Abstract
Objective: Colorectal cancer (CRC) during pregnancy is a rare occurrence, with a reported incidence of 0.8 cases per 100,000 pregnancies. Managing CRC during pregnancy poses substantial challenges for clinicians: the diagnosis is often complicated and delayed due to symptom overlap with pregnancy-related manifestations, and medical imaging is constrained by safety concerns for the foetus. Methods: This article presents two cases of advanced CRC diagnosed and managed during pregnancy. Additionally, we conducted a systematic review of the literature to assess diagnostic and prognostic factors involved in CRC in pregnant individuals. The systematic review, with pre-registration and approval through Prospero, involved an extensive search of medical databases (Pubmed, Web of Science, Scopus and Scholar) and statistical analysis using t-test for continuous variables and chi square for dichotomous variables. Results: A total of 1058 studies were identified. After applying exclusion criteria, sixty-six studies were included. Women whose initial symptoms were severe abdominal pain not responsive to common medical treatments and constipation (acute abdomen) had a mean gestational age at delivery lower than those who presented with paucisymptomatic onset. In our study groups, women who underwent chemotherapy during pregnancy had a higher mean gestational age at delivery and did not experience worse neonatal outcomes compared to those who did not undergo chemotherapy. Conclusions: CRC during pregnancy poses unique diagnostic and therapeutic challenges. Collaborative efforts among various medical disciplines are essential to manage CRC during pregnancy.
1. Introduction
Pregnancy-associated cancer (PAC) is an uncommon and challenging disease that raises crucial clinical, bioethical, and psychological issues, significantly impacting the health of pregnant women and the conceptus [1,2]. Due to bioethical concerns and the rarity of the disease, there is a lack of clinical trials and evidence-based guidelines. The epidemiology of PAC is challenging to study because nationwide registries often underestimate its incidence, databases are poorly comparable, and studies in the literature are heterogeneous because of different inclusion criteria and incoherent time intervals following delivery [3]. Approximately 1 in 2000 pregnancies are associated with a cancer diagnosis [4]. The most frequent cancers diagnosed during pregnancy are breast, melanoma, and cervical cancers, accounting for over 60% of all tumours diagnosed in pregnancy. Much rarer cancers in pregnancy include brain and nervous system tumours (9.3%), lymphomas (68%), thyroid cancer (3.8%), and colorectal cancer (CRC) (2.8%) [5]. Tumours of the gynaecological organs are rare [6]. Moreover, due to the absence of consensus and to the possible risks for the foetal health, treatment options for pregnant women with a diagnosis of cancer are limited: systemic treatment with chemotherapy should be avoided during the first trimester because it is associated with high risk of miscarriage and congenital malformations, but it can be administered during the second and third trimester. Surgery can be safely performed at any time during pregnancy. Radiotherapy should be postponed to the postpartum period regardless of the treated site due to the high risks for the foetus in terms of childhood cancer, foetal death, and mental retardation [7,8]. According to the Global Cancer Observatory-GLOBOCAN statistics, CRC is the second most common cancer in women, but it is a rare form of tumour during pregnancy [9]. In 2023, Sung et al. estimated 71,160 new cases of invasive CRC in American women, including 8990 diagnoses in women younger than 50 years [10]. Since 2010, the incidence of CRC has been increasing by approximately 3% per year in people under 50. We expect this number will continue to rise due to increasing cancer rates, delayed childbearing, and more sensitive screening. The new emerging factors, such as delayed childbearing and enhanced screening, contribute to the increased incidence in pregnant women [11]. Sporadic early onset CRC exhibits similar biological behaviour to later-onset disease. However, there are clinical distinctions [12]: early onset cancer is more likely to present with symptoms such as haematochezia (41%) and abdominal pain [13], with a predominance of left-sided tumours (73%) and advanced cases (27% with distant metastases). These characteristics were also observed in our patients [14]. Based on cancer registry data, survival rates are higher for early onset disease because younger patients are more likely to receive extensive treatments, including surgery and multi-agent adjuvant or neoadjuvant chemotherapies [15].
2. Case Reports
2.1. Case Report 1
A 33-year-old patient with no significant medical history, prior surgical procedures, or family history of oncological disorders presented to the emergency room (ER) at Community Hospital of Barletta (Italy) due to severe abdominal pain unresponsive to common medications, constipation, and bloating (acute abdomen). The patient was 19 weeks pregnant with her second child; her first pregnancy had been uneventful. Obstetric assessment indicated no issues with the ongoing pregnancy. An abdominal Magnetic Resonance Imaging (MRI) revealed signs of intestinal obstruction suggestive of a sigmoid volvulus. She underwent urgent laparotomy with Hartmann sigmoid resection and colostomy the following day. Biopsy of the surgical specimen unveiled a sigmoid adenocarcinoma G2 causing strictures, ulceration, and full-thickness infiltration of the muscular layer with localised high-grade tumour and extension to the subserosal layer. No lymphatic, vascular, or perineural infiltration was detected. Surgical margins were tumour-free, as were the perivisceral lymph nodes. According to the histopathologic Tumour-Node Metastases staging system (pTNM) [14], the final stage was pT3bN0. Following surgery, a chest radiograph (RX) revealed no tumour-related findings, while an abdominal ultrasound identified a hepatic hyperechoic nodule (11 × 7 mm) in segments VII–VIII of uncertain nature. At 23 weeks of pregnancy, it was decided to transfer the patient to the Central Hospital “Policlinico di Bari” (Italy). Upon admission, her clinical condition was stable, with an Eastern Cooperative Oncology Group (ECOG) [16] performance status 1, with no abdominal pain, a functioning colostomy, normal sinus rhythm on electrocardiogram, and a foetus showing no distress. Blood tests indicated mild anaemia (haemoglobin 8.8 g/dL) but otherwise regular values for electrolytes, liver function, renal function, coagulation, platelets, and white blood cells. Tumour markers were assessed, showing an elevation of Alpha-fetoprotein (AFP) 109 ng/mL (normal values 0–7 ng/mL), while other tumour markers, including Carcinoembryonic antigen (CEA), Cancer Antigen 15.3 (CA15.3), Cancer Antigen 125 (CA125), and Cancer Antigen 19.9 (CA19.9), were within the normal range. During the hospitalisation, an abdominal MRI confirmed the presence of multiple hepatic nodules (diameter < 10 mm), suggestive of neoplastic lesions. The MRI, reported in (Figure 1), also showed a nodule on the parietal slope of the amniotic sac (20 mm) of unknown nature. This finding was not confirmed by ultrasound evaluation of the uterus.
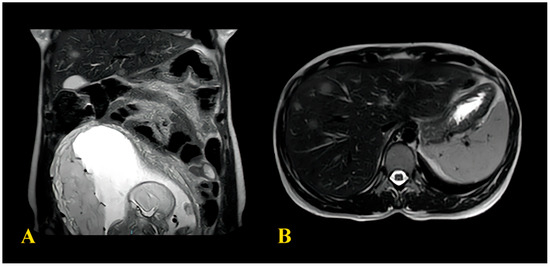
Figure 1.
(A) MRI images showing the formation in amniotic sac; (B) CT image showing the hepatic metastases.
