Abstract
Few studies have reported weight gain in patients with hepatitis C virus (HCV) infection treated with direct-acting antiviral agents (DAAs). This retrospective cohort study identified factors associated with substantial weight gain after DAA treatment in Taiwan. This study involved patients treated using DAAs at the Chiayi and Yunlin branches of Chang Gung Memorial Hospital from 1 January 2017 to 31 October 2020. Body weight data were collected at the start of DAA therapy and 2 years after the confirmation of a sustained virologic response. We performed multiple logistic regression to evaluate the clinical and laboratory parameters associated with a large body mass index (BMI) increase (≥5%). The mean BMI was 25.56 ± 4.07 kg/m2 at baseline and 25.77 ± 4.29 kg/m2 at the endpoint (p = 0.005). A considerable reduction in fibrosis-4 (FIB-4) score was a significant predictor of a large BMI increase (OR: 1.168; 95% CI: 1.047–1.304, p = 0.006). By contrast, older age (OR: 0.979; 95% CI: 0.963–0.996, p = 0.013) and a higher baseline BMI (OR: 0.907; 95% CI: 0.863–0.954, p < 0.001) were associated with a reduced risk of a large increase in BMI at the endpoint. In summary, a larger BMI increase was closely associated with a younger age, lower baseline BMI, and higher FIB-4 score reduction. Notably, differences in DAA regimens did not affect outcomes. Future studies are needed to elucidate the long-term effects and metabolic outcomes associated with this body weight change and investigate the exact underlying mechanisms.
1. Introduction
Chronic hepatitis C virus (HCV) infection can cause various liver diseases, including cirrhosis, liver failure, and liver cancer. Furthermore, it can interfere with host metabolism, increasing the risk of diabetes, chronic kidney disease, cardiovascular disease, and stroke. However, successful treatment of HCV infection reduces the risk of intrahepatic and extrahepatic disease [1,2]. Unlike treatment involving interferon and ribavirin, direct-acting antiviral (DAA) therapy directly interrupts viral replication, has fewer side effects, has a shorter treatment course, and has an ultra-high cure rate (>98%) [3]. In the future, the discussion about HCV will shift from how to treat HCV successfully to how to appropriately track and monitor changes in the liver and related metabolic diseases after successful treatment.
Hepatic steatosis is a characteristic histological manifestation of HCV infection. HCV induces excess production of lipid droplets in infected hepatocytes and uses these droplets as scaffolds for virus assembly, leading to hepatic steatosis [4]. Hepatic steatosis is associated with insulin resistance, liver fibrosis, oxidative stress, and an increased incidence of hepatocellular carcinoma (HCC) [5,6,7]. Notably, insulin resistance is a hallmark feature of obesity and type 2 diabetes [8]. The successful eradication of HCV should lead to these problems being reversed. However, some studies have reported that patients may gain weight after HCV eradication [9,10,11,12]. In addition, there is no relevant literature studying this issue in Taiwan. Therefore, we conducted this study to elucidate the relationship between HCV eradication by DAA and post-treatment body weight changes, identify possible predictive factors, and improve our long-term care of patients after successful HCV treatment.
2. Materials and Methods
2.1. Study Design and Patient Population
In Taiwan, patients with HCV infection receive DAA treatment through a nationwide government-funded program launched in 2017. All patients with HCV and proven active viremia, regardless of the duration and severity of liver disease, are eligible for DAA treatment, with the exception of patients with advanced- or terminal-stage disease or with a limited life expectancy of <6 months. As the availability of different DAAs evolved over time, the approved DAAs in Taiwan include daclatasvir/asunaprevir with or without ribavirin, ombitasvir/paritaprevir/ritonavir/dasabuvir with or without ribavirin, elbasvir/grazoprevir (Zepatier), glecaprevir/pibrentasvir (Maviret), and sofosbuvir-based (SOF-based) regimens including sofosbuvir + ribavirin, sofosbuvir/ledipasvir (Harvoni), and sofosbuvir/velpatasvir (Epclusa). The present retrospective cohort study included patients with HCV who received DAA treatment at the Chiayi or Yunlin branches of Chang Gung Memorial Hospital between 1 January 2017 and 31 October 2020. This study was approved by the Research Ethics Committee of Chang Gung Memorial Hospital and was conducted per the principles of the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice guidelines. Patients with confirmed sustained virologic response (SVR) and available body weight data at the start of DAA therapy and at 2 years after achieving SVR were included in this study. An SVR was defined as having an HCV RNA level below the lower limit of quantification at least 12 weeks after the end of DAA therapy. The study design is shown in Figure 1. Patients who discontinued DAA therapy, had no SVR, died during the follow-up period, or had no available body weight data were excluded from this study.
