Abstract
Vitamin D may have anticancer effects against colorectal cancer (CRC). Bone mineral density (BMD) reflects the long-term vitamin D status. This study investigated the association between osteoporosis and colorectal neoplasms (CRN). The data were obtained from the National Health Insurance Service sample cohort, which included 60,386 osteoporosis patients and 8224 controls who underwent BMD in 2002–2019. The logistic regression models included age, sex, income level, and comorbidity. Sensitivity tests were performed using the data from the National Health Screening Program. In total, 7706 (11.2%) patients were diagnosed with CRN, and the proportion was significantly higher in osteoporosis patients than in controls (11.7% vs. 8.1%). In the multivariate analysis, osteoporosis was associated with an increased risk of CRN (odds ratio (OR) = 1.91, 95% confidence interval = 1.75–2.09, p < 0.0001), which was significant for both colorectal adenomas and CRC (OR = 1.88 and 1.83, respectively). A subgroup analysis by sex revealed a significant association between osteoporosis and CRN in both women and men (OR = 2.06 and 1.66, respectively). The sensitivity tests revealed results similar to those of the original dataset. In conclusion, osteoporosis is significantly associated with CRN risk in both sexes. In high-risk patients with low BMD, appropriate screening for CRN and vitamin D supplementation are required, regardless of sex.
1. Introduction
Colorectal cancer (CRC) is a common cancer with a high mortality rate worldwide, leading to a global focus on its prevention and early detection [1,2]. Colorectal adenomas are precursors to CRC and arise from alterations in the normal mechanisms that regulate DNA repair and cell proliferation, known as the adenoma–carcinoma sequence [3,4]. In addition to the removal of colorectal adenomas and the early detection of CRC, identifying the risk and protective factors for colorectal neoplasms (CRN; including both colorectal adenoma and CRC) and addressing persons at risk are of utmost importance. Although various genetic and environmental factors are associated with the likelihood of developing CRC, several studies have reported that high intakes of vitamin D and calcium are associated with a reduced risk of CRC [5,6].
Osteoporosis commonly occurs in older adults. Increased age, menopause, smoking, decreased physical activity, and a lack of calcium and vitamin D intake are considered important risk factors for osteoporosis. Accordingly, appropriate calcium and vitamin D intake and regular exercise are important for achieving peak bone mass and preventing osteoporosis [7,8]. As the risk factors for osteoporosis are similar to those for CRC, vitamin D status is believed to play an important role in the significant correlation between osteoporosis and CRN. Recently, a cross-sectional study reported that osteoporosis increased the risk of colorectal adenomas in women aged >50 years, and another study found that the development of colorectal adenomas in postmenopausal women was inversely related to vitamin D levels [9,10].
However, most studies on the association between osteoporosis and the risk of CRN have been limited to elderly women or are single-center or multicenter cross-sectional studies that target patients at individual medical institutions [11,12]. In this study, we investigated the association between osteoporosis and CRN using claims data from the National Health Insurance Service (NHIS), which represents the entire population.
2. Materials and Methods
2.1. Study Population
This study used data from the NHIS Sample Cohort database, which is representative of the general South Korean population. Since 2002, the NHIS database has constructed an invaluable resource for patient data, including residence, income level, medical diagnoses, disability- or death-related data, and insurance claims. To investigate osteoporosis, we selected 175,512 individuals who had undergone bone mineral density (BMD) testing among the 1,137,861 individuals registered in the NHIS cohort during the period of 2002–2019. Patients aged <50 years, those who had already been diagnosed with CRC before the BMD test, and those with insufficient baseline information were excluded from the study (Figure 1). The diagnosis of colon cancer was established based on ICD-10 codes. Accordingly, all patients who were assigned a colon cancer diagnosis code even once before subject registration were excluded from the study. According to the World Health Organization diagnostic criteria, osteoporosis is defined as a BMD measured by dual-energy x-ray absorptiometry (DXA) of 2.5 standard deviations below than the reference mean value for young adults (a T-score of ≤−2.5) [13]. The case group (osteoporosis patients) consisted of those who had the M81 and M80 codes of the International Classification of Diseases (ICD-10) of osteoporosis and had a record of being prescribed treatment for osteoporosis during the study period according to the following Anatomical Therapeutic Chemical codes, M05BA04 (alendronic acid), M05BB03 (alendronic acid and cholecalciferol), M05BA07 (risedronic acid), M05BB07 (risedronic acid and cholecalciferol), M05BA06 (ibandronic acid), M05BA08 (zoledronic acid), M05BX04 (denosumab), G03XC01 (raloxifene), and H05AA02 (teriparatide). The control group consisted of individuals who did not have an ICD-10 code for osteoporosis and had never received a prescription for osteoporosis after excluding 51,038 subjects who met the exclusion criteria among the 175,512 individuals who underwent bone mineral density (BMD) testing. This study was approved by the Institutional Review Board of the Dongguk University Ilsan Hospital (IRB no. DUIH 2021-07-021). Because this study analyzed the NHIS secondary data for research and statistical purposes, the requirement for informed consent was waived by the IRB.