Three hepatic biopsies were performed to ascertain the nature of these nodules, but the results were inconclusive. A multidisciplinary team comprising obstetricians, neonatologists, oncologists, and psychologists convened to discuss the case, deciding to initiate adjuvant chemotherapy during the pregnancy. Prior to discharge, the patient received a blood transfusion. Five cycles of folinic acid, fluorouracil, and oxaliplatin (FOLFOX) were administered biweekly (with a 25% dosage reduction as a precaution). Regular monitoring occurred every two weeks post-chemotherapy infusion. The foetus exhibited steady growth throughout treatment, with no signs of distress, and blood tests showed no haematological toxicity. The patient’s overall health remained favourable, and psychological support was provided. After the completion of chemotherapy, an abdomen ultrasound revealed reduced dimensions of the hepatic nodules. Subsequently, the multidisciplinary team scheduled an elective caesarean section (CS) after a three-week pause from the last cycle of chemotherapy at 37 weeks of pregnancy, with concomitant removal of the resectable hepatic nodules. The CS was successfully conducted at 37 weeks + 1 day, resulting in the birth of a 2940 g male infant. The newborn exhibited favourable conditions at birth, with arterial pH of 7.32, BE (B) of 0.8 mmol/L, BEecf of 1.7 mmol/L, and Apgar scores of 8 and 10. Routine assessments showed no distress. The histologic evaluation of hepatic nodules confirmed colic adenocarcinoma localisation. The histopathological analysis of the placenta was negative. After consulting with neonatologists regarding the benefits and risks of breastfeeding, the patient requested to inhibit lactation.
During hospitalisation, a contrast-enhanced chest-abdomen computer tomography (CT) demonstrated numerical and dimensional reduction of hepatic metastases. Given the positive response to chemotherapy, the multidisciplinary team decided to continue with FOLFOX (at full dosage), adding Bevacizumab, which was contraindicated in pregnancy. After six more cycles of chemotherapy, MRI and CT were performed, showing both numerical and dimensional regression of the hepatic nodules. In agreement with the general surgeons, it was decided to perform surgery to excise the liver metastasis and restore bowel continuity. The patient underwent a wedge resection involving segments VIII–V and IVb. After surgery, tumour marker values were within normal range. Given the favourable response to first-line chemotherapy, an additional six cycles of FOLFOX-BEVACIZUMAB were administered. One year post-diagnosis, the patient continues this therapeutic regimen, maintaining an optimal performance status (ECOG performance status 0). Additionally, her infant has reached one year of age, achieving normal developmental milestones.
2.2. Case Report 2
A 43-year-old patient at her fourth pregnancy (preceded by three miscarriages), was admitted to the ER due to abdominal pain at 17 weeks of gestation. Her medical history included intestinal polyp removal via endoscopy and thyroid nodules with current euthyroidism. An abdominal ultrasound revealed a multilocular solid cyst in the right adnexa, exhibiting irregular internal margins and multiple septa and vegetations (Figure 2).
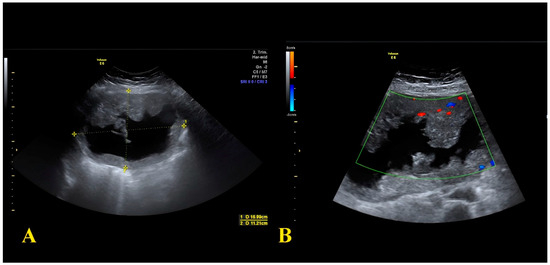
Figure 2.
(A) Sonographic image showing the 16 × 11 cm adnexal cyst with a solid component; (B) Sonographic image showing intralesional vascularization.
The cyst measured 15 cm in maximal diameter with a colour score of 3. Tumour markers assessment showed an elevation of AFP 100 ng/mL (normal range 0–7 ng/mL), CEA 14.6 ng/mL (normal range 0–5.2 ng/mL), and CA125 86.7 U/mL (normal range 0–35 U/mL), while CA19.9 and CA15.3 values were in the normal range. During hospitalisation, the patient underwent an MRI that indicated a sizable right adnexal lesion measuring 19 × 16 × 14 cm, characterised by protruding vegetations and a fluid component. There was not any free fluid in the abdomen nor significant lymphadenopathies. Pharmacological treatment of abdominal pain was unsuccessful; therefore, an urgent laparotomy was performed, during which the right adnexa was resected, alongside omental and peritoneal biopsies. Histological analysis identified an ovarian mucinous tumour with necrotic areas, while the fallopian tube remained tumour-free. Ki67 expression was 40%. Immunohistochemistry results indicated CK20 (+), PAX8 (−), CK7 (−), CDX2 (+), Estrogen Receptor (−), suggesting ovarian metastasis from gastrointestinal carcinoma. After the surgery, a colonoscopy highlighted a colon stenosis at the left flexure, which was biopsied.
Meanwhile, the pregnancy was monitored: foetal growth was regular, and there were no signs of foetal distress. The multidisciplinary team convened to formulate a management strategy: due to the advanced tumour stage and the poor performance status (ECOG 2), it was decided to wait until after the 28th week of gestation to perform a CS and a contextual intestinal resection. The CS was performed at 28 weeks of gestation, delivering a healthy newborn with favourable conditions at birth. Intraoperative inspection revealed multiple foci of cancer in the abdominal cavity, prompting a debulking surgery: left adnexa removal, excision of multiple parietal nodules, left hemicolectomy with colostomy placement, omentectomy, and cholecystectomy. Histopathological assessment unveiled a poorly differentiated adenocarcinoma from the large intestine, invading the serosal layer and perivisceral adipose tissue. Lymphatic, vascular, and perineural infiltration was detected. Twelve lymph nodes showed metastatic involvement. Metastasis were also detected in the omentum and peritoneum. The gallbladder was tumour-free. The final staging was, according to pTNM [17], pT4b, N2, M1b. Immunohistochemistry profiles indicated a mutated K-RAS, wild-type B-RAF and N-RAS, and no microsatellite instability. Following surgery, a contrast-enhanced whole-body CT revealed multiple hepatic metastases. The patient underwent adjuvant chemotherapy consisting of FOLFOX and Bevacizumab. After twelve cycles of chemotherapy, a CT indicated partial response, prompting a maintenance regimen involving 5-fluorouracil + Bevacizumab (five cycles). A subsequent positron emission tomography (PET) scan documented disease progression with multiple new metastases in the liver and lungs. Second-line therapy involving folinic acid, fluorouracil, irinotecan (FOLFIRI), and Aflibercept was recommended. Unfortunately, during this treatment course, the patient’s condition deteriorated, leading to her passing around two years after initial diagnosis.
3. Systematic Review and Metanalysis: Methods
This review was preregistered in the International Prospective Register of Systematic Reviews (PROSPERO) with protocol CRD42023440524 and is made in respect of the Preferred-Reporting-Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18].
3.1. Inclusion and Exclusion Criteria
Two different researchers scoped the existing literature, including studies that met the following inclusion criteria: (1) case reports; (2) written in the English language; (3) published in peer-reviewed journals; (4) with both an available abstract and full text; (5) involving women with a histologic diagnosis of colorectal cancer made during pregnancy; (6) involving women who underwent chemotherapy and/or surgery during pregnancy. We applied the following exclusion criteria: (1) scoping or systematic reviews; (2) literature not in the English language; (3) non-peer-reviewed papers; (4) papers with unavailable full text and abstract; (5) pregnant women with a histological diagnosis different from colorectal cancer; (6) women who were diagnosed or treated for colic cancer not during pregnancy; (7) papers without a histology diagnosis.
3.2. Search Strategy
We conducted searches in the Pubmed, Web of Science, Scopus, and Scholar databases, including a combination of the following terms with synonyms: “colon cancer”, “rectal cancer”, “colorectal cancer”, “pregnancy”, “management”, “surgery”, “maternal outcomes”, and “pediatric outcomes”. All searches were performed in English without a time limit. The initial search was completed in April 2023 and updated in June 2023.