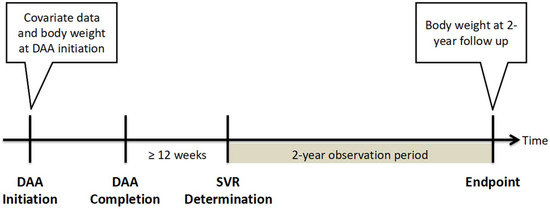
Figure 1.
Study design. Abbreviation: DAA, direct-acting antiviral agent; SVR, sustained virologic response.
2.2. Assessment of Clinical Parameters
The following data were extracted from the electronic medical records of eligible patients: (1) demographic characteristics; (2) body mass index (BMI) at baseline, 12 weeks after DAA completion (SVR12), 1 year after DAA completion, and endpoint; (3) results from laboratory examinations at baseline conducted to determine liver and renal function, fasting plasma glucose levels, glycated hemoglobin (HbA1c) levels, hemogram results, the prothrombin time-international normalized ratio (PT-INR), and alpha-fetoprotein (AFP) levels; (4) fibrosis (FIB)-4 scores at SVR12; and (5) DAA treatment details, including HCV viral profile and DAA treatment regimen. The degree of liver fibrosis was assessed indirectly using FIB-4 scores by applying the following formula: FIB-4 score = [age (years) × aspartate transaminase (AST) (U/L)]/[platelet count (109/L) × alanine aminotransferase (ALT) (U/L)1/2]. A value of <1.45 was considered to indicate the absence of fibrosis or the presence of mild fibrosis, and a value of >3.25 was considered to indicate the presence of advanced fibrosis or cirrhosis. Post-treatment reductions in FIB-4 scores were calculated as the FIB-4 score at baseline minus the FIB-4 score at SVR12. The estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in the renal disease equation.
Body weight change was defined as the difference between BMI at the baseline and endpoint. For baseline data, measurements taken within 90 days before the commencement of DAA therapy were considered, and the measurement taken closest to the start of therapy was used. For endpoint data, measurements taken within 90 days after the end of the follow-up were considered, and the measurement taken closest to the end of the follow-up was used. In cases in which data were not available, two measurements taken within 180 days after the end of the follow-up were interpolated. A study revealed that aging is associated with an expected weight gain of 1 to 2 lbs per year (approximately 0.45 to 0.90 kg per year), which is equivalent to an increase of approximately 1% of total body weight per year with aging [13]. There are some studies that discuss weight and adverse prognosis, including insulin resistance, metabolic syndrome, and related issues, and mention that when the weight increases by more than 5%, the risk begins to increase significantly. One study found that when comparing weight in middle age and age 20, every 5% increase in body weight was associated with a 20% greater risk of developing insulin resistance syndrome [14]. Another study found that when healthy adults gained more than 5% of their weight over a 5-year study period, the amount of weight gain was positively associated with the risk of metabolic syndrome [15]. With consideration of these, we established a 5% increase in BMI as the cutoff for defining a large weight gain during this 2-year follow-up study, which was also beyond the degree of weight gain expected with aging. BMI, calculated as a person’s weight in kilograms divided by the square of their height in meters, is widely employed to assess the severity of obesity. Standard cutoff values were used to categorize individuals as having underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), obesity class I (30–34.9 kg/m2), and obesity class II/III (≥35 kg/m2).
