Deciphering the Autoantibody Response to the OJ Antigenic Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Anti-OJ Autoantibody Testing
2.2. Epitope Mapping
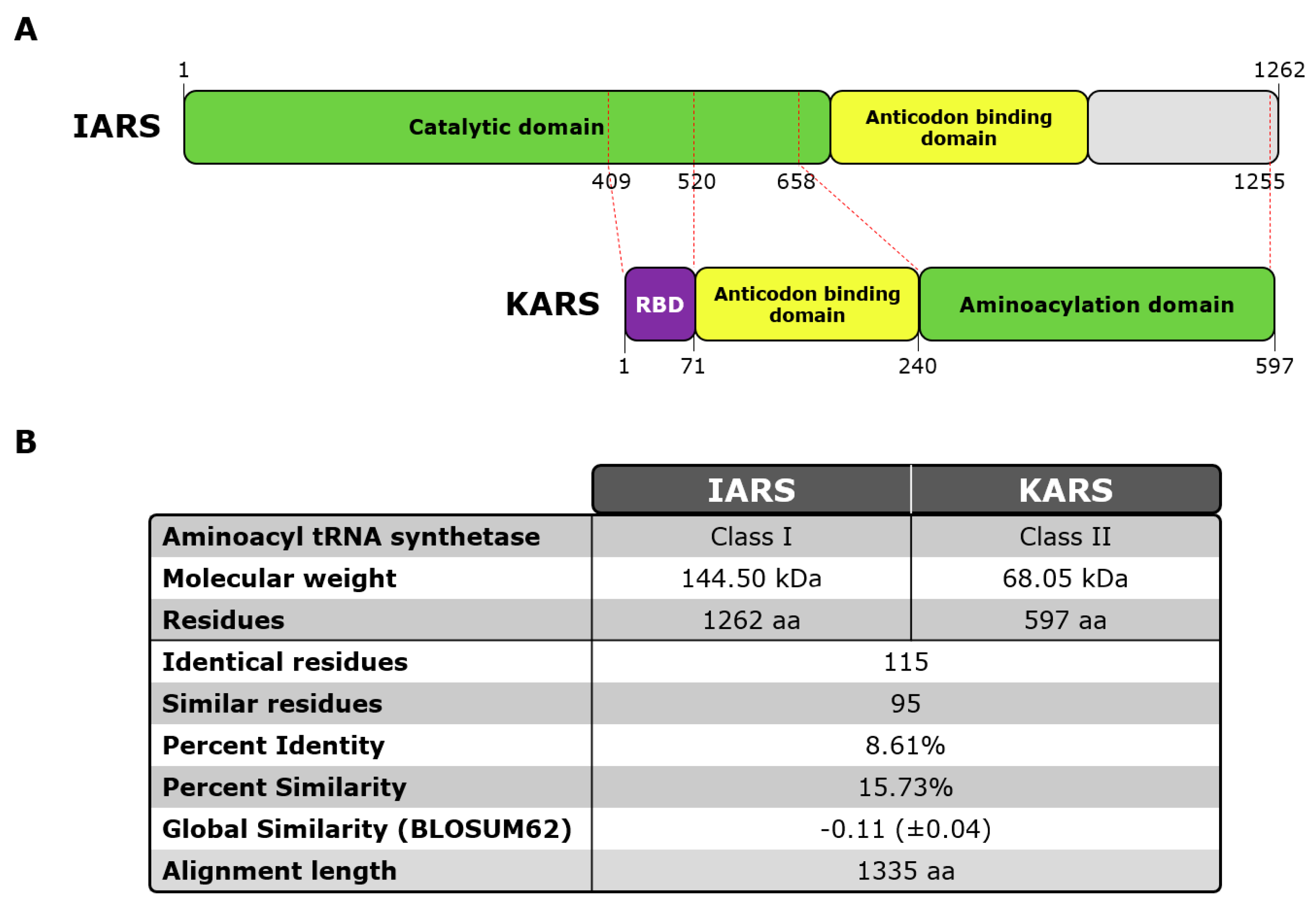
2.3. OJ Antigens
