Can We Achieve More with Less? Parenchymal Sparing Surgery Versus Major Liver Resection for Colorectal Liver Metastases: An Observational Single-Center Study with Propensity Score Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
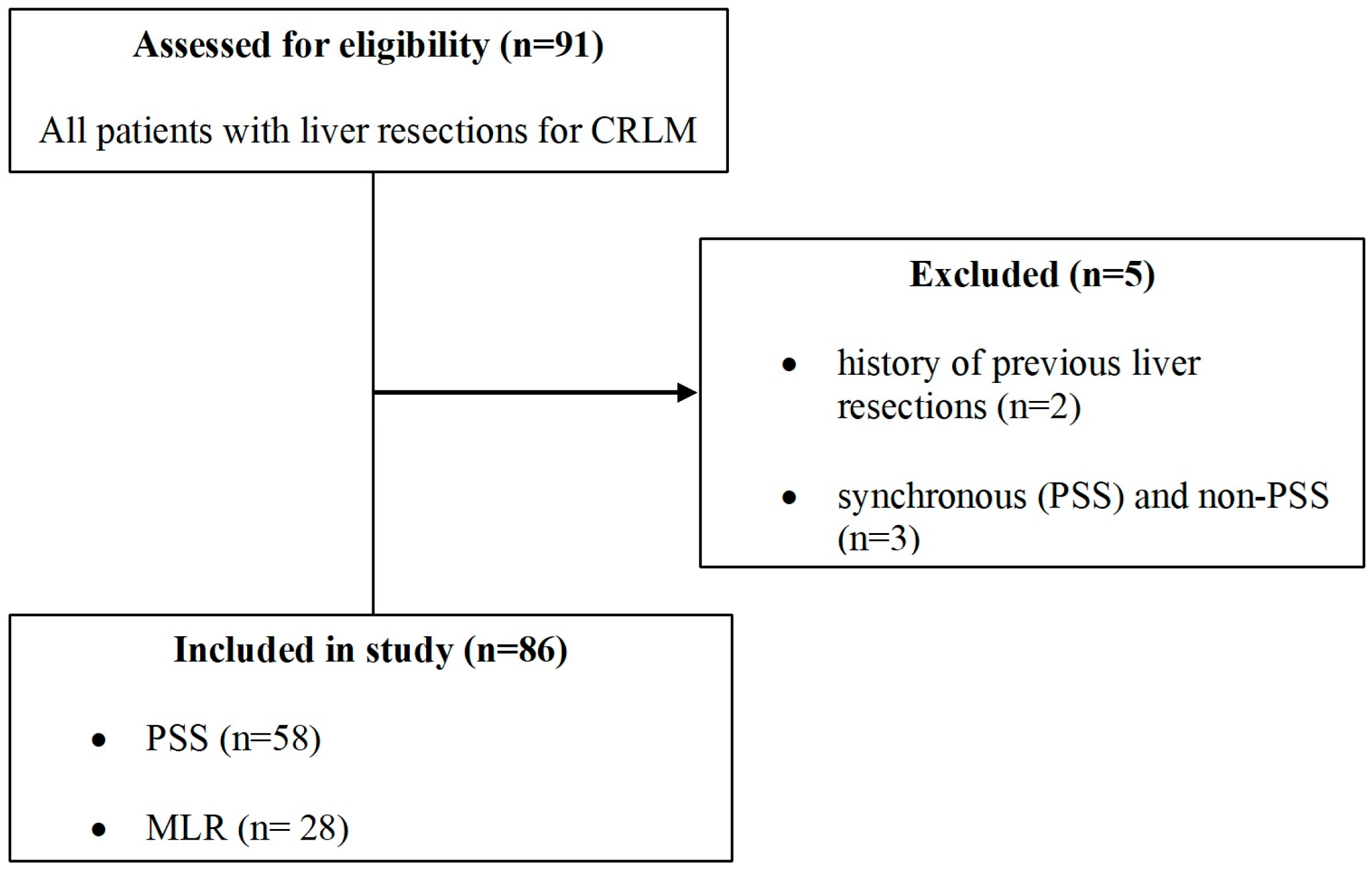
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Postoperative Outcomes
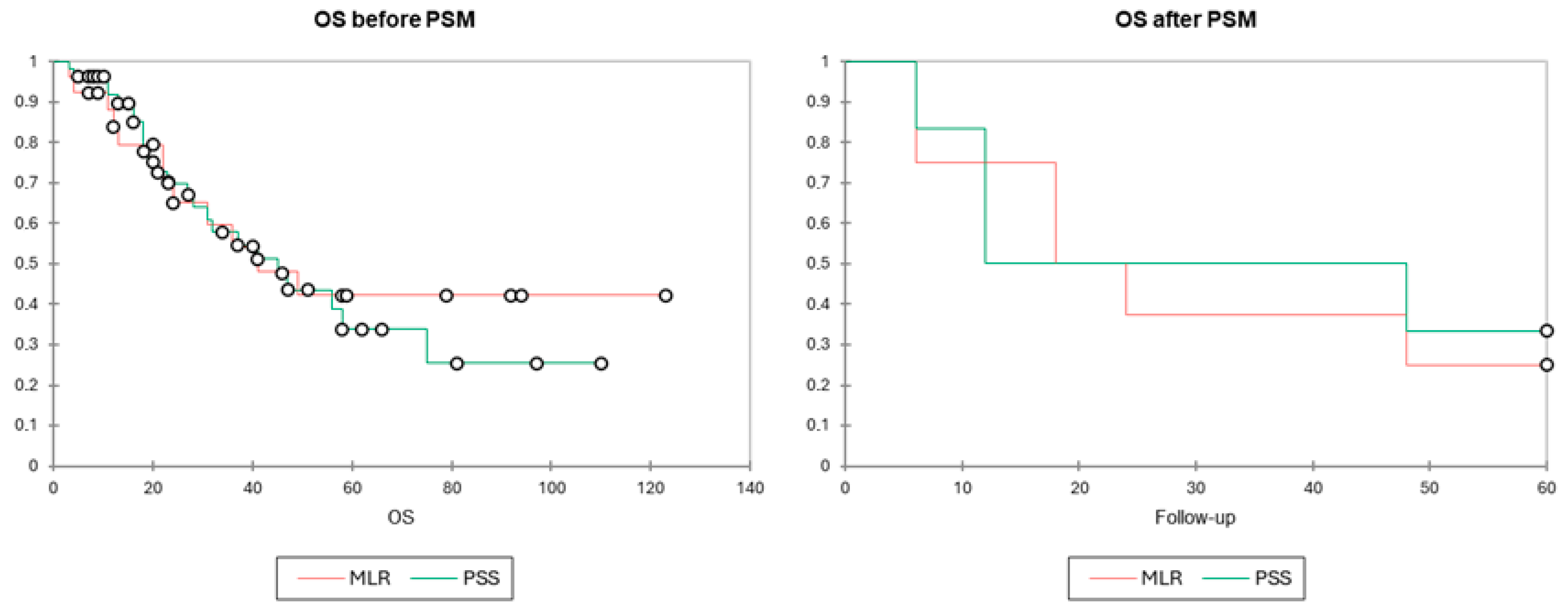
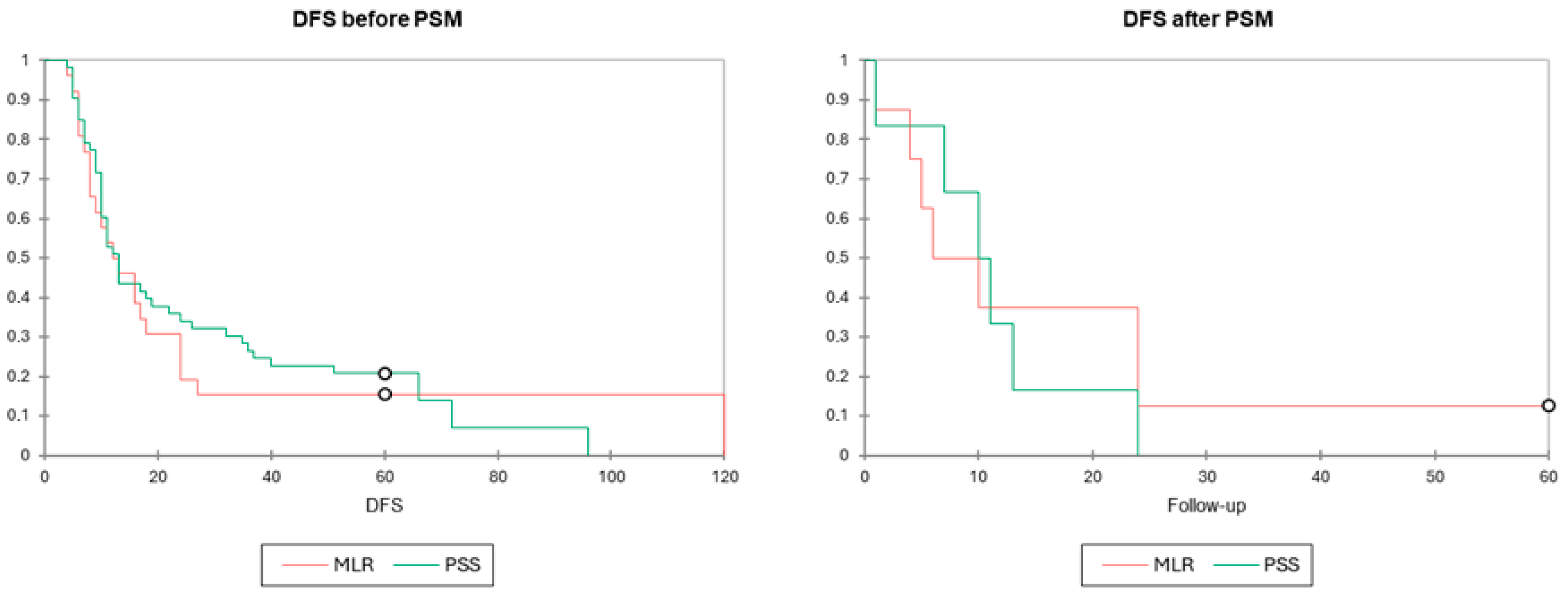
3.3. Long-Term Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Väyrynen, V.; Wirta, E.-V.; Seppälä, T.; Sihvo, E.; Mecklin, J.-P.; Vasala, K.; Kellokumpu, I. Incidence and management of patients with colorectal cancer and synchronous and metachronous colorectal metastases: A population-based study. BJS Open 2020, 4, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Ivey, G.D.; Johnston, F.M.; Azad, N.S.; Christenson, E.S.; Lafaro, K.J.; Shubert, C.R. Current surgical management strategies for colorectal cancer liver metastases. Cancers 2022, 14, 1063. [Google Scholar] [CrossRef] [PubMed]
- Östrand, E.; Rystedt, J.; Engstrand, J.; Frühling, P.; Hemmingsson, O.; Sandström, P.; Sternby Eilard, M.; Tingstedt, B.; Buchwald, P. Importance of resection margin after resection of colorectal liver metastases in the era of modern chemotherapy: Population-based cohort study. BJS Open 2024, 8, zrae035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shindoh, J.; Makuuchi, M.; Matsuyama, Y.; Mise, Y.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J. Hepatol. 2016, 64, 594–600. [Google Scholar] [CrossRef]
- Balzan, S.; Belghiti, J.; Farges, O.; Ogata, S.; Sauvanet, A.; Delefosse, D.; Durand, F. The “50-50 criteria” on postoperative day 5: An accurate predictor of liver failure and death after hepatectomy. Ann. Surg. 2005, 242, 824–828, discussion: 828–829. