become evident.
Taken together, our results suggest that GTI represents a sensitive marker of early myocardial involvement and may serve as a valuable tool to improve the clinical management of patients with TDT. By identifying adverse remodeling at a subclinical stage, GTI could complement established markers such as T2*, offering an opportunity for earlier intervention and potentially better long-term outcomes.
Limitations
This work has several important constraints.
The retrospective nature of the study introduces the possibility of selection bias and incomplete clinical information, with some relevant variables not captured in the database. Advanced CMR mapping techniques such as T1, T2, and extracellular volume quantification were not available at the time of patient enrollment, limiting our ability to perform a complete and sensitive myocardial tissue characterization [74,75,76]. Similarly, myocardial deformation indices were not evaluated. Although strain analysis is increasingly recognized as a sensitive marker of subclinical ventricular dysfunction [77,78], the lack of uniform access to feature tracking software across E-MIOT centers prevented its systematic inclusion.
The cross-sectional design restricts interpretation of causality and precludes conclusions on the temporal evolution of GTI in relation to disease progression. The prognostic significance of GTI in anticipating adverse cardiovascular outcomes was not explored, and longitudinal changes in this parameter remain undefined. Future prospective studies with serial follow-up are required to establish clinically relevant GTI thresholds and to clarify whether its integration with conventional indices, such as myocardial T2*, enhances risk stratification.
5. Conclusions
In patients with TDT, GTI is significantly elevated compared with healthy controls, with male sex and severe myocardial iron overload emerging as its main determinants. Increased GTI is linked to a history of heart failure, suggesting that it may reflect early adverse remodeling and could complement established markers such as myocardial iron load and LV function. Although not sufficient as a standalone tool, GTI shows promise as part of a multiparametric approach to improve risk stratification and guide management in TDT, a role that should be confirmed in prospective studies.
Author Contributions
Conceptualization, A.M.; methodology, A.M.; software, V.P.; formal analysis, A.M.; investigation, A.M.; resources, G.P., M.Z., S.R., P.F., A.V., F.L., A.S., Z.B., V.C., G.M., E.C. and A.C.; data curation, L.P.; writing—original draft preparation, A.M.; writing—review and editing, L.P., G.P., M.Z., S.R., P.F., A.V., F.L., A.S., Z.B., V.C., G.M., E.C., V.P. and A.C.; supervision, A.B. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
The E-MIOT project received “no-profit support” from industrial sponsorships (Chiesi Farmaceutici S.p.A. and Bayer).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Area Vasta Nord Ovest (protocol code 56664, date of approval 8 October 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy.
Acknowledgments
We would like to thank all the colleagues involved in the E-MIOT project (https://emiot.ftgm.it/). We thank all healthy subjects and TDT patients for their cooperation. This project is carried out within the framework of the European Reference Network on Rare Haematological Diseases (ERN-EuroBloodNet)-Project ID No 101085717. ERN-EuroBloodNet is partly co-funded by the European Union within the framework of the Fourth EU Health Programme.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in study design, data collection, analysis, the decision to publish, or the preparation of the manuscript.