3.3. Data Extraction
For each study, the two authors extracted the following data: authorship, year of publication, maternal and gestational age at the time of tumour diagnosis, presence of acute abdomen at the time of diagnosis, type of management (wait, upfront surgery, chemotherapy), type of delivery (caesarean, natural childbirth), neonatal outcomes including live birth, stillbirth, or miscarriage, neonatal major problems at the time of birth, and tumour staging based on the definitive histology.
3.4. Quality of the Studies
The two authors independently evaluated the quality of the included studies using a modified ROB 2 scale [19], assessing five items and the overall risk of bias in the evaluations. The items evaluated were randomisation bias (not applicable), bias in deviation from intended intervention, bias due to missing outcome data, bias in measurement of outcome, and selection bias (not applicable). Each item was rated on a three-point scale: low, some concerns, high, or no information. The overall results are presented in (Figure 3).
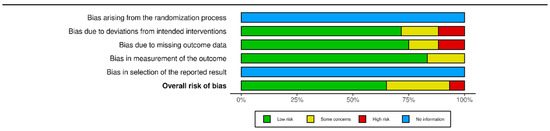
Figure 3.
Summary plot of bias calculated with ROB2 tool via rob vis tool. Modified via [20].
4. Systematic Review and Metanalysis: Results
A total of 1058 studies were initially identified. Firstly, duplicates (n = 239) were removed, resulting in 819 studies for abstract and title screening. Among these, 715 were excluded as they did not meet the inclusion criteria. Out of the remaining 104 studies eligible for full-text analysis, 28 were excluded because they were irrelevant to the topic, 6 due to unreported gestational age and histology, and 4 because the tumour diagnosis was made in the postpartum period. Consequently, 66 studies were ultimately included. See (Figure 4) for a summary of the selection process.
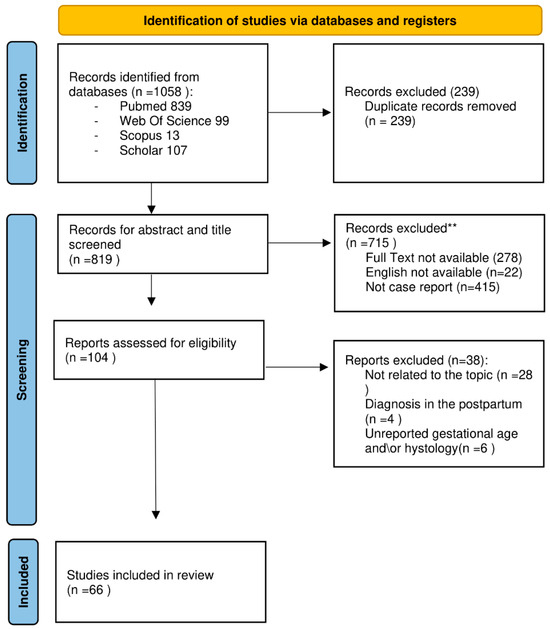
Figure 4.
PRISMA flow diagram for new systematic review-FLOW CHART, modified from [21]. ** The authors manually excluded the records. No automation tools were used.
We enlisted 66 cases of colorectal cancer diagnosed during pregnancy. In (Table 1), we list the main characteristics of the study population.

Table 1.
Main characteristics of the population in the study.
4.1. Primary Outcomes
The mean age at diagnosis was 32.47 ± 4.17 years. The youngest girl diagnosed with PAC was 24 [30], while the oldest was 43 [27]. The mean gestational age at diagnosis was 25 weeks ± 8 weeks. The minimum gestational age was 9 weeks [26], and the maximum was 39 weeks [59]. The mean gestational age at birth was 32 weeks ± 5 weeks. The clinical presentation of patients was mostly non-specific. Acute abdomen at the time of diagnosis was observed in 53% of patients (n = 35). Upfront surgery was performed in 48.5% of patients (n = 32), while 39.9% (n = 27) underwent chemotherapy during pregnancy. The caesarean section was performed in 48.5% of patients (n = 32), while natural delivery occurred in 39.9% (n = 27). Data regarding the delivery modality were missing for 10.6% of patients (n = 7). The neonatal outcomes revealed live births in 75.8% of cases (n = 50), stillbirths in 7.6% of cases (n = 5), and miscarriages in 7.6% of cases (n = 5). In (Table 2), we list the main neonatal outcomes of the study population. The main histological finding was colic adenocarcinoma in 89.4% (n = 59) of cases. More rare histologic types included signet ring cell (n = 3) [37,50], undifferentiated (n = 3) [50,61], and neuroendocrine (n = 1) [50]. A total of 89.4% (n = 59) of the patients in the review were in an advanced stage (III/IV) at diagnosis. Among the patients who underwent upfront surgery, the laparotomic approach was the most commonly chosen method (96.8% of cases, n = 30), and 78% (n = 25) presented with an acute abdomen on the clinical onset. The delivery mode of women who underwent upfront surgery was caesarean section in 28.1% (n = 9) and vaginal delivery in 56.3% (n = 18). The delivery mode was not specified in 15.6% of patients (n = 5). There was no correlation found between mode of delivery and neonatal outcomes (p = 0.83). In (Table 3) we report a summary of the main findings of the review.

Table 2.
Main data on neonatal outcome of the population in the study.

Table 3.
Summary of main findings of the systematic review.
4.2. Subgroup Analysis 1: Chemotherapy
In subgroup analysis 1, we differentiated two groups: women who underwent chemotherapy and women who did not. The first group had a mean gestational age at diagnosis of 22 ± 8 weeks, while the second group had a mean gestational age at diagnosis of 28 ± 6 weeks, with a statistically significant difference (p < 0.004, D Cohen 7.50, CI 95% 0.24–1.27). Regarding gestational age at delivery, women who underwent chemotherapy had a mean gestational age at delivery of 34 ± 4 weeks, while in the other group, it was 31 ± 5 weeks, but the difference was not statistically significant (p = 0.08). We calculated the differences in neonatal outcomes (live birth or stillborn/miscarriage) between the two groups and found no significant difference (p = 0.40). There was no significant difference in delivery modality between the two groups (p = 0.6).
4.3. Subgroup Analysis 2: Upfront Surgery
In subgroup analysis 2, we differentiated women who underwent upfront surgery from those who did not. The two sub-groups had no statistically significant difference in maternal age at diagnosis (p = 0.69) and gestational age at delivery (p = 0.43). However, there was a statistically significant difference in the median gestational age at diagnosis, 22 ± 8 weeks in women who underwent upfront surgery vs. 29 ± 7 weeks in women who did not (p < 0.001). We did not find a statistically significant difference between the two groups regarding neonatal outcomes (p = 0.29). We found a statistically significant difference (Chi-square 8.76, p < 0.003) between the two groups concerning the rate of caesarean sections: 66% (n = 18) of women who underwent upfront surgery had a spontaneous delivery. In contrast, only 28% (n = 9) of women who did not undergo upfront surgery had a spontaneous delivery.
4.4. Subgroup Analysis 3: Acute Abdomen at Diagnosis
In subgroup analysis 3, we differentiated women with acute abdomen at diagnosis vs. women with paucisymptomatic presentations. There was no statistically significant difference between the two groups’ gestational age and maternal age at diagnosis (p = 0.45, p = 0.25). However, there was a statistically significant difference between acute abdomen on onset and gestational age at birth (p = 0.04). Patients with acute abdomen on onset had deliveries at 31 weeks ± 6 weeks, while patients without acute abdomen on onset had a mean gestational age at delivery of 34 weeks ± 4 weeks. The difference between the means of the two groups was statistically significant (p < 0.04, Cohen d = 4.95, CI 95% 0.01–1.0). We calculated the differences in neonatal outcomes (live birth or stillborn/miscarriage) between the two groups and found a significant difference (Chi-square 3.85, p = 0.05). Among women with acute abdomen at diagnosis (n = 31), 25% (n = 8) had adverse neonatal outcomes. In contrast, among women with paucisymptomatic onset of symptoms (n = 29), only 6% (n = 2) had adverse neonatal outcomes. We did not find a statistically significant difference in the caesarean section rate between the two groups (p = 0.23).