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Scoggins, C.R.; Zorzi, D.; Abdalla, E.K.; Andres, A.; Eng, C.; Curley, S.A.; Loyer, E.M.; Muratore, A.; Mentha, G.; et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann. Surg. 2005, 241, 715–722, discussion 722–724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dhir, M.; Lyden, E.R.; Wang, A.; Smith, L.M.; Ullrich, F.; Are, C. Influence of margins on overall survival after hepatic resection for colorectal metastasis: A meta-analysis. Ann. Surg. 2011, 254, 234–242. [Google Scholar] [CrossRef] [PubMed]
- de Haas, R.J.; Wicherts, D.A.; Flores, E.; Azoulay, D.; Castaing, D.; Adam, R. R1 resection by necessity for colorectal liver metastases: Is it still a contraindication to surgery? Ann. Surg. 2008, 248, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Knitter, S.; Schmelzle, M.; Kradolfer, D.; Maurer, M.H.; Auer, T.A.; Fehrenbach, U.; Lachenmayer, A.; Banz, V.; Schöning, W.; et al. Recurrence at surgical margin following hepatectomy for colorectal liver metastases is not associated with R1 resection and does not impact survival. Surgery 2021, 169, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Kingham, T.P.; Correa-Gallego, C.; D’Angelica, M.I.; Gönen, M.; DeMatteo, R.P.; Fong, Y.; Allen, P.J.; Blumgart, L.H.; Jarnagin, W.R. Hepatic parenchymal preservation surgery: Decreasing morbidity and mortality rates in 4152 resections for malignancy. J. Am. Coll. Surg. 2015, 220, 471–479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Memeo, R.; de Blasi, V.; Adam, R.; Goéré, D.; Azoulay, D.; Ayav, A.; Gregoire, E.; Kianmanesh, R.; Navarro, F.; Cunha, A.S.; et al. Parenchymal-sparing hepatectomies (PSH) for bilobar colorectal liver metastases are associated with a lower morbidity and similar oncological results: A propensity score matching analysis. HPB 2016, 18, 781–790. [Google Scholar] [CrossRef]