References
- Angastiniotis, M.; Lobitz, S. Thalassemias: An Overview. Int. J. Neonatal Screen. 2019, 5, 16. [Google Scholar] [CrossRef]
- Weatherall, D.J. Thalassemia as a global health problem: Recent progress toward its control in the developing countries. Ann. N. Y. Acad. Sci. 2010, 1202, 17–23. [Google Scholar] [CrossRef]
- Taher, A.T.; Musallam, K.M.; Cappellini, M.D. beta-Thalassemias. N. Engl. J. Med. 2021, 384, 727–743. [Google Scholar] [CrossRef]
- El-Beshlawy, A.; Dewedar, H.; Hindawi, S.; Alkindi, S.; Tantawy, A.A.; Yassin, M.A.; Taher, A.T. Management of transfusion-dependent β-thalassemia (TDT): Expert insights and practical overview from the Middle East. Blood Rev. 2024, 63, 101138. [Google Scholar] [CrossRef]
- Forni, G.L.; Grazzini, G.; Boudreaux, J.; Agostini, V.; Omert, L. Global burden and unmet needs in the treatment of transfusion-dependent β-thalassemia. Front. Hematol. 2023, 2, 1187681. [Google Scholar] [CrossRef]
- Taher, A.T.; Farmakis, D.; Porter, J.B.; Cappellini, M.D.; Musallam, K.M. Guidelines for the Management of Transfusion-Dependent β-Thalassaemia, 5th ed.; Thalassaemia International Federation: Nicosia, Cyprus, 2025. [Google Scholar]
- Origa, R. β-Thalassemia. Genet. Med. 2017, 19, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Giakoumis, A.; Angastiniotis, M.; Eleftheriou, A. The changing epidemiology of the ageing thalassaemia populations: A position statement of the Thalassaemia International Federation. Eur. J. Haematol. 2020, 105, 16–23. [Google Scholar] [CrossRef]
- Shander, A.; Cappellini, M.D.; Goodnough, L.T. Iron overload and toxicity: The hidden risk of multiple blood transfusions. Vox Sang. 2009, 97, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.T.; Sayani, F.; Trompeter, S.; Drasar, E.; Piga, A. Challenges of blood transfusions in β-thalassemia. Blood Rev. 2019, 37, 100588. [Google Scholar] [CrossRef]
- Yadav, P.K.; Singh, A.K. A Review of Iron Overload in Beta-Thalassemia Major, and a Discussion on Alternative Potent Iron Chelation Targets. Plasmatology 2022, 16, 26348535221103560. [Google Scholar] [CrossRef]
- Borgna-Pignatti, C.; Rugolotto, S.; De Stefano, P.; Zhao, H.; Cappellini, M.D.; Del Vecchio, G.C.; Romeo, M.A.; Forni, G.L.; Gamberini, M.R.; Ghilardi, R.; et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 2004, 89, 1187–1193. [Google Scholar]
- Modell, B.; Khan, M.; Darlison, M.; Westwood, M.A.; Ingram, D.; Pennell, D.J. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2008, 10, 42. [Google Scholar] [CrossRef]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Cecinati, V.; Maggio, A.; Sorrentino, F.; Filosa, A.; Rosso, R.; et al. National networking in rare diseases and reduction of cardiac burden in thalassemia major. Eur. Heart J. 2022, 43, 2482–2492. [Google Scholar] [CrossRef] [PubMed]
- Gammella, E.; Recalcati, S.; Rybinska, I.; Buratti, P.; Cairo, G. Iron-induced damage in cardiomyopathy: Oxidative-dependent and independent mechanisms. Oxid. Med. Cell Longev. 2015, 2015, 230182. [Google Scholar] [CrossRef]
- Ahmed, S.; Peterson, S.J.; Parikh, M.A.; Frishman, W.H. Cardiovascular Manifestations of Hemochromatosis: A Review of Pathophysiology, Mechanisms, and Treatment Options. Cardiol. Rev. 2025, 33, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; El Dahdah, J.; Haroun, E.; Arockiam, A.D.; Safdar, A.; Sorathia, S.; Dong, T.; Griffin, B.; Wang, T.K.M. A Contemporary Review of Clinical Manifestations, Evaluation, and Management of Cardiac Complications of Iron Overload. Hearts 2025, 6, 17. [Google Scholar] [CrossRef]