2.4. Statistical Methods
3. Results
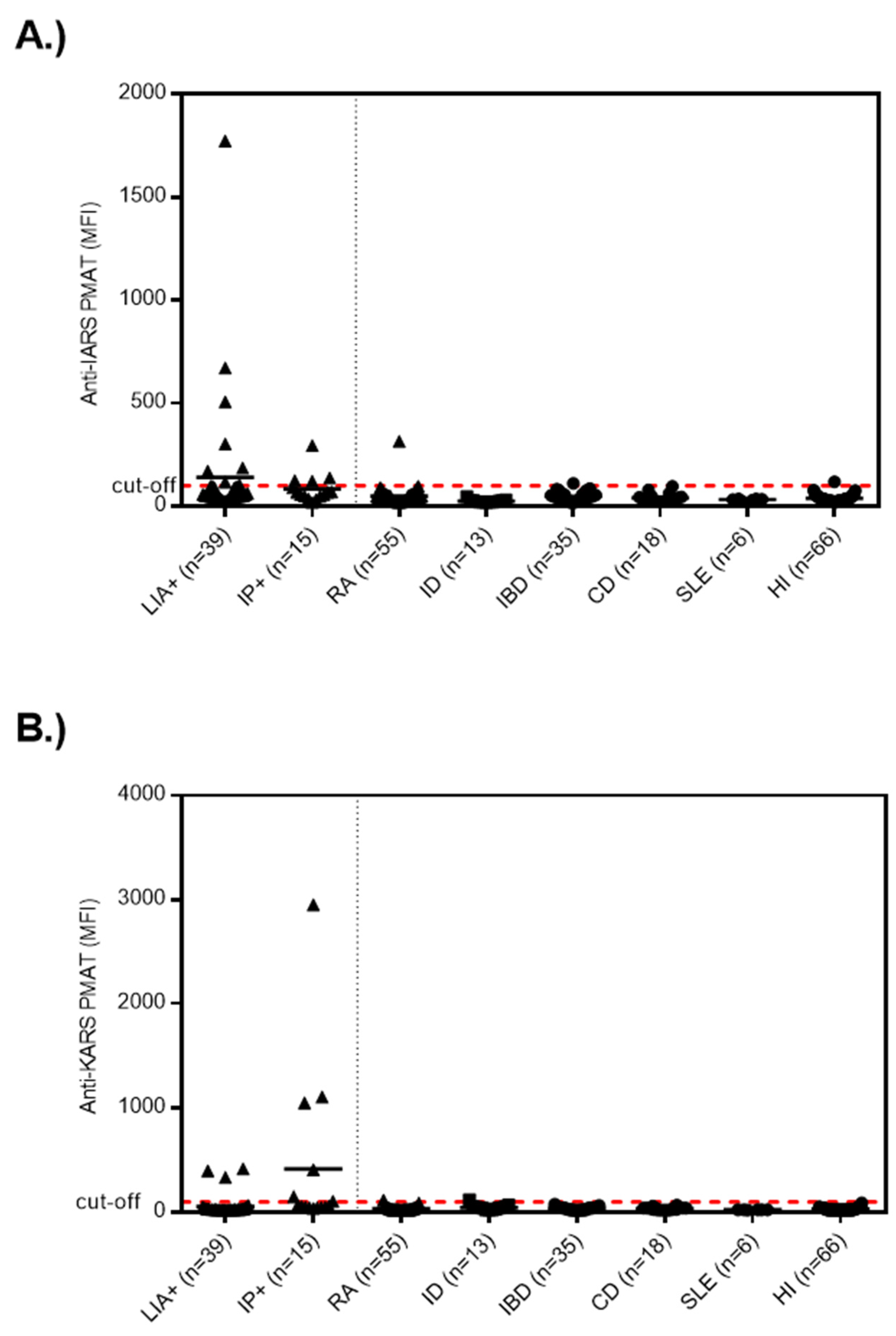
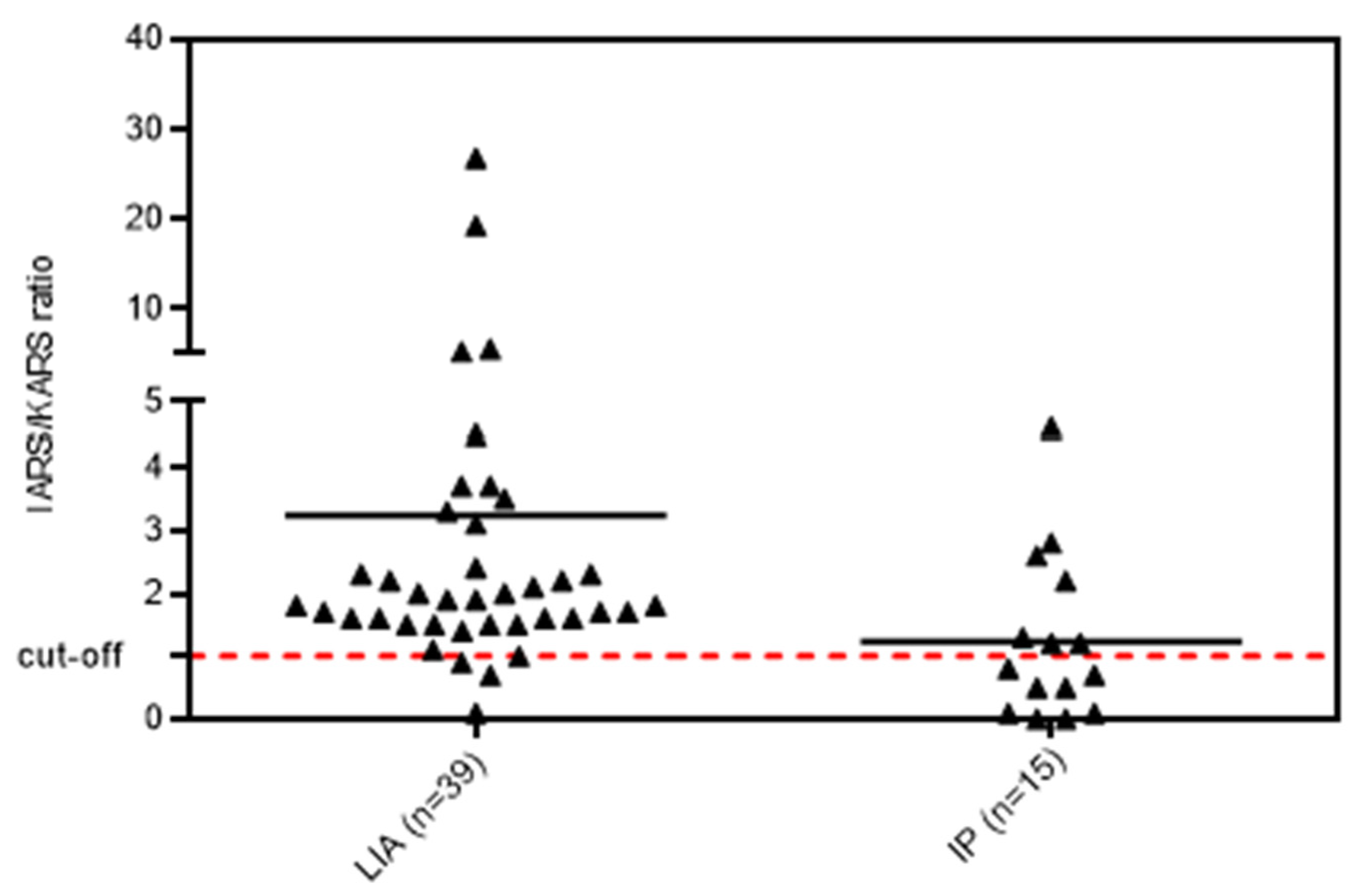