- Andreou, A.; Gloor, S.; Inglin, J.; Di Pietro, M.C.; Banz, V.; Lachenmayer, A.; Kim-Fuchs, C.; Candinas, D.; Beldi, G. Parenchymal-sparing hepatectomy for colorectal liver metastases reduces postoperative morbidity while maintaining equivalent oncologic outcomes compared to non-parenchymal-sparing resection. Surg. Oncol. 2021, 38, 101631. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Y.; Hao, M.; Li, H.; Liang, X.; Yuan, D.; Ding, L. Clinical outcomes of parenchymal-sparing versus anatomic resection for colorectal liver metastases: A systematic review and meta-analysis. World J. Surg. Oncol. 2023, 21, 241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Milazzo, M.; Todeschini, L.; Caimano, M.; Mattia, A.; Cristin, L.; Martinino, A.; Bianco, G.; Spoletini, G.; Giovinazzo, F. Surgical Resection in Colorectal Liver Metastasis: An Umbrella Review. Cancers 2024, 16, 1849. [Google Scholar] [CrossRef]
- Deng, G.; Li, H.; Jia, G.Q.; Fang, D.; Tang, Y.Y.; Xie, J.; Chen, K.; Chen, Z. Parenchymal-sparing versus extended hepatectomy for colorectal liver metastases: A systematic review and meta-analysis. Cancer Med. 2019, 8, 6165–6175. [Google Scholar] [CrossRef]
- Lunca, S.; Morarasu, S.; Ivanov, A.A.; Clancy, C.; O’Brien, L.; Zaharia, R.; Musina, A.M.; Roata, C.E.; Dimofte, G.M. Is Frailty Associated with Worse Outcomes After Major Liver Surgery? An Observational Case-Control Study. Diagnostics 2025, 15, 512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simiras, C.; Morarasu, S.; Simiras, D.M.; Iacob, S.; Ong, W.L.; Musina, A.M.; Velenciuc, N.; Roata, C.E.; Lunca, S.; Dimofte, G.M. Predictive and Prognostic Role of Neutrophil to Lymphocyte Ratio in Rectal Cancer: A Case Control Study with Propensity Score Analysis. Chirurgia 2023, 118, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Livadaru, C.; Morarasu, S.; Frunza, T.C.; Ghitun, F.A.; Paiu-Spiridon, E.F.; Sava, F.; Terinte, C.; Ferariu, D.; Lunca, S.; Dimofte, G.M. Post-operative computed tomography scan-reliable tool for quality assessment of complete mesocolic excision. World J. Gastrointest. Oncol. 2019, 11, 208–226. [Google Scholar] [CrossRef]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Sasaki, K.; Ijzermans, J.N.M.; van Vugt, J.L.A.; Pawlik, T.M.; Choti, M.A.M.; Cameron, J.L.; He, J.; et al. Anatomical resections improve disease-free survival in patients with KRAS-mutated colorectal liver metastases. Ann. Surg. 2017, 266, 641–649. [Google Scholar] [CrossRef]