- Kremastinos, D.T.; Farmakis, D.; Aessopos, A.; Hahalis, G.; Hamodraka, E.; Tsiapras, D.; Keren, A. Beta-thalassemia cardiomyopathy: History, present considerations, and future perspectives. Circ. Heart Fail. 2010, 3, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Triposkiadis, F.; Lekakis, J.; Parissis, J. Heart failure in haemoglobinopathies: Pathophysiology, clinical phenotypes, and management. Eur. J. Heart Fail. 2017, 19, 479–489. [Google Scholar] [CrossRef]
- Akiki, N.; Hodroj, M.H.; Bou-Fakhredin, R.; Matli, K.; Taher, A.T. Cardiovascular Complications in β-Thalassemia: Getting to the Heart of It. Thalass. Rep. 2023, 13, 38–50. [Google Scholar] [CrossRef]
- Aessopos, A.; Berdoukas, V. Cardiac function and iron chelation in thalassemia major and intermedia: A review of the underlying pathophysiology and approach to chelation management. Mediterr. J. Hematol. Infect. Dis. 2009, 1, e2009002. [Google Scholar] [CrossRef]
- Williams, A.M.; Levine, B.D.; Stembridge, M. A change of heart: Mechanisms of cardiac adaptation to acute and chronic hypoxia. J. Physiol. 2022, 600, 4089–4104. [Google Scholar] [CrossRef] [PubMed]
- Oni, O.O.; Adebiyi, A.A.; Aje, A.; Akingbola, T.S. Left ventricular geometry and electrocardiographic criteria in assessing left ventricular hypertrophy in sickle cell anemia patients. J. Natl. Med. Assoc. 2022, 114, 504–511. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Rossi, G.; Caruso, V.; Cuccia, L.; Spasiano, A.; Gerardi, C.; Zuccarelli, A.; D’Ascola, D.G.; Grimaldi, S.; et al. Cardiac complications and diabetes in thalassaemia major: A large historical multicentre study. Br. J. Haematol. 2013, 163, 520–527. [Google Scholar] [CrossRef]
- Barbero, U.; Ajassa, M.; Gaglioti, C.M.; Piga, A.; Ferrero, G.B.; Longo, F. The Influence of Cardiovascular Risk Factors and Hypogonadism on Cardiac Outcomes in an Aging Population of Beta-Thalassemia Patients. J. Cardiovasc. Dev. Dis. 2021, 9, 3. [Google Scholar] [CrossRef]
- Wood, J.C. Cardiac complications in thalassemia throughout the lifespan: Victories and challenges. Ann. N. Y. Acad. Sci. 2023, 1530, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Pennell, D.J.; Udelson, J.E.; Arai, A.E.; Bozkurt, B.; Cohen, A.R.; Galanello, R.; Hoffman, T.M.; Kiernan, M.S.; Lerakis, S.; Piga, A.; et al. Cardiovascular function and treatment in beta-thalassemia major: A consensus statement from the American Heart Association. Circulation 2013, 128, 281–308. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.I.; Kallifatidis, A.; Kourtidou, S.; Lama, N.; Christidi, A.; Detorakis, E.; Chatzantonis, G.; Vrachliotis, T.; Karamitsos, T.; Kouskouras, K.; et al. Cardiovascular magnetic resonance for the evaluation of patients with cardiovascular disease: An overview of current indications, limitations, and procedures. Hell. J. Cardiol. 2023, 70, 53–64. [Google Scholar] [CrossRef]
- Liu, C.; Ferrari, V.A.; Han, Y. Cardiovascular Magnetic Resonance Imaging and Heart Failure. Curr. Cardiol. Rep. 2021, 23, 35. [Google Scholar] [CrossRef]