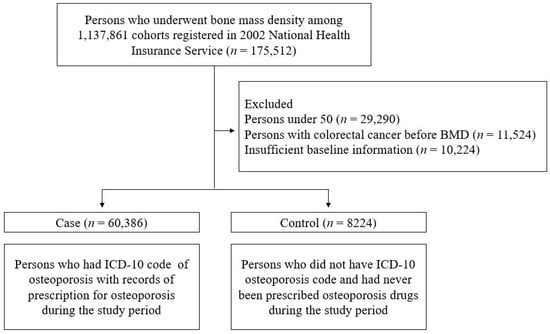
Figure 1.
Schemes of the study.
2.2. Study Variables
The main outcome was the diagnosis of CRN after the BMD test. Based on the ICD-10 codes, CRN included tubular, serrated, or villous adenomas (D12.0, D12.2–12.8), adenoma with high-grade dysplasia or carcinoma in situ (D01.0–01.2), and CRC (C18–20).
This study was designed to compare the incidence of CRN between the case and control groups. Several characteristics recorded in the NHIS cohort were compared. Age was divided into seven groups: 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and ≥80 years. All citizens of South Korea are obliged to subscribe to the National Health Insurance and pay insurance premiums based on their salary and property. The income levels were determined by the health insurance premium decile of the claims data (0–3, 4–7, and 8–10; higher scores indicate higher levels). The Charlson Comorbidity Index (CCI) was calculated by scoring comorbid conditions such as cardiovascular disease, diabetes, chronic lung disease, liver disease, kidney disease, and cancer, which are highly correlated with patient death. In this study, CCI was classified into four groups according to the score: 0 (low risk), 1, 2, and 3 or more (high risk).
2.3. Statistical Analyses
A chi-squared test was performed to compare the baseline characteristics and occurrence of CRN between osteoporosis patients and controls. Univariate and multivariate logistic regression analyses were used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) to determine the association between osteoporosis and CRN, followed by sub-analyses according to histological classification (low-grade adenoma, high-grade adenoma/carcinoma in situ, and invasive CRC). The models included age, sex, income, and the CCI score. We also performed subgroup analyses according to sex, age, income, and CCI scores to determine how the ORs from the regression model differed according to baseline characteristics. Moreover, to check the reliability of the national data, sensitivity analyses were performed using the sub-data set included in the national health screening program (NHSP). Statistical significance was set at p value less than 0.05. All statistical analyses were performed using SAS 9.4 version (SAS Institute, Cary, NC, USA).
3. Results
3.1. Baseline Characteristics
Overall, 68,610 individuals (60,386 patients and 8224 controls) were included in this study. Table 1 summarizes the baseline clinical characteristics of the study groups. A total of 7706 (11.2%) patients were diagnosed with CRN, and this proportion was significantly higher in the osteoporosis group than in the control group (11.7% vs. 8.1%, p < 0.0001). Significant differences were observed in the subclasses of colorectal adenomas and CRC. In a comparison of the other variables, the proportion of women was higher in the osteoporosis group than in the control group (89.8% vs. 52.2%). Osteoporosis tended to become more common with increasing age (p < 0.0001). The CCI score was slightly lower in the osteoporosis group. The osteoporosis patients and the control group did not differ significantly in terms of income levels.

Table 1.
Baseline characteristics of the study population.