- Hamady, Z.Z.; Lodge, J.P.; Welsh, F.K.; Toogood, G.J.; White, A.; John, T.; Rees, M. One-millimeter cancer-free margin is curative for colorectal liver metastases: A propensity score case-match approach. Ann. Surg. 2014, 259, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Kokudo, N.; Tada, K.; Seki, M.; Ohta, H.; Azekura, K.; Ueno, M.; Matsubara, T.; Takahashi, T.; Nakajima, T.; Muto, T. Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am. J. Surg. 2001, 181, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.D.; O’Suilleabhain, C.B.; Madhavan, K.K.; Wigmore, S.J.; Parks, R.W.; Garden, O.J. The extent of resection influences outcome following hepatectomy for colorectal liver metastases. Eur. J. Surg. Oncol. 2004, 30, 370–376. [Google Scholar] [CrossRef] [PubMed]
- She, W.H.; Cheung, T.T.; Ma, K.W.; Tsang, S.H.Y.; Dai, W.C.; Chan, A.C.Y.; Lo, C.M. Anatomical versus nonanatomical resection for colorectal liver metastasis. World J. Surg. 2020, 44, 2743–2751. [Google Scholar] [CrossRef]
- Aguirre-Ghiso, J.A. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 2007, 7, 834e46. [Google Scholar] [CrossRef]
- Mise, Y.; Aloia, T.A.; Brudvik, K.W.; Schwarz, L.; Vauthey, J.N.; Conrad, C. Parenchymal-sparing Hepatectomy in Colorectal Liver Metastasis Improves Salvageability and Survival. Ann. Surg. 2016, 263, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Makuuchi, M.; Hasegawa, H.; Yamazaki, S. Ultrasonically guided subsegmentectomy. Surg. Gynecol. Obstet. 1985, 161, 346–350. [Google Scholar]
- Torzilli, G.; Garancini, M.; Donadon, M.; Cimino, M.; Procopio, F.; Montorsi, M. Intraoperative ultrasonographic detection of communicating veins between adjacent hepatic veins during hepatectomy for tumours at the hepatocaval confluence. Br. J. Surg. 2010, 97, 1867–1873. [Google Scholar] [CrossRef]