2.3. Statistical Analysis
All statistical analyses were conducted using SPSS (version 22, IBM, Chicago, IL, USA). Continuous data were expressed as means (standard deviations), and categorical data were expressed as numbers (percentages). The chi-square test was employed to analyze categorical variables, and the Student’s t-test was used to analyze normally distributed continuous data. The paired t-test was used to compare baseline and endpoint BMIs. Univariate and stepwise multivariate logistic regression analyses were performed to evaluate factors related to BMI changes at the endpoint. The results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). All statistical tests were two-tailed, and p < 0.05 was considered statistically significant.
3. Results
3.1. Baseline Characteristics of the Study Population
A total of 735 patients were enrolled in this study (Figure 2). The baseline characteristics of the study population are presented in Table 1. The mean age was 66.1 years, and 45.7% of the participants were men. The mean BMI was 25.6 kg/m2. The most prevalent weight category was normal (48.7%). The mean HCV-RNA titer was 10.9 × 106 IU/mL and 493 (67.1%) patients were infected with HCV genotype 1b.
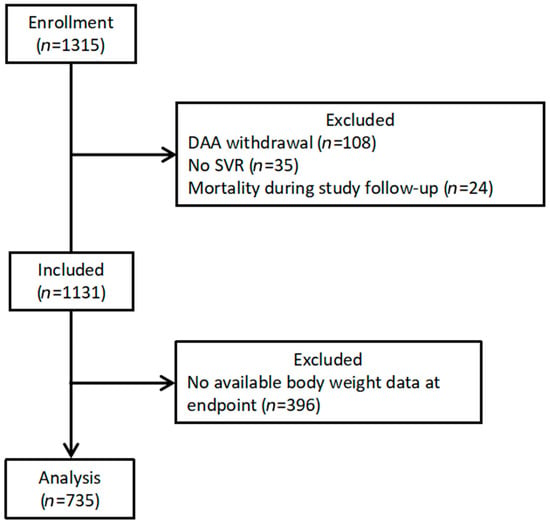
Figure 2.
Patient selection flow diagram. Abbreviation: DAA, direct-acting antiviral agent; SVR, sustained virologic response.

Table 1.
Baseline characteristics.
Comparing baseline data between two subgroups, patients with a large BMI increase (an increase of ≥5% from baseline) had a lower body weight (62.3 kg vs. 65.4 kg, p = 0.05), had a lower BMI (24.5 kg/m2 vs. 25.9 kg/m2, p < 0.001), had a higher ratio of normal BMI (62.0% vs. 44.9%, p < 0.001), lower ratio of overweight (27.6% vs. 38.1%, p = 0.014) or obesity class I (4.9% vs. 12.6%, p = 0.004), had a higher AST level (66.0 U/L vs. 55.7 U/L, p = 0.013), had a lower serum albumin level (4.20 g/dL vs. 4.30 g/dL, p = 0.030), had a lower platelet count (163.8 × 103/μL vs. 182 × 103/μL, p = 0.003), had a higher FIB-4 score (3.68 vs. 3.03, p = 0.006), had a higher FIB-4 score reduction (1.1 vs. 0.6, p = 0.002), had lower log-transformed HCV titers (5.7 vs. 6.0, p < 0.001), had a higher ratio of mixed genotype (5.5% vs. 2.3%, p = 0.039), and had a lower hemoglobin level (13.5 g/dL vs. 13.9 g/dL, p = 0.034) than the patients with no or a mild BMI increase (an increase of <5% from baseline).
3.2. BMI Change
The changes in BMI from baseline to the endpoint are presented in Figure 3. The mean BMI of the 735 patients was 25.56 ± 4.07 kg/m2 at baseline and 25.77 ± 4.29 kg/m2 at the endpoint (p = 0.005). For the patients who experienced a large BMI increase, the BMI curve continued to increase slowly throughout the tracking period.
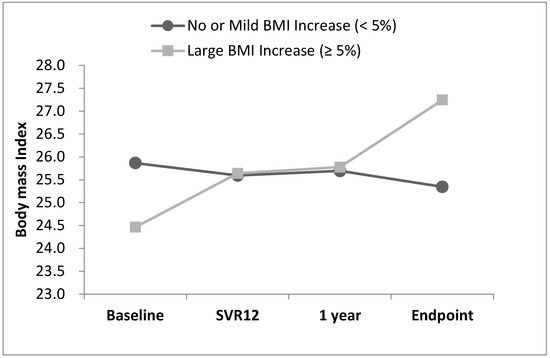
Figure 3.