4.5. Subgroup Analysis 4: Surgery versus Chemotherapy
In subgroup analysis 4, we differentiated patients who underwent surgery vs. patients who underwent chemotherapy. We found a significant difference in gestational age between the two groups at the time of the delivery (p < 0.03, CI 95% 0.4–8.7, Cohen d = 5.03). Patients who underwent surgery had a mean gestational age at the time of the delivery of 29 weeks ± 5 weeks, while patients who underwent chemotherapy had a mean gestational age at the time of delivery of 34 weeks ± 4 weeks. No other significant differences were found among the two groups of patients.
5. Discussion
5.1. Case Studies
Managing cancer during pregnancy is a complex task that requires a multidisciplinary approach involving oncologists, gynaecologists, paediatricians, and psychologists [70,71]. PAC diagnosis can be challenging for gynaecologists and primary care physicians and should adhere to current cancer guidelines applicable to general oncological patients [72,73]. Often, the typical signs and symptoms of cancer, such as changes in appetite, nausea, constipation, abdominal discomfort, pain, and fatigue, can be easily mistaken for pregnancy-related physiological changes [74]. This overlap in clinical presentation may contribute to delayed diagnosis and management of cancer during pregnancy [75,76]. In our cases, the patients were under 50 years old and had no family history of neoplastic diseases or significant medical/obstetrical personal history. Their pregnancies were physiological and under regular obstetrical check-ups and screenings. The cancer was diagnosed in both cases in the ER during the diagnostic work-up of acute abdominal pain. In case 1, the lesion causing acute pain was located in the bowel, and histologic findings indicated intestinal cancer with resection margins and perivisceral lymph nodes free of disease. Given the limited number of metastatic sites, the gestational age (25 weeks at the beginning of chemotherapy), and the favourable clinical conditions of the patient, chemotherapy with FOLFOX was initiated and continued at 75% dosage until she reached the term of the pregnancy. In case 2, the diagnosis was more challenging because the cyst removed during the urgent surgery was identified as mucinous cancer, with immunomorphological characteristics suggesting ovarian metastasis from gastrointestinal carcinoma. Subsequently, a colonoscopy revealed colon stenosis at the left flexure level, compatible with primary disease. The approach in case 2 differed significantly due to the earlier gestational age at the moment of the diagnosis (she was 17 weeks pregnant), the longer diagnostic process to find the primitive disease, and the more invasive disease. The multidisciplinary team decided to wait until the 28th week of pregnancy to deliver the foetus and initiate chemotherapy afterwards. The clinical approach was significantly different between the two cases, given that in case 1, the primary lesion was surgically removed in the initial surgery, and there were doubts about hepatic nodules, but the tumour was oligometastatic. In contrast, in case 2, only one metastasis was removed in the initial surgery, and imaging did not provide a clear picture of the actual extension of the disease. In case 1, chemotherapy was initiated during pregnancy, whereas in case 2, it was decided to wait until the 28th week to deliver the foetus and then re-evaluate the patient and start therapy. In both cases, we arranged caesarean sections simultaneous to the general neoplastic surgery. The newborns in both cases showed no clinical issues at birth and underwent regular neonatal follow-ups without any reported concerns.
5.2. Diagnostic Work-Up
The diagnostic work-up and staging of CRC during pregnancy is challenging; the importance of the information that invasive procedures, ionising radiation, and contrast agents can provide to the clinician should be carefully weighed against the risk for foetal health. Radiological procedures are categorised by the American College of Obstetricians and Gynaecologists (ACOG) into three main groups: very low dose (<0.1 mGy), low to moderate dose (<0.1–10 mGy), and higher dose (10.50 mGy) [75]. The maximum cumulative radiation is 100 mGys, defined as the threshold dose. While very low-dose procedures like chest radiography and mammography are considered safe, low-to-moderate dose procedures such as abdominal X-rays, chest CT, and lumbar spine radiography should be performed only when the benefits outweigh the risks to foetal health. In contrast, procedures associated with higher doses, including abdominal CT, pelvic CT, and whole-body PET/CT, should generally be avoided during pregnancy [77]. Contrast agents are generally discouraged during pregnancy due to the potential placental passage. However, the ACOG guidelines permit contrast use in specific situations where the diagnostic performance of imaging is expected to improve maternal-foetal outcomes. Among contrast agents, gadolinium appears safer, whereas iodinated contrasts are generally contraindicated due to a lack of studies in humans [78]. To mitigate the risks, ultrasonography and magnetic resonance imaging (MRI) techniques are preferred in pregnant women to avoid the exposition to ionising radiation. Serum tumour markers like CA 125, CA 15.3, AFP, and SCC are generally not significant during pregnancy, but markers like AMH, CEA, CA 19-9, and HE4 usually do not exhibit increased levels during pregnancy, making them potentially applicable [79]. In conclusion, chest X-rays with abdominal shielding and MRI imaging without contrast can be safely employed for staging oncologic patients during pregnancy. Serum tumour markers may be helpful in selected cases. The emerging popularity of cell-free DNA screening increases cancer diagnosis in asymptomatic pregnant women. Amant et al. reported three cases of cancer (ovarian cancer and two forms of lymphoma) among a cohort of 4000 pregnant asymptomatic women who underwent non-invasive prenatal testing (NIPT) [80]. These findings question the utility of NIPT tests like a cancer soft marker, but further studies are needed to assess the clinical reliability and diagnostic and prognostic value of this new approach [81].
5.3. Treatment Options: Upfront Surgery
Oncologic treatment during pregnancy may include surgery, especially after the first trimester, systemic treatment with medications compatible with pregnancy, and, in selected cases, radiotherapy. Radical surgery is one of the most effective strategies to improve cancer outcomes. In a 6018 cohort of second- and third-trimester pregnant women who underwent laparoscopic and laparotomic abdominal surgery, statistically significant (p < 0.001) benefits were observed in the laparoscopic group in terms of operative time, need for blood transfusion, and hospital stays compared to the laparotomy group [82]. Laparoscopy also results in fewer adverse foetal and maternal complications compared to laparotomy [83]. However, managing PAC can pose challenges, including an increased rate of severe maternal morbidity associated with cancer, often necessitating transfusions, hysterectomy, and ventilation complications [84,85].