- Viganò, L.; Torzilli, G.; Troisi, R.; Aldrighetti, L.; Ferrero, A.; Majno, P.; Toso, C.; Figueras, J.; Cherqui, D.; Adam, R.; et al. Minor hepatectomies: Focusing a blurred picture: Analysis of the outcome of 4471 open resections in patients without cirrhosis. Ann. Surg. 2019, 270, 842–851. [Google Scholar] [CrossRef]
- Torzilli, G.; McCormack, L.; Pawlik, T. Parenchyma-sparing liver resections. Int. J. Surg. 2020, 82S, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Evrard, S.; Torzilli, G.; Caballero, C.; Bonhomme, B. Parenchymal sparing surgery brings treatment of colorectal liver metastases into the precision medicine era. Eur. J. Cancer 2018, 104, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hisaka, T.; Ishikawa, H.; Sakai, H.; Kawahara, R.; Goto, Y.; Nomura, Y.; Yasunaga, M.; Kinugasa, T.; Fujita, F.; Mizobe, T.; et al. Sinusoidal obstruction syndrome and postoperative complications resulting from preoperative chemotherapy for colorectal cancer liver metastasis. Anticancer. Res. 2019, 39, 4549–4554. [Google Scholar] [CrossRef] [PubMed]
- Duwe, G.; Knitter, S.; Pesthy, S.; Beierle, A.; Bahra, M.; Schmelzle, M.; Schmuck, R.; Lohneis, P.; Raschzok, N.; Öllinger, R.; et al. Hepatotoxicity following systemic therapy for colorectal liver metastases and the impact of chemotherapy-associated liver injury on outcomes after curative liver resection. Eur. J. Surg. Oncol. 2017, 43, 1668–1681. [Google Scholar] [CrossRef]
- Hilal, M.A.; Aldrighetti, L.; Dagher, I.; Edwin, B.; Troisi, R.I.; Alikhanov, R.; Aroori, S.; Belli, G.; Besselink, M.; Briceno, J.; et al. The Southampton consensus guidelines for laparoscopic liver surgery: From indication to implementation. Ann. Surg. 2018, 268, 11–18. [Google Scholar] [CrossRef]