BMI trend from baseline to endpoint.
The distribution of the BMI change rate is presented in Figure 4. The average BMI change rate was 1.0% ± 8.3%. Specifically, the average BMI change rate was −0.5% ± 1.3% in the patients with no or a mild BMI increase and 2.78% ± 1.87% in the patients with a large BMI increase. For the top 10% of patients with the greatest weight gain, the average rate of BMI increase was 17.5% ± 9.9%.
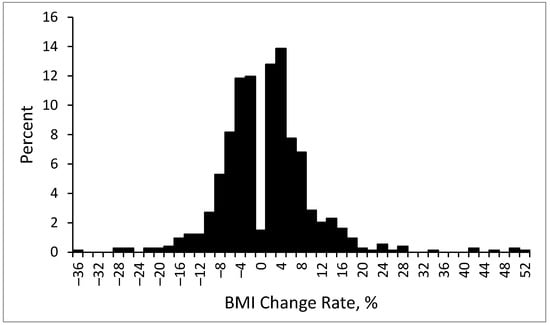
Figure 4.
Distribution of BMI change rate at endpoint.
The changes in weight categories at each time point (baseline, SVR12, 1 year after SVR, and 2 years after SVR [endpoint]) are presented in Figure 5. During the 2-year follow-up period, the proportion of patients with overweight and obesity increased from 49.4% to 54.3%.
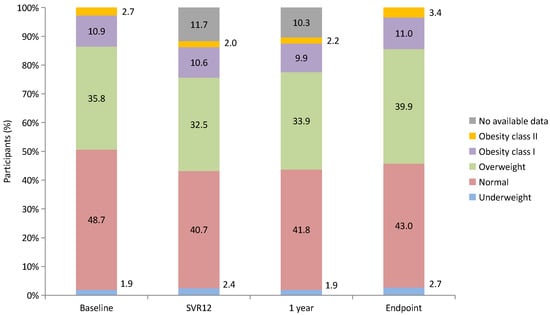
Figure 5.
Changes in weight categories from baseline to endpoint.
The changes in weight category from baseline to the endpoint after stratification by weight category at baseline are presented in Figure 6. Of the 735 patients, 549 (74.6%) experienced no change in the weight category, whereas 111 (15.1%) were in a higher weight category at the endpoint. Among the patients with a normal BMI at baseline, at the endpoint, 19.6% had developed overweight, whereas 1% had developed obesity. Conversely, for the patients with overweight and obesity, more patients had an improved BMI than had a worse BMI. At the end of the follow-up period, 14.1% of the patients with overweight at baseline had a normal BMI. Among those with class I obesity at baseline, 1.3% had a normal BMI at the endpoint, and 25% were overweight.
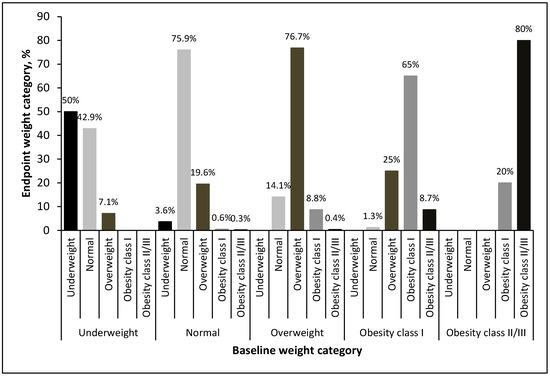
Figure 6.
Weight category change at endpoint by weight category at baseline.