3.2. Risks for Colonic Neoplasms in Osteoporosis; Analyses by Histologic Subclasses
Table 2 shows the univariate and multivariate logistic regression analyses of the association between osteoporosis and CRN according to the histological subclasses. The CRN rates were significantly higher in the osteoporosis patients than in the controls (OR = 1.49, 95% CI = 1.37–1.62, p < 0.0001). In terms of histologic subclass, both non-invasive the CRN, including all adenomas and carcinoma in situ (CIS) (OR = 1.38, 95% CI = 1.27–1.51, p < 0.0001), and the invasive CRC (OR = 1.90, 95% CI = 1.57–2.30, p < 0.0001) showed a significant increase with osteoporosis. In the multivariate analysis adjusted by other covariates (sex, age, CCI, and income level), osteoporosis was associated with an increased risk of CRN (OR = 1.91, 95% CI = 1.75–2.09, p < 0.0001), which was also significant in terms of both low-grade adenoma and invasive CRC (OR = 1.89 & OR = 1.83, both p < 0.0001). Noninvasive CRN was associated with osteoporosis (OR = 1.88, p < 0.0001); high-grade adenoma/CIS alone did not show an association with osteoporosis, which was related to the small sample size.

Table 2.
Univariate and multivariate logistic regression analyses for the association between osteoporosis and colonic neoplasms: subgroup by histology.
3.3. Subgroup Analyses by Baseline Characteristics
Next, we performed a subgroup analysis by age, sex, CCI, and income level to determine an association between osteoporosis and CRN, despite the differences in baseline characteristics between the subgroups (Table 3). The association between osteoporosis and CRN was statistically significant in all the age groups except those aged 80 years or older (OR = 1.30, 95% CI = 0.78–2.16, p = 0.3202). Osteoporosis was also a risk factor for CRN in both sexes, regardless of CCI and income levels. The risk of osteoporosis-related CRN was higher in women than in men (OR = 2.06 vs. 1.66, both p < 0.0001). The ORs tended to increase as the income levels increased.

Table 3.
Multivariate logistic regression analysis for the association between osteoporosis and colonic neoplasms: subgroups by baseline characteristics.
3.4. Sensitivity Analysis
Of the 68,610 study subjects, sensitivity tests were performed on only 35,099 individuals who had participated in the NHSP for cancer screening (30,490 osteoporosis patients and 4609 controls). The baseline characteristics are shown in Supplementary Table S1. In the univariate analysis, CRN had a significant correlation with osteoporosis (OR = 1.37, 95% CI = 1.23–1.53, p < 0.0001) (Table 4). After adjustment for sex, age, CCI, income level, body mass index (BMI), and smoking and drinking habits, significant results were also observed (OR = 1.75, 95% CI = 1.56–1.97, p < 0.0001). In the analysis of each histological subclass, low-grade adenoma, noninvasive CRN, and invasive CRC were all significantly associated with osteoporosis, which was consistent with the main outcomes of the study.

Table 4.
Sensitivity analysis using data from the National Health Screening Program (n = 35,099).
4. Discussion
This nationwide, retrospective cohort study used claims data from a sample cohort from the NHIS to investigate the relationship between osteoporosis and CRN. Osteoporosis was significantly correlated with the risk of CRN, noninvasive CRN, and invasive CRC. In the subgroup analyses according to baseline characteristics, osteoporosis increased the risk of CRN in most age groups, regardless of sex, CCI, or income level. The main results of this study are consistent with the sensitivity analyses using the NHSP data.
To the best of our knowledge, this was the first study to demonstrate on a nationwide scale that osteoporosis is associated with an increased risk of CRN, regardless of sex. Taiwan and Denmark also published national cohort studies in 2012; however, all were limited to women taking oral bisphosphonates [14,15]. In the latter, the incidence and mortality rates for CRC were significantly lower in women taking oral bisphosphonates. Although the study did not assess BMD, it can be assumed that increased bone density is associated with CRC prevention, which is consistent with our findings. A multicenter study in Korea confirmed that osteoporosis is an independent risk factor for colorectal and high-risk adenomas. However, in the subgroup analysis according to sex, osteoporosis increased the risk of adenoma only in women (OR = 1.66, p = 0.034 vs. OR = 1.45, p = 0.417), which is believed to be due to the limited sample size [12]. Our study targeted a larger sample size and found that osteoporosis increased the risk of CRN regardless of sex, although the OR for this risk was higher in women than in men (OR = 2.06 vs. OR = 1.66, both p < 0.0001). This statistical difference provides some support for the previous multicenter study, but it may also be due to higher statistical power in women from a larger sample.