- Fretland, Å.A.; Dagenborg, V.J.; Bjørnelv, G.M.W.; Kazaryan, A.M.; Kristiansen, R.; Fagerland, M.W.; Hausken, J.; Tønnessen, T.I.; Abildgaard, A.; Barkhatov, L.; et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg. 2018, 267, 199–207. [Google Scholar] [CrossRef]
- Morarasu, S.; Clancy, C.; Gorgun, E.; Yilmaz, S.; Ivanecz, A.; Kawakatsu, S.; Musina, A.M.; Velenciuc, N.; Roata, C.E.; Dimofte, G.M.; et al. Laparoscopic versus open resection of primary colorectal cancers and synchronous liver metastasis: A systematic review and meta-analysis. Int. J. Colorectal. Dis. 2023, 38, 90. [Google Scholar] [CrossRef] [PubMed]
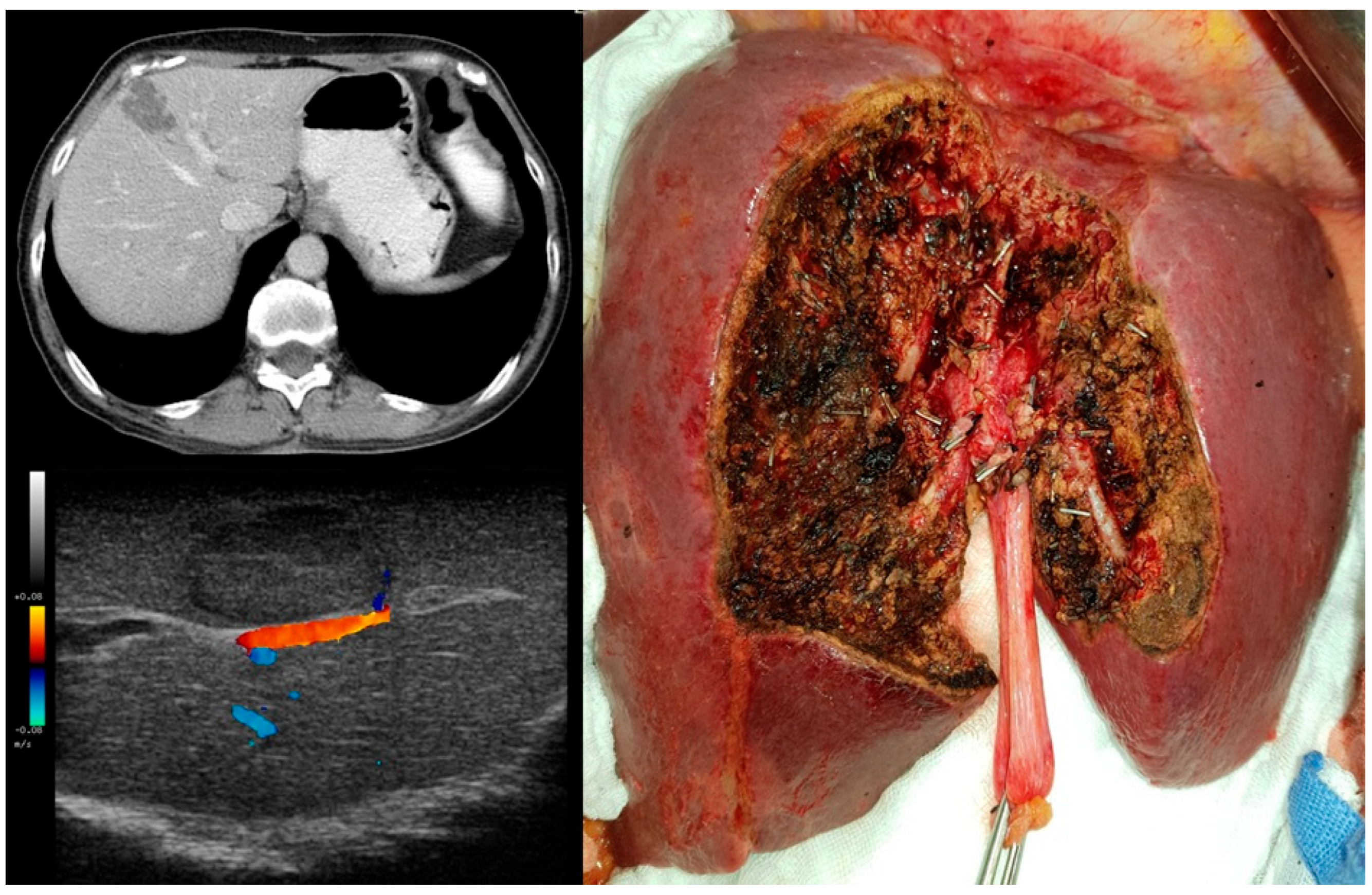
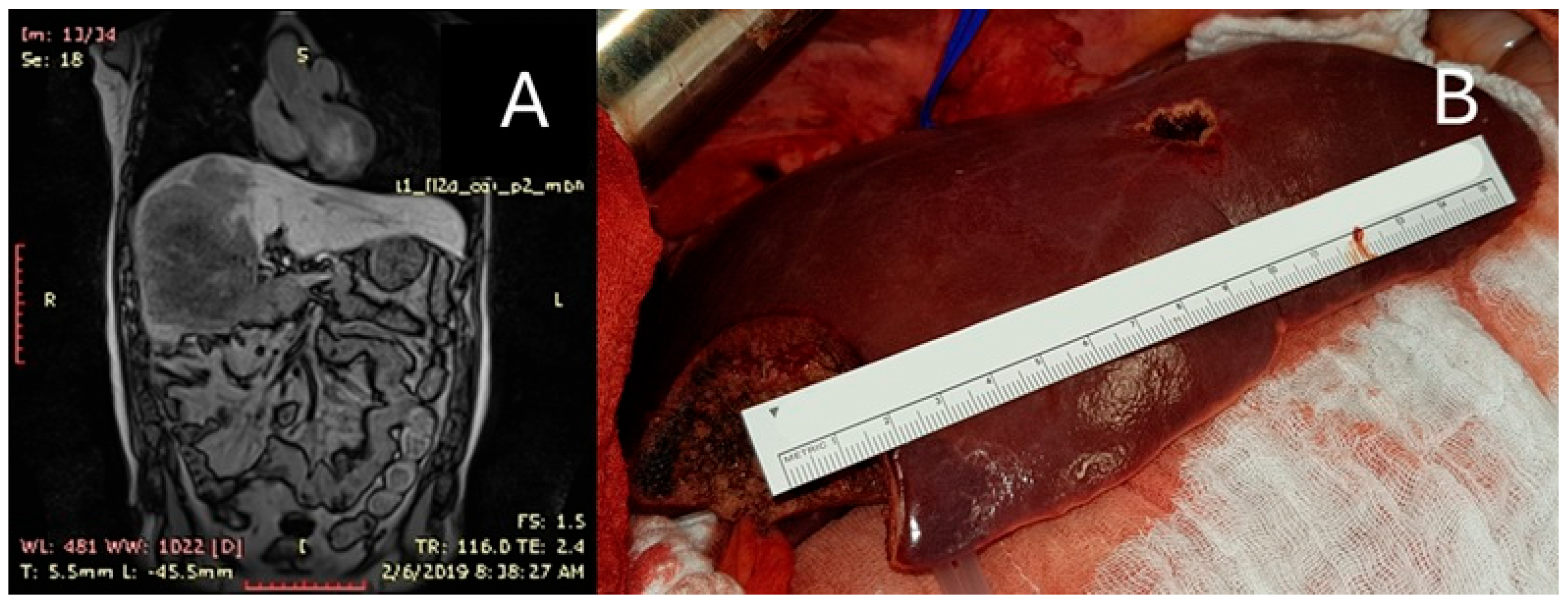
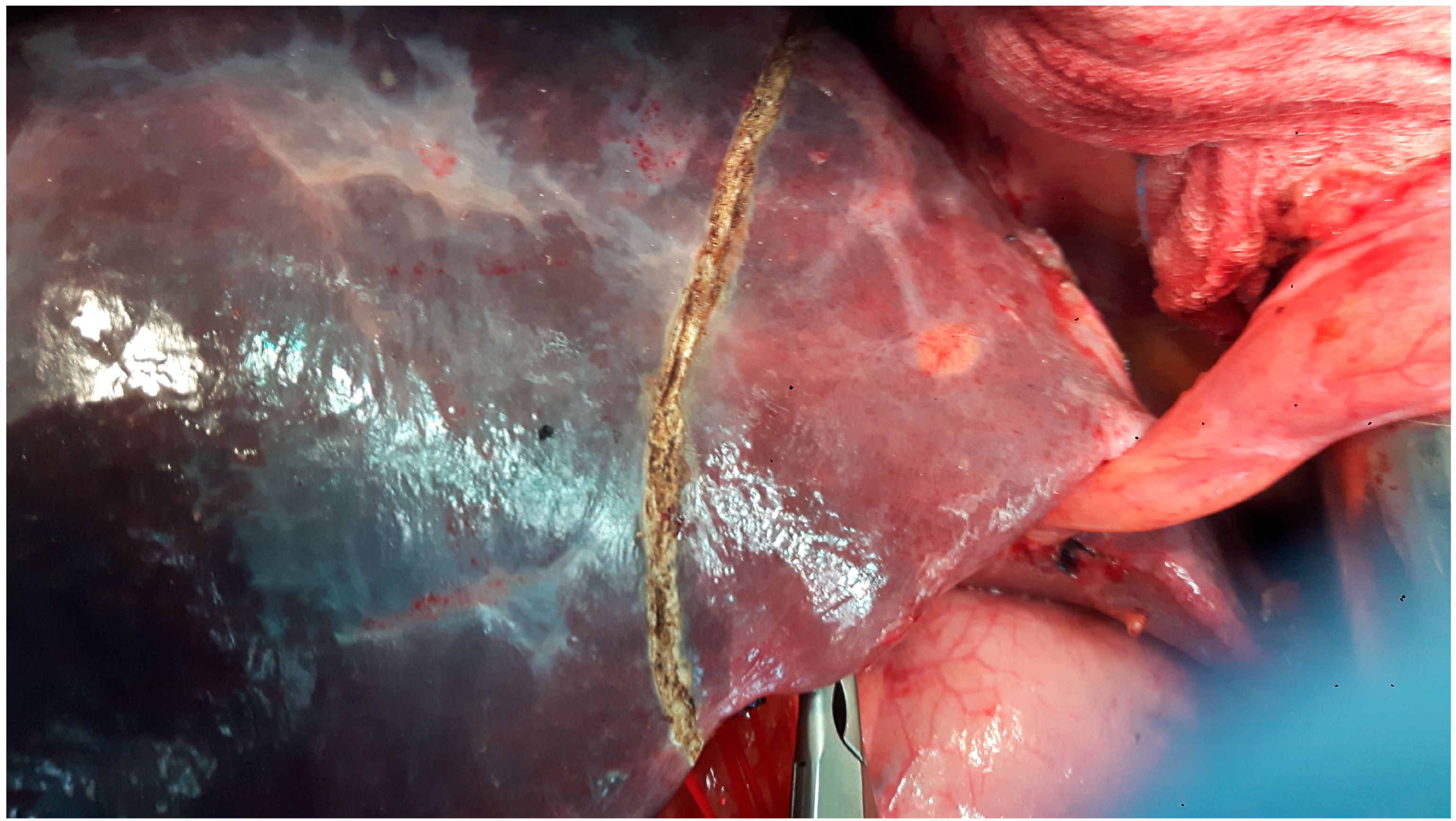
| PSS n (%)/Mean (SD) | MLR n (%)/Mean (SD) | p-Value | |
|---|---|---|---|
| Total | 58 (100) | 28 (100) | |
| Gender (males) | 39 (67.2) | 12 (42.8) | p = 0.031 |
| Age, mean (SD) | 60.7 (10.2) | 59.8 (7.6) | p = 0.679 |
| BMI | 25.2 (3.8) | 26 (3.3) | p = 0.343 |
| mFI ≥ 2 | 6 (10.3) | 7 (25) | p = 0.107 |
| Comorbidities | |||
| ALD | 1 (1.7) | 0 | p = 1 |
| Viral Hepatitis | 0 | 2 (7.1) | p = 0.103 |
| Cirrhosis | 0 | 0 | |
| Diabetes | 0 | 0 | |
| CHF | 3 (5.1) | 2 (7.1) | p = 0.658 |
| COPD | 2 (3.4) | 1 (3.5) | p = 1 |
| CKD | 6 (10.3) | 2 (7.1) | p = 1 |
| Smoking | 4 (6.8) | 5 (17.8) | p = 0.143 |
| Neoadjuvant therapy | 44 (75.8) | 25 (89.2) | p = 0.246 |
| ASA 2 | 10 (17.2) | 6 (21.4) | p = 0.768 |
| ASA 3 | 43 (74.1) | 20 (71.4) | p = 0.799 |
| Preoperative blood tests | |||
| Total Bilirubin (mg/dL) | 0.70 (0.3) | 0.76 (0.77) | p = 0.604 |
| Albumin (g/dL) | 4.54 (0.7) | 4.6 (0.4) | p = 0.675 |
| INR | 1.00 (0.07) | 1.03 (0.06) | p = 0.054 |
| PLT (mmc) × 103 | 227 (67) | 258 (96) | p = 0.085 |
| AST | 29.9 (12.1) | 33.7 (14.4) | p = 0.203 |
| ALT | 29.6 (21.7) | 32.7 (19.8) | p = 0.525 |
| Operative factors | |||
| No. of liver lesions | 1.6 (1.04) | 1.67 (1.61) | p = 0.808 |
| Operative time (min) | 235.2 (77.9) | 302.6 (127.3) | p = 0.003 |
| Blood loss (mL) | 567.6 (422.2) | 632.6 (533.8) | p = 0.541 |