3.3. Multivariate Regression Analysis for Notable BMI Increase
The predictors of a notable BMI increase are listed in Table 2. Univariate analyses revealed a lower body weight (p = 0.005), lower BMI (p < 0.001), higher AST level (p = 0.014), lower albumin level (p = 0.017), lower platelet count (p = 0.002), higher FIB-4 score reduction (p = 0.001), lower HCV titer (p < 0.001), and lower hemoglobin level (p = 0.035) at baseline to be associated with a considerable BMI increase at the endpoint. Additionally, individuals with overweight or obesity class I at baseline had a lower likelihood of experiencing a notable BMI increase than those with a normal BMI (OR: 0.525 and 0.283, respectively, p = 0.001). Multivariate analyses revealed that a higher FIB-4 score reduction (OR: 1.168; 95% CI: 1.047–1.304, p = 0.006) was associated with an increased risk of having a notably higher BMI at the endpoint. Conversely, older age (OR: 0.979; 95% CI: 0.963–0.996, p = 0.013) and a higher baseline BMI (OR: 0.907; 95% CI: 0.863–0.954, p < 0.001) were associated with a lower risk of having a notably higher BMI at the endpoint. Although the HCV titer was discovered to be significantly associated with a notable increase in BMI in multivariable regression, its OR was very close to 1.000, indicating that a unit increase in the predictor variable is likely to have a minimal effect on the outcome.

Table 2.
Logistic regression analysis for notable BMI increase.
4. Discussion
In our study, patients with HCV who received DAA therapy and achieved SVR were noted to experience a significant increase in BMI after 2 years of follow-up. The average BMI change rate in the overall study population from baseline to the endpoint was 1.0% ± 8.3%, which is consistent with the rates reported in previous studies [9,10,11,12]. Research indicates that the weight changes that occur after DAA treatment are significantly different from those that occur after interferon treatment. Patients often experience varying degrees of weight loss during interferon treatment and return to their pretreatment weight 6 months after stopping interferon treatment [16]. To the best of our knowledge, no study has investigated the long-term weight changes that occur after interferon treatment.
The mechanisms underlying weight gain during DAA therapy remain under investigation. However, possible mechanisms include improvements in chronic inflammation, improvements in hepatic anabolic function, and improvements in quality of life after treatment [17,18,19]. The weight gain that occurs in patients undergoing DAA therapy may be similar to that of those undergoing combination antiretroviral therapy (cART) for HIV infection; patients undergoing cART often gain weight after treatment due to the reversal of the catabolic effects of HIV infection. After successful treatment, the weight distribution of individuals with HIV infection is similar to those without HIV infection [20,21].
In this study, a younger age, lower baseline BMI, and higher FIB-4 score reduction were predictive of a greater risk of having an increased BMI at the endpoint. The finding that younger patients are more susceptible to weight gain after HCV eradication is consistent with that of another study [10]. HCV infection adversely affects the body’s energy balance, resulting in a decrease in somatic and visceral protein amounts and an increase in body fat percentage [22,23]. Additionally, a study has reported a significant increase in skeletal muscle mass index after DAA treatment [24]. Given that the outcome measure in our study was BMI changes, we were unable to determine which part of the body composition was responsible for weight gain. However, if weight gain is assumed to be caused by an increase in skeletal muscle mass, then such a gain in mass could explain why younger adults gain more weight after treatment than older adults do. However, further research is required to validate this assumption.
Our finding that patients with lower baseline BMI were more likely to experience a notable increase in BMI after HCV eradication, possibly because such patients have lower anabolic status at baseline relative to other patients. When liver damage and infection burden are reduced or eliminated, patients with lower baseline BMI have more room to gain weight than other patients. However, our results were inconsistent with those of other studies that reported a higher BMI being associated with greater weight gain after therapy [10,12]. Different population ethnicities, cultural influences on health-promoting behaviors, and prevalences of chronic diseases may have contributed to these discrepancies. Our study revealed that overweight and obesity class I were associated with reduced odds of experiencing a notable increase in BMI after therapy. A study demonstrated that people with a BMI ≤ 30 are at risk of weight gain after HCV treatment, whereas people with a BMI > 30 do not gain weight [11]. This study assumed that people who are overweight or obese are physically inactive and, therefore, less likely to gain skeletal muscle mass and body weight. However, this assumption does not account for our observation that some individuals with overweight or obesity lost weight or even had a normal BMI at the endpoint. Our lack of data on body composition, exercise habits, diet, complete chronic medical history, and socioeconomic status limits our ability to explain this finding, and the underlying mechanism must be investigated in more extensive and detailed research.