5.4. Treatment Options: Chemotherapy
The placenta functions as a biological interface between the mother and the foetus, providing nutrients and oxygen and acting as an immunologic barrier [86,87]. Under physiological conditions, maternal and foetal blood are functionally connected but separated by the placental barrier, which regulates the passage of xenobiotics, including antineoplastic agents [88,89]. The transfer of anticancer agents across the placental barrier is influenced by their properties such as molecular weight, ionisation, binding to plasma proteins, and hydro-lipophilicity. Highly lipophilic molecules with a molecular weight of less than 500 Daltons, without ionisation, and bound to plasma proteins are likely to cross the placental barrier through passive diffusion, a process that does not require energy and is directly dependent on drug concentration in the maternal circulation [90,91]. Larger-molecular-weight hydrophilic molecules may be actively transported across the placental barrier by ATP-powered transporters against the concentration gradient, such as multidrug resistance protein, glycoprotein-p, and breast cancer resistance protein [92]. Antineoplastic toxicity depends mainly on the timing of exposure. Within the first ten days after conception, there is an “all-or-nothing” phenomenon, where the embryo’s survival depends on the extent of cell destruction [93]. The administration of antineoplastic drugs is generally contraindicated from 10 days after conception and until 14 weeks of pregnancy due to the high risk of major malformations (heart, neural tube, upper and lower limbs, eyes, palate, and ears) that may occur during the organogenesis [94]. According to the third consensus on gynaecological cancers, chemotherapy is contraindicated during the first trimester to avoid damage during organogenesis. Dosing of chemotherapeutic drugs during pregnancy should consider molecular weight, without significant differences in dosing compared to non-pregnant patients [6]. Chemotherapy is generally not recommended beyond 35 weeks of pregnancy. It is essential to allow a three-week interval between the last cycle of chemotherapy and delivery to allow maternal and foetal bone marrow to recover. The standard chemotherapy for advanced colorectal carcinoma is based on FOLFOX or FOLFIRI regimens (5-FU, leucovorin, and oxaliplatin or irinotecan) [95]. There are no specific guidelines for the management of colorectal cancer during pregnancy, but studies consider the FOLFOX and FOLFIRI regimens feasible during the second and third trimesters [96]. The human safety of 5-FU has been evaluated retrospectively through a literature review of case reports, case series, and surveys [97]. Exposure during pregnancy was associated with a 1% overall rate of major malformations when chemotherapy was administered during the second or third trimester, which was not significantly different from the rate in the general population. Platinum-based therapies, such as oxaliplatin, have also been associated with relatively safe foetal outcomes when administered in the second and third trimesters, with agents such as cisplatin and carboplatin demonstrating rates of major foetal malformation of 1% and 0%, respectively [97]. Oxaliplatin has been associated with an increased incidence of the foetal condition of small for gestational age [98]. Among four patients registered in the International Network on Cancer, Infertility, and Pregnancy database who received combination FOLFOX treatment in the second or third trimesters, all of them delivered healthy infants [99]. Two reports of irinotecan administered during the second trimester did not note any complications or malformations. Indeed, 5-FU is considered to not result in significant long-term disabilities, apart from the tendency for newborns to be smaller than those who are not exposed, and oxaliplatin and irinotecan have also been reported to be safe [46].
6. Conclusions
We presented these cases and the accompanying review to shed light on the complexities of managing PAC. We want to underscore the importance of early cancer diagnosis and individualised treatment plans: the collaboration of multidisciplinary healthcare specialists should be encouraged to optimise clinical outcomes for both pregnant women and foetuses.
The successful management of case 1, including upfront surgery and adjuvant chemotherapy during pregnancy, made it possible to reach the pregnancy term (37 weeks) and deliver a healthy newborn. This case demonstrates the effectiveness of a coordinated and well-executed healthcare strategy. Case 2 highlights that despite the multidisciplinary efforts, in some cases of advanced PAC, the aggressiveness of the disease and poor performance status of the patient can contraindicate invasive management of the disease. This case emphasises the importance of early detection and underscores the need for ongoing research and advancements in maternal-foetal medicine and oncology to improve outcomes in such complex cases.
Our systematic review provides clinically significant insights into the characteristics and management approaches for pregnant women diagnosed with colorectal cancer. We demonstrate the variability in presentation, treatment modalities, and outcomes. Gestational age at diagnosis, mode of delivery, and acute symptoms appear to influence maternal and neonatal outcomes. Our statistical analysis revealed the importance of early diagnosis of PAC: the cases where the diagnosis was delayed often led to upfront surgery due to acute abdomen, resulting in poor maternal and neonatal outcomes with higher caesarean rates and earlier gestational age at the delivery. In contrast, we found that women with an early diagnosis were more likely to undergo chemotherapy during pregnancy, resulting in better control of the disease and later gestational age at delivery. Despite the uncertainties about the effects of chemotherapy on the foetus, we did not find different neonatal outcomes between those exposed to chemotherapy during pregnancy and those not. Chemotherapy during pregnancy is a feasible and safe treatment for PAC, allowing the clinician to treat the maternal disease and concomitantly delay foetal delivery to a safer gestational age.
In the author’s current knowledge, this is one of the studies on PAC with the most significant sample size and longest time extension; our statistical analysis highlighted some topics of emerging clinical importance in managing pregnant women diagnosed with CRC. The study’s main limitations are the high heterogeneity of included articles, the low/moderate overall risk of bias, mainly because of unreported data, and the absence of long-term outcomes for both the patients and the newborns. Because of ethical concerns and the rarity of PAC, there is a lack of prospective or randomised studies on CRC during pregnancy; this causes a lack of evidence-based guidelines for managing the disease. Medical knowledge and multidisciplinary care advancements continue to offer new options to improve outcomes in these rare and intricate situations. Further efforts in oncological gynaecology are needed to refine our understanding and improve care for pregnant individuals facing this challenging disease.
Author Contributions
A.G., M.C. and F.M. collected the data of case studies and performed the scoping of literature on medical databases; A.G., M.C. and G.R.D. procured images and tables and performed statistical analysis; A.G., M.C., M.D. and V.P. executed the formal analysis of the literature; A.V., E.C., G.C., L.M.S. and M.M. supervised the work, conceptualized the research analysis, and devised the methodology; A.G. and M.C. wrote the original draft; A.G., M.C., A.V., L.M.S. and M.M. reviewed the original draft; L.M.S., M.M., A.V., E.C. and G.C. verified the overall replication/reproducibility of results; A.G., M.C. and A.V. reviewed the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethics Committee approval was not necessary since the study was a summary of data and outcomes of routine management (without direct intervention) and not an experimental protocol. We ensured the complete anonimacy of the patients.
Informed Consent Statement
Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
All data are reported in the text.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Murgia, F.; Marinaccio, M.; Cormio, G.; Loizzi, V.; Cicinelli, R. Pregnancy related cancer in Apulia. A population based linkage study. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 3, 100025. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.; Anic, K.; Hasenburg, A. Cancer and pregnancy: A comprehensive review. Cancers 2021, 13, 3048. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, N.A. Coexistence of pregnancy and malignancy. Oncologist 2002, 7, 279–287. [Google Scholar] [CrossRef]
- Stensheim, H.; Møller, B.; van Dijk, T.; Fosså, S.D. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: A registry-based cohort study. J. Clin. Oncol. 2009, 27, 45–51. [Google Scholar] [CrossRef]
- Eibye, S.; Kjær, S.K.; Mellemkjær, L. Incidence of pregnancy-associated cancer in Denmark, 1977–2006. Obstet. Gynecol. 2013, 122, 608–617. [Google Scholar] [CrossRef]
- Amant, F.; Berveiller, P.; Boere, I.A.; Cardonick, E.; Fruscio, R.; Fumagalli, M.; Halaska, M.J.; Hasenburg, A.; Johansson, A.L.V.; Lambertini, M.; et al. Gynecologic cancers in pregnancy: Guidelines based on a third international consensus meeting. Ann. Oncol. 2019, 30, 1601–1612. [Google Scholar] [CrossRef]
- Sorouri, K.; Loren, A.W.; Amant, F.; Partridge, A.H. Patient-Centered Care in the Management of Cancer During Pregnancy. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e100037. [Google Scholar] [CrossRef]
- Peccatori, F.A.; Azim, H.A.; Orecchia, R., Jr.; Hoekstra, H.J.; Pavlidis, N.; Kesic, V.; Pentheroudakis, G.; ESMO Guidelines Working Group. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2013, 24, vi160–vi170. [Google Scholar] [CrossRef]
- The International Agency for Research on Cancer IARC. Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 23 January 2023).