| Positive resection margin | 8 (13.8) | 2 (7.14) | p = 0.487 |
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| PSS n (%)/Mean (SD) | MLR n (%)/Mean (SD) | p-Value | PSS n (%)/Mean (SD) | MLR n (%)/Mean (SD) | p-Value | |
| Total | 58 (100) | 28 (100) | 10 (100) | 10 (100) | ||
| Morbidity | 11 (18.9) | 16 (57.1) | p = 0.001 | 2 (20) | 8 (80) | p = 0.023 |
| Clavien–Dindo III–V | 0 | 5 (17.8) | p = 0.005 | 0 | 2 (20) | p = 0.473 |
| Surgical complications | ||||||
| Bile leak | 1 (1.7) | 2 (7.1) | p = 0.246 | 0 | 0 | |
| PHLF | 0 | 2 | 0 | 0 | ||
| Bleeding/Hematoma | 0 | 2 (7.1) | p = 0.103 | 0 | 0 | |
| IAC | 1 (1.7) | 2 (7.1) | p = 0.246 | 0 | 0 | |
| SSI | 0 | 1 (3.5) | p = 0.325 | 0 | 1 (10) | p = 1 |
| HAI | 4 (6.8) | 3 (10.7) | p = 0.690 | 1 (10) | 3 (30) | p = 0.582 |
| Medical complications | ||||||
| DVT/PE | 0 | 0 | 0 | 0 | 0 | |
| HAP | 3 (5.1) | 1 (3.5) | p = 1 | 1 (10) | 1 (10) | p = 1 |
| Cardiac | 0 | 1 (3.5) | p = 0.325 | 0 | 1 (10) | p = 1 |
| AKI | 2 (3.4) | 3 (10.7) | p = 0.324 | 0 | 1 (10) | p = 1 |
| MODS | 0 | 1 (3.5) | p = 0.325 | 0 | 1 (10) | p = 1 |
| Reintervention | 0 | 3 (10.7) | p = 0.032 | 0 | 2 (20) | p = 0.473 |
| LOS | 12.3 (4.1) | 14.1 (6.1) | p = 0.109 | 14.7 (6.2) | 13.3 (4.8) | p = 0.579 |
| Length of ICU stay | 2.1 (1.7) | 3.5 (4.7) | p = 0.046 | 2.6 (2.2) | 5 (7.7) | p = 0.355 |
| 30-day mortality | 0 | 0 | 0 | 0 | ||
| Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|
| OS (months) | DFS (months) | OS (months) | DFS (months) | |||||
| Median (Range) | Wilcoxon text | Median (Range) | Wilcoxon text | Median (Range) | Wilcoxon text | Median (Range) | Wilcoxon text | |
| PSS | 31 (3–110) | p = 0.884 | 25.2 (4–96) | p = 0.519 | 40.9 (8–97) | p = 0.741 | 24.3 (7–72) | p = 0.653 |
| MLR | 37.1 (3–123) | 22.2 (4–120) | 21.2 (3–59) | 13.8 (6–24) | ||||
| OS | |||
|---|---|---|---|
| Variable | Wald Chi2 | p-value | Hazard ratio |
| PSS | 3.662 | 0.056 | 2.177 [0.981–4.827] |