Our finding that patients with higher reductions in FIB-4 scores are more likely to gain weight after HCV eradication has not been discussed in previous studies. In general, an eventual FIB-4 score reduction after HCV eradication can be considered to indicate fibrosis regression. However, a large reduction in FIB-4 score over a short period of time is generally caused by a reduction in transaminases, which is a consequence of inflammation remission rather than fibrosis resolution [25]. In such cases, after successful eradication, inflammation is relieved, and patients return to a relatively healthy state, which may lead to weight gain. The phenomenon that changes in inflammatory markers predicts disease prognosis is also seen in HIV-infected patients. For example, higher pretreatment C-reactive protein (CRP) levels indicate more severe disease states, and a greater decrease in CRP levels after HIV treatment indicates better disease control [26]. A study found that in underweight participants, an increase of 1 unit in body mass index was associated with a decrease of 9.32 mg/L in CRP levels [27].
During the observation period of this study, DAAs were not used. In other words, after the initial DAAs were completely metabolized, the DAAs themselves had almost no interference with weight changes. In the regression analysis of risk factors associated with weight gain, differences in DAA regimens also did not affect the results. Interestingly, we noted that the group with a large increase in BMI showed stable weight gain even during DAA treatment. In addition to the effect of DAA treatment, other confounding factors that may contribute to weight changes should also be considered, such as exercise habits, diet, or current medications. However, as this was a retrospective study, data on these confounding factors are incomplete. Additionally, we did not measure body weight regularly during treatment (e.g., once a week), so we do not know whether weight continued to increase throughout treatment, only started to increase later in treatment, or some other trajectory. The specific changes and possible causes need to be investigated through broader and more detailed studies in the future.
In the long term, we should evaluate whether people with increased BMI are at increased risk of diabetes, cardiovascular disease, or other metabolic diseases. If the risk of such diseases is unaffected or even reduced, it means that the patient is recovering from an unhealthy state. However, if the risk of such diseases does increase, it means that patients with identified predictors, such as younger age, lower baseline BMI, and higher FIB-4 score reduction, must pay greater attention to their weight after HCV eradication. Such patients should take a more proactive approach to avoid obesity, which may negate the benefits of HCV eradication.
This study has some limitations. First, this was a retrospective study, and the availability of weight data was limited by the irregularity of the post-treatment follow-up visits by the majority of the target patients, which then limited the number of patients that could be included. In addition, covariates that influence body weight, including exercise habits, diet, personal income, and education level, were not recorded and could not be assessed. Second, the observation period of our study was only two years, and longer-term monitoring of weight change may show a different trajectory. In addition, assessing the long-term effects of weight gain on hepatic and metabolic diseases, such as metabolic dysfunction-associated fatty liver disease, cirrhosis, HCC, diabetes, and cardiovascular disease, is crucial. Additionally, specific targets requiring timely intervention must be identified. Third, comparative studies should be conducted with matched cohorts of untreated hepatitis C patients. However, when DAA therapy is so effective, delaying HCV treatment solely for research purposes is not justified. In addition, there is no historical control group available for comparison. Fourth, BMI is an imperfect measure of obesity because it does not distinguish body fat from lean body mass. Previous studies have associated body fat with unfavorable health outcomes, whereas fat-free mass was associated with improved health [28]. Studies based on body composition rather than BMI may be appropriate to assess the complexities of body weight changes after HCV treatment.
5. Conclusions
This study revealed that treatment with DAA resulted in SVR and a significant increase in BMI after 2 years of follow-up in patients with HCV infection. A younger age, lower baseline BMI, and high FIB-4 score reduction after HCV treatment were associated with a more notable increase in BMI. Differences in DAA regimens did not affect outcomes. Future studies are required to elucidate the long-term effects and metabolic outcomes associated with such a change, and the exact mechanisms underlying the change must be investigated.