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cao, S.; Okekpe, C.C.; Dombrovsky, I.; Valenzuela, G.J.; Roloff, K. Colorectal cancer diagnosed during pregnancy with delayed treatment. Cureus 2020, 12, e8261. [Google Scholar] [CrossRef]
- National Cancer Institute Statistics. The Surveillance, Epidemiology, and End Results (SEER) Program. Available online: https://seer.cancer.gov/index.html (accessed on 8 July 2023).
- Chen, F.W.; Sundaram, V.; Chew, T.A.; Ladabaum, U. Advanced-stage colorectal cancer in persons younger than 50 years not associated with longer duration of symptoms or time to diagnosis. Clin. Gastroenterol. Hepatol. 2017, 15, 728–737.e723. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Chatila, W.K.; Yaeger, R.; Walch, H.; Fernandes, G.D.S.; Krishnan, A.; Palmaira, L.; Maio, A.; Kemel, Y.; Srinivasan, P.; et al. A comprehensive comparison of early-onset and average-onset colorectal cancers. J. Natl. Cancer Inst. 2021, 113, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Habermann, E.B.; Rothenberger, D.A.; Kwaan, M.; Weinberg, A.D.; Parsons, H.M.; Gupta, P.; Al-Refaie, W.B. Adjuvant chemotherapy for stage III colon cancer in the oldest old. Cancer 2013, 119, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.; Creech, R.; Tormey, D.; Horton, J.; Davis, T.; McFadden, E.; Carbone, P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 1139–1167. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.; Savović, J.; Page, M.; Elbers, R. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- RoB 2; ROBINS-I; ROBINS-E; ROB ME. Risk of Bias Visualization Tool. Available online: https://www.riskofbias.info/welcome/robvis-visualization-tool (accessed on 18 August 2023).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Morales Santana, D.A.; Czigany, Z.; Meister, F.A.; Wiltberger, G.J.; Caspers, R.; Enzensberger, C.; Stickeler, E.; Neumann, U.P.; Lambertz, A. Bowel Obstruction Due to Stenotic Sigmoid Colon Cancer in a 32-Year-Old Patient Presenting in the Third Trimester of Pregnancy: A Case Report of an Interval Surgical Approach. Am. J. Case Rep. 2022, 23, e935920. [Google Scholar] [CrossRef]
- Tarik, I.A.; Henry, F.J.; Shabery, N.A.; Omar, Z.; Ibrahim, O.E. Colonic mucinous adenocarcinoma in a pregnant woman presented as pseudo Sister Mary Joseph’s nodule: A case report. Med. J. Malays. 2022, 77, 258–260. [Google Scholar]
- Yang, H.; Han, X. Colorectal cancer in pregnancy: A case report and literature review. J. Gastrointest. Oncol. 2021, 12, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Maqueira, A.J.; Rifai, A.O.; Albury, C.; Kantrales, W.A.; Rydell, D.; Breland, H. Delayed Diagnosis of Colonic Adenocarcinoma in a 30-Year-Old Postpartum Woman Misdiagnosed with HELLP Syndrome. Case Rep. Surg. 2020, 2020, 3085413. [Google Scholar] [CrossRef] [PubMed]
- Alkhamis, W.H.; Naama, T.; Arafah, M.A.; Abdulghani, S.H. Good Outcome of Early-Stage Rectal Cancer Diagnosed during Pregnancy. Am. J. Case Rep. 2020, 21, e925673. [Google Scholar] [CrossRef]
- Frydenberg, H.; Harsem, N.K.; Ofigsbø, Å.; Skoglund, H.; Brændengen, M.; Kaasa, S.; Guren, M.G. Chemotherapy during Pregnancy for Advanced Colon Cancer: A Case Report. Clin. Color. Cancer 2020, 19, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, P.; Zizzo, R.; Tavassoli, E.; Sutera, M.; Tin, M.C.F.; Petruzzelli, L.; Benedetto, M.; D’Anna, M.R.; De Paolis, P.; Menato, G. Colon Adenocarcinoma during Pregnancy: A Case Report and Review of the Literature. Case Rep. Obs. Gynecol. 2020, 2020, 8894722. [Google Scholar] [CrossRef]
- Sravanthi, M.V.; Suma Kumaran, S.; Palle, A.; Bojanapally, P. Adenocarcinoma of Sigmoid Colon Diagnosed in Pregnancy: A Case Report. Cureus 2020, 12, e9491. [Google Scholar] [CrossRef]
- Ibargüengoitia Ochoa, F.; Miranda Dévora, G.; Silva Lino, L.; Sepulveda Rivera, C.; González Vázquez, D.; Pérez Quintanilla, M. Colorectal Signet Ring Cell Carcinoma in a Young Pregnant Woman. Case Rep. Oncol. 2020, 13, 182–187. [Google Scholar] [CrossRef]
- Lee, S.F.; Burge, M.; Eastgate, M. Metastatic colorectal cancer during pregnancy: A tertiary center experience and review of the literature. Obs. Med. 2019, 12, 38–41. [Google Scholar] [CrossRef]
- Munteanu, O.; Voicu, D.; Voiculescu, D.I.; Negreanu, L.; Georgescu, T.A.; Sajin, M.; Berceanu, C.; Mehedinţu, C.; Brătilă, E.; Istrate-Ofiţeru, A.M.; et al. Colon cancer in pregnancy: A diagnostic and therapeutic challenge. Rom. J. Morphol. Embryol. 2019, 60, 307–317. [Google Scholar]
- Žegarac, Ž.; Duić, Ž.; Stasenko, S. Nonobstetrical Acute Abdomen during Pregnancy as a Consequence of Colorectal Carcinoma Perforation: Case Report and Review of the Literature. Case Rep. Gastrointest. Med. 2019, 2019, 3682980. [Google Scholar] [CrossRef]
- Murphy, J.E.; Shampain, K.; Riley, L.E.; Clark, J.W.; Basnet, K.M. Case 32-2018: A 36-Year-Old Pregnant Woman with Newly Diagnosed Adenocarcinoma. N. Engl. J. Med. 2018, 379, 1562–1570. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, B.; Shen, K. Adenocarcinoma of the ascending colon in a 31-year-old pregnant woman: A case report. Medicine 2018, 97, e13707. [Google Scholar] [CrossRef]
- Makhijani, R.; Bhagat, V.H.; Fayek, M. Colon cancer presenting as pseudo-obstruction during pregnancy—A case report. Obs. Med. 2017, 10, 198–200. [Google Scholar] [CrossRef]
- Ye, W.; Tang, Y.; Yao, C.; Shi, J.; Xu, Y.; Jiang, J. Advanced gastrointestinal carcinoma with massive ascites and hydrothorax during pregnancy: A case report and review of the literature. Medicine 2017, 96, e9354. [Google Scholar] [CrossRef]
- Jones, A.; Povlow, M.R. Colorectal Cancer Presenting with Constipation During Pregnancy. Cureus 2017, 9, e1190. [Google Scholar] [CrossRef]
- Gabriel, I.; Olejek, A.; Drozdzowska, B. Colon cancer in pregnancy—A difficult diagnosis. Eur. J. Obs. Gynecol. Reprod. Biol. 2016, 203, 340–341. [Google Scholar] [CrossRef]
- Ossendorp, R.R.; Silvis, R.; van der Bij, G.J. Advanced colorectal cancer resulting in acute bowel obstruction during pregnancy; A case report. Ann. Med. Surg. 2016, 8, 18–20. [Google Scholar] [CrossRef]
- Makoshi, Z.; Perrott, C.; Al-Khatani, K.; Al-Mohaisen, F. Chemotherapeutic treatment of colorectal cancer in pregnancy: Case report. J. Med. Case Rep. 2015, 9, 140. [Google Scholar] [CrossRef]