- Merlo, M.; Gagno, G.; Baritussio, A.; Bauce, B.; Biagini, E.; Canepa, M.; Cipriani, A.; Castelletti, S.; Dellegrottaglie, S.; Guaricci, A.I.; et al. Clinical application of CMR in cardiomyopathies: Evolving concepts and techniques: A position paper of myocardial and pericardial diseases and cardiac magnetic resonance working groups of Italian society of cardiology. Heart Fail. Rev. 2023, 28, 77–95. [Google Scholar] [CrossRef]
- Azpiroz Franch, M.J.; Casas Masnou, G.; Romero, A.; Gonzalez Del Hoyo, M.I.; Larranaga Moreira, J.M.; Escalona Silva, R.; Guala, A.; Limeres Freire, J.; Bayes De Luna, A.; Zorio Grima, E.; et al. Prognostic role of cardiac magnetic resonance in left ventricular non compaction. Eur. Heart J. 2022, 43, ehac544.254. [Google Scholar] [CrossRef]
- Klem, I.; Shah, D.J.; White, R.D.; Pennell, D.J.; van Rossum, A.C.; Regenfus, M.; Sechtem, U.; Schvartzman, P.R.; Hunold, P.; Croisille, P.; et al. Prognostic Value of Routine Cardiac Magnetic Resonance Assessment of Left Ventricular Ejection Fraction and Myocardial Damage. Circ. Cardiovasc. Imaging 2011, 4, 610–619. [Google Scholar] [CrossRef]
- Boretto, P.; Patel, N.H.; Patel, K.; Rana, M.; Saglietto, A.; Soni, M.; Ahmad, M.; Sin Ying Ho, J.; De Filippo, O.; Providencia, R.A.; et al. Prognosis prediction in cardiac amyloidosis by cardiac magnetic resonance imaging: A systematic review with meta-analysis. Eur. Heart J. Open 2023, 3, oead092. [Google Scholar] [CrossRef]
- Dohy, Z.; Szabo, L.; Toth, A.; Czimbalmos, C.; Horvath, R.; Horvath, V.; Suhai, F.I.; Geller, L.; Merkely, B.; Vago, H. Prognostic significance of cardiac magnetic resonance-based markers in patients with hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2021, 37, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Kasa, G.; Teis, A.; Juncà, G.; Aimo, A.; Lupón, J.; Cediel, G.; Santiago-Vacas, E.; Codina, P.; Ferrer-Sistach, E.; Vallejo-Camazón, N.; et al. Clinical and prognostic implications of left ventricular dilatation in heart failure. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 849–856. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Rossi, G.; Midiri, M.; Missere, M.; Valeri, G.; Sorrentino, F.; D’Ascola, D.G.; Spasiano, A.; Filosa, A.; et al. Prediction of cardiac complications for thalassemia major in the widespread cardiac magnetic resonance era: A prospective multicentre study by a multi-parametric approach. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, P.L.M.; van de Ven, P.M.; Yoo, B.; Peace, R.A.; Heyndrickx, G.R.; Handly, N. Ejection fraction as related to basic components in the left and right ventricular volume domains. Int. J. Cardiol. 2018, 255, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H. Ejection Fraction Pros and Cons: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2360–2379. [Google Scholar] [CrossRef]
- Ojha, V.; Ganga, K.P.; Seth, T.; Roy, A.; Naik, N.; Jagia, P.; Gulati, G.S.; Kumar, S.; Sharma, S. Role of CMR feature-tracking derived left ventricular strain in predicting myocardial iron overload and assessing myocardial contractile dysfunction in patients with thalassemia major. Eur. Radiol. 2021, 31, 6184–6192. [Google Scholar] [CrossRef]
- Meloni, A.; Saba, L.; Positano, V.; Pistoia, L.; Campanella, A.; Spasiano, A.; Putti, M.C.; Fotzi, I.; Cossu, A.; Corigliano, E.; et al. Global longitudinal strain by cardiac magnetic resonance is associated with cardiac iron and complications in beta-thalassemia major patients. Int. J. Cardiol. 2024, 413, 132319. [Google Scholar] [CrossRef]