Author Contributions
Conceptualization, T.-S.C.; Methodology, C.-H.C. and T.-S.C.; Validation, S.-N.L. and C.-H.H.; Formal analysis, Y.-Y.H., Y.-J.D. and C.-H.H.; Investigation, W.-M.C., K.-L.W. and C.-H.H.; Resources, S.-N.L.; Writing—original draft, C.-H.C.; Writing—review & editing, C.-H.C.; Visualization, K.-C.C.; Supervision, C.-H.S. and T.-S.C.; Project administration, T.-S.C.; Funding acquisition, T.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research grant from Chang Gung Memorial Hospital (CMRPG6M0202).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Ethics Committee of Chang Gung Memorial Hospital (202101641B0C502). Approved date on 24 April 2023.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohanty, A.; Salameh, S.; Butt, A.A. Impact of direct acting antiviral agent therapy upon extrahepatic manifestations of hepatitis C virus infection. Curr. HIV/AIDS Rep. 2019, 16, 389–394. [Google Scholar] [CrossRef]
- Lanini, S.; Scognamiglio, P.; Pisapia, R.; Minosse, C.; Agresta, A.; Ippolito, G. Recovery of metabolic impairment in patients who cleared chronic hepatitis C infection after direct-acting antiviral therapy. Int. J. Antimicrob. Agents 2019, 53, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, L.; Schulte, B.; Manns, M.P.; Maasoumy, B. Treatment of chronic hepatitis C: Efficacy, side effects and complications. Visc. Med. 2019, 35, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Miyanari, Y.; Atsuzawa, K.; Usuda, N.; Watashi, K.; Hishiki, T.; Zayas, M.; Bartenschlager, R.; Wakita, T.; Hijikata, M.; Shimotohno, K. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 2007, 9, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Cortez-Pinto, H. Insulin resistance and steatosis in chronic hepatitis C. Ann. Hepatol. 2009, 8, S67–S75. [Google Scholar] [CrossRef] [PubMed]
- Vidali, M.; Tripodi, M.-F.; Ivaldi, A.; Zampino, R.; Occhino, G.; Restivo, L.; Sutti, S.; Marrone, A.; Ruggiero, G.; Albano, E.; et al. Interplay between oxidative stress and hepatic steatosis in the progression of chronic hepatitis C. J. Hepatol. 2008, 48, 399–406. [Google Scholar] [CrossRef]
- Moriishi, K.; Mochizuki, R.; Moriya, K.; Miyamoto, H.; Mori, Y.; Abe, T.; Murata, S.; Tanaka, K.; Miyamura, T.; Suzuki, T.; et al. Critical role of PA28γ in hepatitis C virus-associated steatogenesis and hepatocarcinogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 1661–1666. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- El Kassas, M.; Alboraie, M.; Naguib, M.; Omar, H.; El Tahan, A.; Moaz, I.; Abdellah, M.; Ezzat, S.; Wifi, M.-N.; Sherief, A.F.; et al. A significant upsurge of body mass index in patients with chronic hepatitis C successfully treated with direct-acting antiviral regimens. Turk. J. Gastroenterol. 2019, 30, 708–713. [Google Scholar] [CrossRef]
- Do, A.; Esserman, D.A.; Krishnan, S.; Lim, J.K.; Taddei, T.H.; Hauser, R.G.; Tate, J.P.; Re, V.L.; Justice, A.C. Excess weight gain after cure of hepatitis C infection with direct-acting antivirals. J. Gen. Intern. Med. 2020, 35, 2025–2034. [Google Scholar] [CrossRef]
- Schlevogt, B.; Boeker, K.H.W.; Mauss, S.; Klinker, H.; Heyne, R.; Link, R.; Simon, K.-G.; Sarrazin, C.; Serfert, Y.; Manns, M.P.; et al. Weight Gain after Interferon-Free Treatment of Chronic Hepatitis C—Results from the German Hepatitis C-Registry (DHC-R). Biomedicines 2021, 9, 1495. [Google Scholar] [CrossRef] [PubMed]
- Nkwocha, C.L.; Carter, P.S.; Blair, S.; Blackwell, J.M.; Fasanmi, E.O. Understanding the effect of direct-acting antiviral therapy on weight in patients with chronic hepatitis C. Antivir. Ther. 2022, 27, 13596535221115253. [Google Scholar] [CrossRef]