- Chouaib, N.; Rafai, M.; El Bouti, A.; Belkouch, A.; El bakkali, H.; Belyamani, L. Discovery of a small bowel tumor following an acute intestinal obstruction in a pregnant woman: Report of a case. Pan Afr. Med. J. 2015, 20, 340. [Google Scholar] [CrossRef]
- Kocián, P.; Hoch, J.; Halaska, M. Sigmoid colon cancer in pregnancy—A case report. Rozhl. Chir. 2015, 94, 170–173. [Google Scholar]
- Dogan, N.U.; Tastekin, D.; Kerimoglu, O.S.; Pekin, A.; Celik, C. Rectal cancer in pregnancy: A case report and review of the literature. J. Gastrointest. Cancer 2013, 44, 354–356. [Google Scholar] [CrossRef]
- Ho, M.Y.; Cassano-Bailey, A.; Czaykowski, P. Colorectal Cancer in Pregnancy: Driven by Pregnancy-Associated Growth Factors? J. Gastrointest. Cancer 2012, 43 (Suppl. S1), S239–S242. [Google Scholar] [CrossRef]
- Jeppesen, J.B.; Østerlind, K. Successful twin pregnancy outcome after in utero exposure to FOLFOX for metastatic colon cancer: A case report and review of the literature. Clin. Color. Cancer 2011, 10, 348–352. [Google Scholar] [CrossRef]
- Gensheimer, M.; Jones, C.A.; Graves, C.R.; Merchant, N.B.; Lockhart, A.C. Administration of oxaliplatin to a pregnant woman with rectal cancer. Cancer Chemother. Pharmacol. 2009, 63, 371–373. [Google Scholar] [CrossRef]
- Kanate, A.S.; Auber, M.L.; Higa, G.M. Priorities and uncertainties of administering chemotherapy in a pregnant woman with newly diagnosed colorectal cancer. J. Oncol. Pharm. Pr. 2009, 15, 5–8. [Google Scholar] [CrossRef]
- Taylor, J.; Amanze, A.; Di Federico, E.; Verschraegen, C. Irinotecan use during pregnancy. Obs. Gynecol. 2009, 114, 451–452. [Google Scholar] [CrossRef]
- Duffy, A.; Shia, J.; Klimstra, D.; Temple, L.; O’Reilly, E.M. Collision tumor of the large bowel in the context of advanced pregnancy and ulcerative colitis. Clin. Color. Cancer 2008, 7, 402–405. [Google Scholar] [CrossRef]
- Mechery, J.; Ikhena, S.E. Cancer of the descending colon during pregnancy. J. Obstet. Gynaecol. 2007, 27, 311–312. [Google Scholar] [CrossRef]
- Lolis, E.D.; Likoudis, P.; Voiniadis, P.; Hassiakos, D.; Samanides, L. Synchronous rectal and colon cancer caused by familial polyposis coli during pregnancy. J. Obs. Gynaecol. Res. 2007, 33, 199–202. [Google Scholar] [CrossRef]
- Chêne, G.; Tardieu, A.S.; Favard, A.; Lebel, A.; Voitellier, M. Colorectal cancer discovered during pregnancy. J. Gynecol. Obs. Biol. Reprod. 2006, 35, 504–512. [Google Scholar] [CrossRef]
- Penney, D.; Ganapathy, R.; Jonas-Obichere, M.; El-Refeay, H. Intussusception: A rare cause of abdominal pain in pregnancy. Ultrasound Obs. Gynecol. 2006, 28, 723–725. [Google Scholar] [CrossRef]
- Harma, M.; Uzunkoy, A. Colorectal cancer presenting with uncommon soft tissue invasion during pregnancy. Acta Obs. Gynecol. Scand. 2005, 84, 491–493. [Google Scholar] [CrossRef]
- Minter, A.; Malik, R.; Ledbetter, L.; Winokur, T.S.; Hawn, M.T.; Saif, M.W. Colon cancer in pregnancy. Cancer Control 2005, 12, 196–202. [Google Scholar] [CrossRef]
- Papathanasiou, K.; Tolikas, A.; Savvidis, A.; Traianos, V.; Tzafettas, J. Advanced sigmoid colon cancer in pregnancy presented as an abscess. J. Obs. Gynaecol. 2004, 24, 319–320. [Google Scholar] [CrossRef]
- Kömürcü, S.; Ozet, A.; Oztürk, B.; Arpaci, F.; Altundağ, M.K.; Tezcan, Y. Colon cancer during pregnancy. A case report. J. Reprod. Med. 2001, 46, 75–78. [Google Scholar]
- Kitoh, T.; Nishimura, S.; Fukuda, S.; Hirabuki, S.; Kaganoi, J.; Tokunaga, Y.; Ohsumi, K. The incidence of colorectal cancer during pregnancy in Japan: Report of two cases and review of Japanese cases. Am. J. Perinatol. 1998, 15, 165–171. [Google Scholar] [CrossRef]
- Rojansky, N.; Shushan, A.; Livni, N.; Jurim, O.; Sulam, M.; Galun, E. Pregnancy associated with colon carcinoma overexpressing p53. Gynecol. Oncol. 1997, 64, 516–520. [Google Scholar] [CrossRef]
- Vitoratos, N.; Louridas, K.; Zourlas, P.A. Carcinoma of the colon during pregnancy. Int. J. Gynaecol. Obs. 1994, 44, 72–73. [Google Scholar] [CrossRef]
- Heres, P.; Wiltink, J.; Cuesta, M.A.; Burger, C.W.; van Groeningen, C.J.; Meijer, S. Colon carcinoma during pregnancy: A lethal coincidence. Eur. J. Obs. Gynecol. Reprod. Biol. 1993, 48, 149–152. [Google Scholar] [CrossRef]
- Shioda, Y.; Koizumi, S.; Furuya, S.; Akira, S.; Kameyama, N.; Hamano, N.; Takahashi, W.; Tokunaga, A.; Tanaka, N.; Onda, M. Intussusception caused by a carcinoma of the cecum during pregnancy: Report of a case and review of the literature. Surg. Today 1993, 23, 556–561. [Google Scholar] [CrossRef]
- Heise, R.H.; Van Winter, J.T.; Wilson, T.O.; Ogburn, P.L., Jr. Colonic cancer during pregnancy: Case report and review of the literature. Mayo Clin. Proc. 1992, 67, 1180–1184. [Google Scholar] [CrossRef]
- Gonsoulin, W.; Mason, B.; Carpenter, R.J., Jr. Colon cancer in pregnancy with elevated maternal serum alpha-fetoprotein level at presentation. Am. J. Obs. Gynecol. 1990, 163, 1172–1173. [Google Scholar] [CrossRef]
- Jaffe, R.; Schwartz, I.; Freund, U.; Cohen, I.; Rosen, D.; Fejgin, M. Perforated adenocarcinoma of the colon during pregnancy. Int. J. Gynaecol. Obs. 1989, 30, 371–373. [Google Scholar] [CrossRef]
- Tsukamoto, N.; Uchino, H.; Matsukuma, K.; Kamura, T. Carcinoma of the colon presenting as bilateral ovarian tumors during pregnancy. Gynecol. Oncol. 1986, 24, 386–391. [Google Scholar] [CrossRef]
- Nesbitt, J.C.; Moise, K.J.; Sawyers, J.L. Colorectal carcinoma in pregnancy. Arch. Surg. 1985, 120, 636–640. [Google Scholar] [CrossRef]
- Hill, J.A.; Kassam, S.H.; Talledo, O.E. Colonic cancer in pregnancy. South. Med. J. 1984, 77, 375–378. [Google Scholar] [CrossRef]
- Oduncu, F.S.; Kimmig, R.; Hepp, H.; Emmerich, B. Cancer in pregnancy: Maternal-fetal conflict. J. Cancer Res. Clin. Oncol. 2003, 129, 133–146. [Google Scholar] [CrossRef]
- Vimercati, A.; Greco, P.; Loverro, G.; Lopalco, P.; Pansini, V.; Selvaggi, L. Maternal complications after caesarean section in HIV infected women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 90, 73–76. [Google Scholar] [CrossRef]