Epidemiologic studies have suggested that lower BMD is associated with an increased risk for colorectal adenoma/cancer, especially in postmenopausal women [9,11,12]. The biological mechanisms linking bone mass to the risk of CRC are not fully understood; however, cumulative exposure to estrogen may play a role, and so may vitamin D deficiency. Vitamin D is a steroidal hormone that plays a major role in the regulation of bone metabolism. Calcitriol (1.25(OH)2D) is an active form of vitamin D that is considered to exert anticancer effects through its function as a transcription factor and a regulator of differentiation, as well as immune response. Several studies have reported that vitamin D deficiency increases CRC incidence [16,17,18,19]. A prospective cohort study showed that vitamin D intake was associated with a reduced risk of early-onset CRC before the age of 50 years and CRC precursors (i.e., conventional adenomas and serrated polyps) [20]. Furthermore, a meta-analysis of case–control studies suggested an inverse association between serum 25(OH)D levels, the circulating form of vitamin D in the body, and colorectal adenomas in both Western and Asian populations [21]. Thus, adequate vitamin D supplementation is important for the prevention of CRC.
The biologically active form of vitamin D, calcitriol (1.25(OH)2D), binds to the vitamin D3 receptor (VDR). The VDR–RXR heterodimers, in which VDR and retinoid X receptor (RXR) are combined, bind to the vitamin D response element (VDRE) and activate or inhibit gene expression to produce vitamin D anti-neoplastic activity [17]. In colorectal cancer, 1.25(OH)2D exerts antitumor effects by suppressing cell proliferation, sensitizing apoptosis, and inhibiting angiogenesis [22]. Vitamin D is primarily produced upon exposure to sunlight. Therefore, the circulating 25(OH)D concentration is lower in winter than in summer, with less sun exposure, and can even change depending on the day, night, and next morning [23,24]. Moreover, medications such as antiepileptic drugs, bile acid sequestrants, metformin, and glucocorticoids can affect circulating 25(OH)D [25,26]. Bone mineral density changes throughout life, increases rapidly during adolescence, plateaus in the 30s, and then gradually decreases with age. In addition, BMD can change due to pregnancy, lactation, or medications that affect bone metabolism (e.g., contraceptives, glucocorticoids, and antiepileptic drugs), but it occurs slowly, over several months to years, compared to serum 25(OH)D concentration [27].
Because serum 25(OH)D has a short half-life of several weeks, it may not be indicative of an individual’s overall vitamin D status. A randomized controlled trial confirmed that there was a dose effect on 25(OH)D concentration when comparing the monthly intake of vitamin D after 12 months, but the study concluded that there was no between-group difference in BMD [28]. However, another double-blind, randomized controlled trial demonstrated that the BMD significantly increased after 15 and 30 months of continuous administration of calcium and vitamin D compared to that in the placebo group [29]. At this point, BMD can be considered a reflection of long-term vitamin D status over months to years, and is used as a more reliable biomarker to confirm the anti-neoplastic effect on CRN. Of course, if medication data were available, the vitamin D hypothesis could be directly addressed instead of being indirectly hypothesized via osteoporosis, and the correlation between BMD and vitamin D levels could also be assessed. It is unfortunate that the vitamin D levels of osteoporosis patients and controls could not be compared in this study. It would be worth including this assessment in subsequent studies.
In this study, we used claims data extracted from 1,137,861 cohorts registered in the NHIS database. Most Koreans (97%) are enrolled in the NHIS program, and the greatest advantage of these data is that they cover almost the entire population and are the closest to real-world data [30]. Consequently, the claims data used in this study are considered reliable and statistically significant.
Our study had several limitations. First, the secondary data we used had not been collected for specific research purposes. The omission of any important covariates might have resulted in residual confounding [31]. Therefore, when organizing the case and control groups, we checked not only whether osteoporosis was diagnosed, but also whether osteoporosis medication was taken during the study period. Moreover, considering that the sensitivity analysis was consistent with the main results of the study, the limitations of the secondary data were overcome to some extent. Second, the baseline characteristics of the case and control groups differed. In the control group, the male-to-female ratio was relatively even (male: female = 47.8% vs. 52.2%); however, in the case group, there were more females (M: F = 10.2% vs. 89.8%). The patients with osteoporosis were older than the control group. This difference can naturally occur when osteoporosis is common in both women and elderly individuals. To overcome this difference, we conducted subgroup analyses for sex, age, CCI, and income level as baseline characteristics, and most of these factors provided significant results. Finally, because only those aged 50 years or older who underwent BMD were included, the number of osteoporosis patients was much higher than that in the control group. In this regard, a selection bias might have occurred, but it was considered negligible because this was a nationwide database that did not target patients in hospitals.