| PRM | 0.732 | 0.392 | 0.608 [0.195–1.899] |
| Size | 20.727 | <0.0001 | 6.895 [3.003–15.832] |
| Number | 1.398 | 0.237 | 1.672 [0.713–3.918] |
| DFS | |||
| Variable | Wald Chi2 | p-value | Hazard ratio |
| PSS | 0.007 | 0.935 | 0.978 [0.570–1.676] |
| PRM | 0.154 | 0.695 | 0.855 [0.392–1.866] |
| Size | 1.254 | 0.263 | 1.395 [0.779–2.497] |
| Number | 7.763 | 0.005 | 2.451 [1.305–4.606] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunca, S.; Morarasu, S.; Zaharia, R.; Ivanov, A.-A.; Clancy, C.; O’Brien, L.; Ong, W.L.; Dimofte, G.-M. Can We Achieve More with Less? Parenchymal Sparing Surgery Versus Major Liver Resection for Colorectal Liver Metastases: An Observational Single-Center Study with Propensity Score Analysis. Diagnostics 2025, 15, 1334. https://doi.org/10.3390/diagnostics15111334
Lunca S, Morarasu S, Zaharia R, Ivanov A-A, Clancy C, O’Brien L, Ong WL, Dimofte G-M. Can We Achieve More with Less? Parenchymal Sparing Surgery Versus Major Liver Resection for Colorectal Liver Metastases: An Observational Single-Center Study with Propensity Score Analysis. Diagnostics. 2025; 15(11):1334. https://doi.org/10.3390/diagnostics15111334
Chicago/Turabian StyleLunca, Sorinel, Stefan Morarasu, Raluca Zaharia, Andreea-Antonina Ivanov, Cillian Clancy, Luke O’Brien, Wee Liam Ong, and Gabriel-Mihail Dimofte. 2025. "Can We Achieve More with Less? Parenchymal Sparing Surgery Versus Major Liver Resection for Colorectal Liver Metastases: An Observational Single-Center Study with Propensity Score Analysis" Diagnostics 15, no. 11: 1334. https://doi.org/10.3390/diagnostics15111334
APA StyleLunca, S., Morarasu, S., Zaharia, R., Ivanov, A.-A., Clancy, C., O’Brien, L., Ong, W. L., & Dimofte, G.-M. (2025). Can We Achieve More with Less? Parenchymal Sparing Surgery Versus Major Liver Resection for Colorectal Liver Metastases: An Observational Single-Center Study with Propensity Score Analysis. Diagnostics, 15(11), 1334. https://doi.org/10.3390/diagnostics15111334