- Hepner, A.; Negrini, D.; Hase, E.A.; Exman, P.; Testa, L.; Trinconi, A.F.; Filassi, J.R.; Francisco, R.P.V.; Zugaib, M.; O’Connor, T.L.; et al. Cancer during pregnancy: The oncologist overview. World J. Oncol. 2019, 10, 28–34. [Google Scholar] [CrossRef]
- Giorgione, V.; Cavoretto, P.; Cormio, G.; Valsecchi, L.; Vimercati, A.; De Gennaro, A. Prenatal Diagnosis of Twin Pregnancies with Complete Hydatidiform Mole and Coexistent Normal Fetus: A Series of 13 Cases. Gynecol. Obstet. Investig. 2017, 82, 404–409. [Google Scholar] [CrossRef]
- Lutterodt, M.C.; Kähler, P.; Kragstrup, J.; Nicolaisdottir, D.R.; Siersma, V.; Ertmann, R.K. Examining to what extent pregnancy-related physical symptoms worry women in the first trimester of pregnancy: A cross-sectional study in general practice. BJGP Open 2019, 3, bjgpopen19X101674. [Google Scholar] [CrossRef]
- de Haan, J.; Vandecaveye, V.; Han, S.N.; Van de Vijver, K.K.; Amant, F. Difficulties with diagnosis of malignancies in pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 2016, 33, 19–32. [Google Scholar] [CrossRef]
- Vimercati, A.; De Nola, R.; Trerotoli, P.; Metta, M.; Cazzato, G.; Resta, L.; Malvasi, A. COVID-19 Infection in Pregnancy: Obstetrical Risk Factors and Neonatal Outcomes-A Monocentric, Single-Cohort Study. Vaccines 2022, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, E.; Thérasse, E.; Thomassin-Naggara, I.; Trop, I. Quality initiatives: Guidelines for use of medical imaging during pregnancy and lactation. RadioGraphics 2012, 32, 3. [Google Scholar] [CrossRef] [PubMed]
- Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet. Gynecol. 2017, 130, e210–e216. [CrossRef]
- Han, S.; Lotgerink, A.; Gziri, M.; Van Calsteren, K.; Hanssens, M.; Amant, F. Physiologic variations of serum tumor markers in gynecological malignancies during pregnancy: A systematic review. BMC Med. 2012, 10, 86. [Google Scholar] [CrossRef]
- Amant, F.; Verheecke, M.; Wlodarska, I.; Dehaspe, L.; Brady, P.; Brison, N.; Van Den Bogaert, K.; Dierickx, D.; Vandecaveye, V.; Tousseyn, T.; et al. Presymptomatic Identification of Cancers in Pregnant Women During Noninvasive Prenatal Testing. JAMA Oncol. 2015, 1, 814–819. [Google Scholar] [CrossRef]
- Heesterbeek, C.J.; Aukema, S.M.; Galjaard, R.-J.H.; Boon, E.M.J.; Srebniak, M.I.; Bouman, K.; Faas, B.H.W.; Govaerts, L.C.P.; Hoffer, M.J.V.; den Hollander, N.S.; et al. Noninvasive Prenatal Test Results Indicative of Maternal Malignancies: A Nationwide Genetic and Clinical Follow-Up Study. J. Clin. Oncol. 2022, 40, 22. [Google Scholar] [CrossRef]
- Shigemi, D.; Aso, S.; Matsui, H.; Fushimi, K.; Yasunaga, H. Safety of Laparoscopic Surgery for Benign Diseases during Pregnancy: A Nationwide Retrospective Cohort Study. J. Minim. Invasive Gynecol. 2019, 26, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Obermair, A.; Asher, R.; Pareja, R.; Frumovitz, M.; Lopez, A.; Moretti-Marques, R. Incidence of adverse events in minimally invasive vs open radical hysterectomy in early cervical cancer: Results of a randomized controlled trial. Am. J. Obstet. Gynecol. 2020, 222, 249.e1–249.e10. [Google Scholar] [CrossRef]
- Haas, J. Pregnancy in association with a newly diagnosed cancer: A population-based epidemiologic assessment. Int. J. Cancer 1984, 34, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Klar, M.; Youssefzadeh, A.C.; Mandelbaum, R.S.; Roman, L.D.; Ouzounian, J.G.; Wright, J.D. Assessment of severe maternal morbidity and mortality in pregnancies complicated by cancer in the US. JAMA Oncol. 2022, 8, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Karahoda, R. Trophoblast: The central unit of fetal growth, protection and programming. Int. J. Biochem. Cell Biol. 2018, 105, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Triarico, S.; Rivetti, S.; Capozza, M.A.; Romano, A.; Maurizi, P.; Mastrangelo, S.; Attinà, G.; Ruggiero, A. Transplacental passage and fetal effects of antineoplastic treatment during pregnancy. Cancers 2022, 14, 3103. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 2015, 213, S115–S122. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Sun, X.; Dey, S. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Benoit, L.; Mir, O.; Vialard, F.; Berveiller, P. Cancer during Pregnancy: A Review of Preclinical and Clinical Transplacental Transfer of Anticancer Agents. Cancers 2021, 13, 1238. [Google Scholar] [CrossRef]
- Pacifici, G.M.; Nottoli, R. Placental transfer of drugs administered to the mother. Clin. Pharmacokinet. 1995, 28, 235–269. [Google Scholar] [CrossRef]
- Syme, M.; Paxton, J.; Keelan, J. Drug transfer and metabolism by the human placenta. Clin. Pharmacokinet. 2004, 43, 487–514. [Google Scholar] [CrossRef]
- Adam, M. The all-or-none phenomenon revisited. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 664–669. [Google Scholar] [CrossRef]
- Ruggiero, S.; Ripetti, V.; Bianchi, A.; La Vaccara, V.; Alloni, R.; Coppola, R. A singular observation of a giant benign Brenner tumor of the ovary. Arch. Gynecol. Obs. 2011, 284, 513–516. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Pellino, G.; Simillis, C.; Kontovounisios, C.; Baird, D.; Nikolaou, S.; Warren, O.; Tekkis, P.; Rasheed, S. Colorectal cancer diagnosed during pregnancy: Systematic review and treatment pathways. Eur. J. Gastroenterol. Hepatol. 2017, 29, 743–753. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. NTP Monograph: Developmental Effects and Pregnancy Outcomes Associated with Cancer Chemotherapy Use During Pregnancy. NTP Monogr. 2013, i-214, NIH Publication No. 13-5956. [Google Scholar]
- de Haan, J.; Verheecke, M.; Van Calsteren, K.; Van Calster, B.; Shmakov, R.G.; Mhallem Gziri, M.; Halaska, M.J.; Fruscio, R.; Lok, C.A.R.; Boere, I.A. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: A 20-year international cohort study of 1170 patients. Lancet Oncol. 2018, 19, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kocián, P.; de Haan, J.; Cardonick, E.H.; Uzan, C.; Lok, C.A.R.; Fruscio, R.; Halaska, M.J.; Amant, F.; On behalf of the Writing Committee of the International Network on Cancer. Infertility and Pregnancy (INCIP) on this particular manuscript. Management and outcome of colorectal cancer during pregnancy: Report of 41 cases. Acta Chir. Belg. 2019, 119, 166–175. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).