- Dutton, G.R.; Kim, Y.; Jacobs Jr, D.R.; Li, X.; Loria, C.M.; Reis, J.P.; Carnethon, M.; Durant, N.H.; Gordon-Larsen, P.; Shikany, J.M.; et al. 25-year weight gain in a racially balanced sample of US adults: The CARDIA study. Obesity 2016, 24, 1962–1968. [Google Scholar] [CrossRef]
- Everson, S.; Glodberg, D.E.; Helmrich, S.P.; Lakka, T.A.; Lynch, J.W.; Kaplan, G.A.; Salonen, J.T. Weight gain and the risk of developing insulin resistance syndrome. Diabetes Care 1998, 21, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chen, J.D.; Chen, P.C. Excessive 5-year weight gain predicts metabolic syndrome development in healthy middle-aged adults. World J. Diabetes 2011, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Seyam, M.S.; Freshwater, D.A.; O’Donnell, K.; Mutimer, D.J. Weight loss during pegylated interferon and ribavirin treatment of chronic hepatitis C. J. Viral Hepat. 2005, 12, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Zampino, R.; Marrone, A.; Restivo, L.; Guerrera, B.; Sellitto, A.; Rinaldi, L.; Romano, C.; E Adinolfi, L. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J. Hepatol. 2013, 5, 528–540. [Google Scholar] [CrossRef]
- Foster, G.R.; Goldin, R.D.; Thomas, H.C. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology 1998, 27, 209–212. [Google Scholar] [CrossRef]
- Musialik, J.; Suchecka, W.; Klimacka-Nawrot, E.; Petelenz, M.; Hartman, M.; Błońska-Fajfrowska, B. Taste and appetite disorders of chronic hepatitis C patients. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1400–1405. [Google Scholar] [CrossRef]
- Kumar, S.; Samaras, K. The impact of weight gain during HIV treatment on risk of pre-diabetes, diabetes mellitus, cardiovascular disease, and mortality. Front. Endocrinol. 2018, 9, 705. [Google Scholar] [CrossRef]
- Erlandson, K.M.; Carter, C.C.; Melbourne, K.; Brown, T.T.; Cohen, C.; Das, M.; Esser, S.; Huang, H.; Koethe, J.R.; Martin, H.; et al. Weight change following antiretroviral therapy switch in people with viral suppression: Pooled data from randomized clinical trials. Clin. Infect. Dis. 2021, 73, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.; Ali, I.; Ali, S.; Alam, I.; Naseem, F.; Naseem, Z. Impact of combination interferon therapy on the body weight, body fat and lean body mass of chronic HCV infected patients. J. Antivir. Antiretrovir. 2013, 6, 1–5. [Google Scholar]
- Chen, Y.-Y.; Fang, W.-H.; Wang, C.-C.; Kao, T.-W.; Chang, Y.-W.; Yang, H.-F.; Wu, C.-J.; Sun, Y.-S.; Chen, W.-L. Increased body fat percentage in patients with hepatitis B and C virus infection. PLoS ONE 2018, 13, e0200164. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, R.; Iwasa, M.; Hara, N.; Tamai, Y.; Yoshikawa, K.; Ogura, S.; Tanaka, H.; Eguchi, A.; Yamamoto, N.; Kobayashi, Y.; et al. Changes in liver function and body composition by direct-acting antiviral therapy for hepatitis C virus infection. Hepatol. Res. 2018, 48, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, S.; Butt, M.U.; Trujillo, S.; Asaad, I. FIB-4 regression with direct-acting antiviral therapy in patients with hepatitis C infection: A safety-net hospital experience. Front. Med. 2020, 7, 359. [Google Scholar] [CrossRef]
- Lau, B.; Sharrett, A.R.; Kingsley, L.A.; Post, W.; Palella, F.J.; Visscher, B.; Gange, S.J. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch. Intern. Med. 2006, 166, 64–70. [Google Scholar] [CrossRef]
- Mave, V.; Erlandson, K.M.; Gupte, N.; Balagopal, A.; Asmuth, D.M.; Campbell, T.B.; Smeaton, L.; Kumarasamy, N.; Hakim, J.; Santos, B.; et al. Inflammation and change in body weight with antiretroviral therapy initiation in a multinational cohort of HIV-infected adults. J. Infect. Dis. 2016, 214, 65–72. [Google Scholar] [CrossRef]
- Wada, R.; Tekin, E. Body composition and wages. Econ. Hum. Biol. 2010, 8, 242–254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).