- Arenja, N.; Fritz, T.; Andre, F.; Riffel, J.H.; Aus dem Siepen, F.; Ochs, M.; Paffhausen, J.; Hegenbart, U.; Schönland, S.; Müller-Hennessen, M.; et al. Myocardial contraction fraction derived from cardiovascular magnetic resonance cine images-reference values and performance in patients with heart failure and left ventricular hypertrophy. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1414–1422. [Google Scholar] [CrossRef]
- Lu, D.Y.; Huang, W.M.; Wang, W.T.; Hung, S.C.; Sung, S.H.; Chen, C.H.; Yang, Y.J.; Niu, D.M.; Yu, W.C. Reduced global longitudinal strain as a marker for early detection of Fabry cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 487–495. [Google Scholar] [CrossRef]
- Saran, A.C.; Tormin, S.C.; Vogel, J.; Krakauer, R.; Salles, J.E.N. Left Ventricular Global Longitudinal Strain: An Early Marker of Diabetic Cardiomyopathy. ABC Imagem Cardiovasc. 2025, 38, e20240082. [Google Scholar] [CrossRef]
- Fischer, K.; Obrist, S.J.; Erne, S.A.; Stark, A.W.; Marggraf, M.; Kaneko, K.; Guensch, D.P.; Huber, A.T.; Greulich, S.; Aghayev, A.; et al. Feature Tracking Myocardial Strain Incrementally Improves Prognostication in Myocarditis Beyond Traditional CMR Imaging Features. JACC Cardiovasc. Imaging 2020, 13, 1891–1901. [Google Scholar] [CrossRef]
- Chadalavada, S.; Fung, K.; Rauseo, E.; Lee, A.M.; Khanji, M.Y.; Amir-Khalili, A.; Paiva, J.; Naderi, H.; Banik, S.; Chirvasa, M.; et al. Myocardial Strain Measured by Cardiac Magnetic Resonance Predicts Cardiovascular Morbidity and Death. J. Am. Coll. Cardiol. 2024, 84, 648–659. [Google Scholar] [CrossRef]
- Lundin, M.; Heiberg, E.; Nordlund, D.; Gyllenhammar, T.; Steding-Ehrenborg, K.; Engblom, H.; Carlsson, M.; Atar, D.; van der Pals, J.; Erlinge, D.; et al. Prognostic utility and characterization of left ventricular hypertrophy using global thickness. Sci. Rep. 2023, 13, 22806. [Google Scholar] [CrossRef]
- Meloni, A.; De Marchi, D.; Pistoia, L.; Grassedonio, E.; Peritore, G.; Preziosi, P.; Restaino, G.; Righi, R.; Riva, A.; Renne, S.; et al. Multicenter validation of the magnetic resonance T2* technique for quantification of pancreatic iron. Eur. Radiol. 2019, 29, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Martini, N.; Positano, V.; D’Angelo, G.; Barison, A.; Todiere, G.; Grigoratos, C.; Barra, V.; Pistoia, L.; Gargani, L.; et al. Myocardial T1 Values at 1.5 T: Normal Values for General Electric Scanners and Sex-Related Differences. J. Magn. Reson. Imaging 2021, 54, 1486–1500. [Google Scholar] [CrossRef]
- Meloni, A.; Luciani, A.; Positano, V.; De Marchi, D.; Valeri, G.; Restaino, G.; Cracolici, E.; Caruso, V.; Dell’amico, M.C.; Favilli, B.; et al. Single region of interest versus multislice T2* MRI approach for the quantification of hepatic iron overload. J. Magn. Reson. Imaging 2011, 33, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; De Marchi, D.; Positano, V.; Neri, M.G.; Mangione, M.; Keilberg, P.; Lendini, M.; Cirotto, C.; Pepe, A. Accurate estimate of pancreatic T2* values: How to deal with fat infiltration. Abdom. Imaging 2015, 40, 3129–3136. [Google Scholar] [CrossRef]