5. Conclusions
Osteoporosis is a risk factor for CRN regardless of sex. Although vitamin D supplements or bisphosphonates may improve bone density, the results of this study do not directly prove the effectiveness of the use of supplements or medications for CRN. However, this study shows that improving bone density is associated with a decrease in CRN and provides indirect evidence for the need for vitamin D supplementation. The advantage of the study is that it was conducted on a large nationwide scale, which has not been reported before. Accordingly, appropriate screening for CRN is required in high-risk groups with low BMD, and sufficient vitamin D intake is recommended. Further large-scale prospective studies are needed to confirm our findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics14060666/s1, Table S1: Baseline characteristics of the data used in sensitivity analysis.
Author Contributions
Conceptualization, J.H.N.; data curation and formal analysis, G.U.P.; investigation, J.H.N., S.H.Y. and D.J.O.; methodology, G.U.P. and J.H.N.; writing—original draft, S.H.Y. and J.H.N.; writing—review and editing, D.J.O. and S.H.K.; supervision, H.W.K., J.H.K. and Y.J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Dongguk University Research Fund (2021) and a National Research Foundation of Korea (NRF) grant (2021R1G1A1094851) funded by the Korean Government (MSIT).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Dongguk University Ilsan Hospital (IRB no. DUIH 2021-07-021 (19 February 2021).
Informed Consent Statement
Patient consent was waived because this study analyzed the NHIS secondary data for research and statistical purpose.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The author Geun U Park works for GN Co. The other authors declare no conflicts of interest.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Kanth, P.; Inadomi, J.M. Screening and prevention of colorectal cancer. Br. Med. J. 2021, 374, n1855. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Goel, A.; Chung, D.C. Pathways of colorectal carcinogenesis. Gastroenterology 2020, 158, 291–302. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Duong, H.Q. The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy. Oncol. Lett. 2018, 16, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260. [Google Scholar] [CrossRef]
- Mondul, A.M.; Weinstein, S.J.; Layne, T.M.; Albanes, D. Vitamin D and cancer risk and mortality: State of the science, gaps, and challenges. Epidemiol. Rev. 2017, 39, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194 (Suppl. S2), S3–S11. [Google Scholar] [CrossRef]
- Lane, J.M.; Russell, L.; Khan, S.N. Osteoporosis. Clin. Orthop. Relat. Res. 2000, 372, 139–150. [Google Scholar] [CrossRef]
- Lim, J.U.; Cha, J.M.; Lee, J.I.; Joo, K.R.; Shin, H.P.; Park, J.J.; Jeon, J.W. Osteoporosis is associated with the risk of colorectal adenoma in women. Dis. Colon. Rectum. 2013, 56, 169–174. [Google Scholar] [CrossRef]
- Marques da Costa, P.; Martins, I.; Neves, J.; Cortez-Pinto, H.; Velosa, J. Serum vitamin D levels correlate with the presence and histological grading of colorectal adenomas in peri and postmenopausal women. Clin. Nutr. 2019, 38, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.; Lipka, S.; Davis-Yadley, A.H.; Shen, H.; Silpe, J.; White, A.; Satler, S.; Luebbers, D.; Statler, J.; Zheng, A.; et al. Low bone mineral density linked to colorectal adenomas: A cross-sectional study of a racially diverse population. J. Gastrointest. Oncol. 2015, 6, 165–171. [Google Scholar]
- Nam, J.H.; Koh, M.; Kang, H.W.; Ryu, K.H.; Lee, D.S.; Kim, S.H.; Jang, D.K.; Jeong, J.B.; Kim, J.W.; Lee, K.L. Osteoporosis is associated with an increased risk of colorectal adenoma and high-risk adenoma: A retrospective, multicenter, cross-sectional, case-control study. Gut Liver 2022, 16, 269–276. [Google Scholar] [CrossRef]
- WHO Scientific Group on the Prevention and Management of Osteoporosis. Prevention and Management of Osteoporosis: Report of a WHO Scientific Group; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Chiang, C.H.; Huang, C.C.; Chan, W.L.; Huang, P.H.; Chen, T.J.; Chung, C.M.; Lin, S.J.; Chen, J.W.; Leu, H.B. Oral alendronate use and risk of cancer in postmenopausal women with osteoporosis: A nationwide study. J. Bone Miner. Res. 2012, 27, 1951–1958. [Google Scholar] [CrossRef]