- Meloni, A.; Restaino, G.; Borsellino, Z.; Caruso, V.; Spasiano, A.; Zuccarelli, A.; Valeri, G.; Toia, P.; Salvatori, C.; Positano, V.; et al. Different patterns of myocardial iron distribution by whole-heart T2* magnetic resonance as risk markers for heart complications in thalassemia major. Int. J. Cardiol. 2014, 177, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Tyzka, J.M.; Carson, S.; Nelson, M.D.; Coates, T.D. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005, 106, 1460–1465. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [PubMed]
- Meloni, A.; Righi, R.; Missere, M.; Renne, S.; Schicchi, N.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Spasiano, A.; Roberti, M.G.; et al. Biventricular Reference Values by Body Surface Area, Age, and Gender in a Large Cohort of Well-Treated Thalassemia Major Patients Without Heart Damage Using a Multiparametric CMR Approach. J. Magn. Reson. Imaging 2021, 53, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Moss, J.; Thisted, R. Predictors of body surface area. J. Clin. Anesth. 1992, 4, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Marsella, M.; Borgna-Pignatti, C.; Meloni, A.; Caldarelli, V.; Dell’Amico, M.C.; Spasiano, A.; Pitrolo, L.; Cracolici, E.; Valeri, G.; Positano, V.; et al. Cardiac iron and cardiac disease in males and females with transfusion-dependent thalassemia major: A T2* magnetic resonance imaging study. Haematologica 2011, 96, 515–520. [Google Scholar] [CrossRef]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Elsedfy, H.; Yaarubi, S.A.; Skordis, N.; Khater, D.; El Kholy, M.; Stoeva, I.; Fiscina, B.; Angastiniotis, M.; et al. The ICET-A Recommendations for the Diagnosis and Management of Disturbances of Glucose Homeostasis in Thalassemia Major Patients. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016058. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Vogel, M.; Anderson, L.J.; Holden, S.; Deanfield, J.E.; Pennell, D.J.; Walker, J.M. Tissue Doppler echocardiography in patients with thalassaemia detects early myocardial dysfunction related to myocardial iron overload. Eur. Heart J. 2003, 24, 113–119. [Google Scholar] [CrossRef]
- Liguori, C.; Pitocco, F.; Di Giampietro, I.; de Vivo, A.E.; Schena, E.; Cianciulli, P.; Zobel, B.B. Relationship between myocardial T2 values and cardiac volumetric and functional parameters in β-thalassemia patients evaluated by cardiac magnetic resonance in association with serum ferritin levels. Eur. J. Radiol. 2013, 82, e441–e447. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Frazer, D.M.; Bowden, D.K.; Anderson, G.J. Transfusion suppresses erythropoiesis and increases hepcidin in adult patients with β-thalassemia major: A longitudinal study. Blood 2013, 122, 124–133. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Tower, J.; Pomatto, L.C.D.; Davies, K.J.A. Sex differences in the response to oxidative and proteolytic stress. Redox Biol. 2020, 31, 101488. [Google Scholar] [CrossRef] [PubMed]
- Pennell, D.J.; Porter, J.B.; Cappellini, M.D.; Chan, L.L.; El-Beshlawy, A.; Aydinok, Y.; Ibrahim, H.; Li, C.K.; Viprakasit, V.; Elalfy, M.S.; et al. Deferasirox for up to 3 years leads to continued improvement of myocardial T2* in patients with beta-thalassemia major. Haematologica 2012, 97, 842–848. [Google Scholar] [CrossRef]
- Berdoukas, V.; Chouliaras, G.; Moraitis, P.; Zannikos, K.; Berdoussi, E.; Ladis, V. The efficacy of iron chelator regimes in reducing cardiac and hepatic iron in patients with thalassaemia major: A clinical observational study. J. Cardiovasc. Magn. Reson. 2009, 11, 20. [Google Scholar] [CrossRef]