- Pazianas, M.; Abrahamsen, B.; Eiken, P.A.; Eastell, R.; Russell, R.G. Reduced colon cancer incidence and mortality in postmenopausal women treated with an oral bisphosphonate--Danish National Register Based Cohort Study. Osteoporos. Int. 2012, 23, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.; Ng, K.; Giovannucci, E.L.; Manson, J.E.; Qian, Z.R.; Ogino, S. Vitamin D and colorectal cancer: Molecular, epidemiological and clinical evidence. Br. J. Nutr. 2016, 115, 1643–1660. [Google Scholar] [CrossRef] [PubMed]
- Klampfer, L. Vitamin D and colon cancer. World J. Gastrointest. Oncol. 2014, 6, 430–437. [Google Scholar] [CrossRef]
- Giovannucci, E. Epidemiology of vitamin D and colorectal cancer. Anticancer Agents Med. Chem. 2013, 13, 11–19. [Google Scholar] [CrossRef]
- Emmanouilidou, G.; Kalopitas, G.; Bakaloudi, D.R.; Karanika, E.; Theocharidou, E.; Germanidis, G.; Chourdakis, M. Vitamin D as a chemopreventive agent in colorectal neoplasms. A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Ther. 2022, 237, 108252. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lipsyc-Sharf, M.; Zong, X.; Wang, X.; Hur, J.; Song, M.; Wang, M.; Smith-Warner, S.A.; Fuchs, C.; Ogino, S.; et al. Total vitamin D intake and risks of early-onset colorectal cancer and precursors. Gastroenterology 2021, 161, 1208–1217. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, Y.H.; Cho, C.H.; Kim, S.H.; Lee, J.E. Circulating levels of vitamin D and colorectal adenoma: A case-control study and a meta-analysis. World J. Gastroenterol. 2015, 21, 8868–8877. [Google Scholar] [CrossRef]
- Pereira, F.; Larriba, M.J.; Muñoz, A. Vitamin D and colon cancer. Endocr. Relat. Cancer 2012, 19, R51–R71. [Google Scholar] [CrossRef]
- Kroll, M.H.; Bi, C.; Garber, C.C.; Kaufman, H.W.; Liu, D.; Caston-Balderrama, A.; Zhang, K.; Clarke, N.; Xie, M.; Reitz, R.E.; et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS ONE 2015, 10, e0118108. [Google Scholar] [CrossRef]
- Jadayil, S.A.; Jadayel, B.A.; Takruri, H.; Muwalla, M.; McGrattan, A.M. Study of the fluctuation of serum vitamin D concentration with time during the same day and night on a random sample of healthy adults. Clin. Nutr. ESPEN 2021, 46, 499–504. [Google Scholar] [CrossRef]
- Mazahery, H.; von Hurst, P.R. Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients 2015, 7, 5111–5142. [Google Scholar] [CrossRef]
- Wakeman, M. A Literature review of the potential impact of medication on vitamin D status. Risk Manag. Healthc. Policy 2021, 14, 3357–3381. [Google Scholar] [CrossRef]
- Watts, N.B.; Binkley, N.; Owens, C.D.; Al-Hendy, A.; Puscheck, E.E.; Shebley, M.; Schlaff, W.D.; Simon, J.A. Bone mineral density changes associated with pregnancy, lactation, and medical treatments in premenopausal women and effects later in life. J. Women’s Health 2021, 30, 1416–1430. [Google Scholar] [CrossRef]
- Aspray, T.J.; Chadwick, T.; Francis, R.M.; McColl, E.; Stamp, E.; Prentice, A.; von Wilamowitz-Moellendorff, A.; Schoenmakers, I. Randomized controlled trial of vitamin D supplementation in older people to optimize bone health. Am. J. Clin. Nutr. 2019, 109, 207–217. [Google Scholar] [CrossRef]
- Di Daniele, N.; Carbonelli, M.G.; Candeloro, N.; Iacopino, L.; De Lorenzo, A.; Andreoli, A. Effect of supplementation of calcium and vitamin D on bone mineral density and bone mineral content in peri- and post-menopause women; a double-blind, randomized, controlled trial. Pharmacol. Res. 2004, 50, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Kyoung, D.S.; Kim, H.S. Understanding and utilizing claim data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) database for research. J. Lipid Atheroscler. 2022, 11, 103–110. [Google Scholar] [PubMed]
- Cheng, H.G.; Phillips, M.R. Secondary analysis of existing data: Opportunities and implementation. Shanghai Arch. Psychiatry 2014, 26, 371–375. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).