- Pennell, D.J.; Porter, J.B.; Piga, A.; Lai, Y.R.; El-Beshlawy, A.; Elalfy, M.; Yesilipek, A.; Kilinc, Y.; Habr, D.; Musallam, K.M.; et al. Sustained improvements in myocardial T2* over 2 years in severely iron-overloaded patients with beta thalassemia major treated with deferasirox or deferoxamine. Am. J. Hematol. 2015, 90, 91–96. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Pistoia, L.; Cuccia, L.; Gamberini, M.R.; Lisi, R.; D’Ascola, D.G.; Rosso, R.; Allo, M.; Spasiano, A.; et al. MRI multicentre prospective survey in thalassaemia major patients treated with deferasirox versus deferiprone and desferrioxamine. Br. J. Haematol. 2018, 183, 783–795. [Google Scholar] [CrossRef]
- Entezari, S.; Haghi, S.M.; Norouzkhani, N.; Sahebnazar, B.; Vosoughian, F.; Akbarzadeh, D.; Islampanah, M.; Naghsh, N.; Abbasalizadeh, M.; Deravi, N. Iron Chelators in Treatment of Iron Overload. J. Toxicol. 2022, 2022, 4911205. [Google Scholar] [CrossRef] [PubMed]
- Origa, R.; Cinus, M.; Pilia, M.P.; Gianesin, B.; Zappu, A.; Orecchia, V.; Clemente, M.G.; Pitturru, C.; Denotti, A.R.; Corongiu, F.; et al. Safety and Efficacy of the New Combination Iron Chelation Regimens in Patients with Transfusion-Dependent Thalassemia and Severe Iron Overload. J. Clin. Med. 2022, 11, 2010. [Google Scholar] [CrossRef]
- Antohi, E.-L.; Chioncel, O. Understanding cardiac systolic performance beyond left ventricular ejection fraction. Explor. Med. 2020, 1, 75–84. [Google Scholar] [CrossRef]
- Batouty, N.M.; Tawfik, A.M.; Sobh, D.M.; Gadelhak, B.N.; El-Ashwah, S.; Hussein, M.A.; Gad, M.; Aziz, A.; El-Shahed, M.A.; Karam, R. Global and regional cardiac magnetic resonance feature tracking left ventricular strain analysis in assessing early myocardial disease in β thalassemia major patients. J. Cardiovasc. Imaging 2024, 32, 18. [Google Scholar] [CrossRef]
- Meloni, A.; Saba, L.; Positano, V.; Taccori, M.; Pistoia, L.; De Marco, E.; Sanna, P.M.G.; Longo, F.; Giovangrossi, P.; Gerardi, C.; et al. Left ventricular diastolic and systolic functions by cardiac magnetic resonance in beta-thalassemia major: Correlation with clinical findings and cardiac complications. Int. J. Cardiovasc. Imaging 2025, 41, 847–857. [Google Scholar] [CrossRef]
- Meloni, A.; Martini, N.; Positano, V.; De Luca, A.; Pistoia, L.; Sbragi, S.; Spasiano, A.; Casini, T.; Bitti, P.P.; Allò, M.; et al. Myocardial iron overload by cardiovascular magnetic resonance native segmental T1 mapping: A sensitive approach that correlates with cardiac complications. J. Cardiovasc. Magn. Reson. 2021, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [PubMed]
- Ferreira, V.M.; Piechnik, S.K. CMR Parametric Mapping as a Tool for Myocardial Tissue Characterization. Korean Circ. J. 2020, 50, 658–676. [Google Scholar] [CrossRef] [PubMed]
- Kraigher-Krainer, E.; Shah, A.M.; Gupta, D.K.; Santos, A.; Claggett, B.; Pieske, B.; Zile, M.R.; Voors, A.A.; Lefkowitz, M.P.; Packer, M.; et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 63, 447–456. [Google Scholar] [CrossRef]
- Xu, J.; Yang, W.; Zhao, S.; Lu, M. State-of-the-art myocardial strain by CMR feature tracking: Clinical applications and future perspectives. Eur. Radiol. 2022, 32, 5424–5435. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).