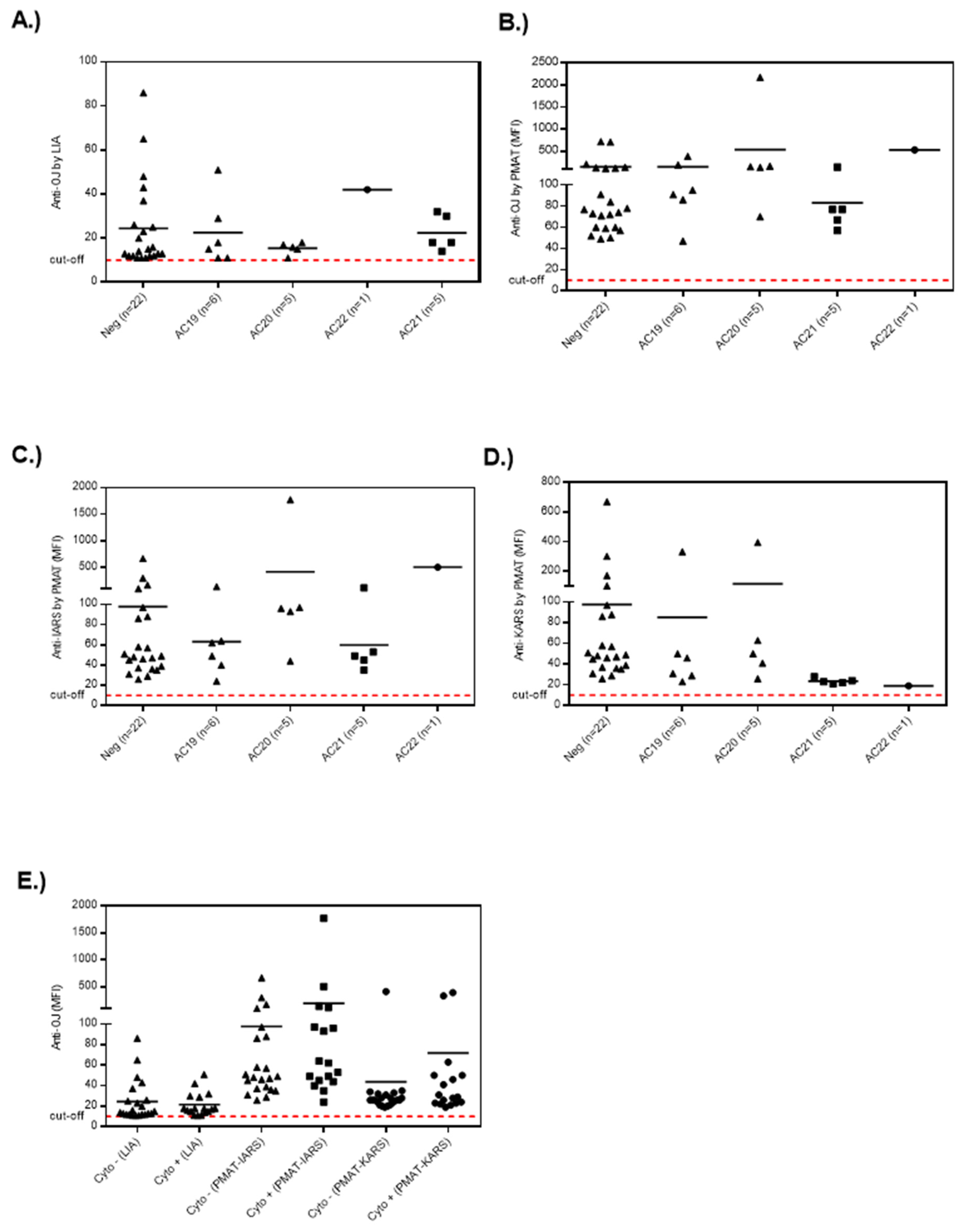
3.1. Anti-OJ Positive Samples Identified by LIA vs. IP
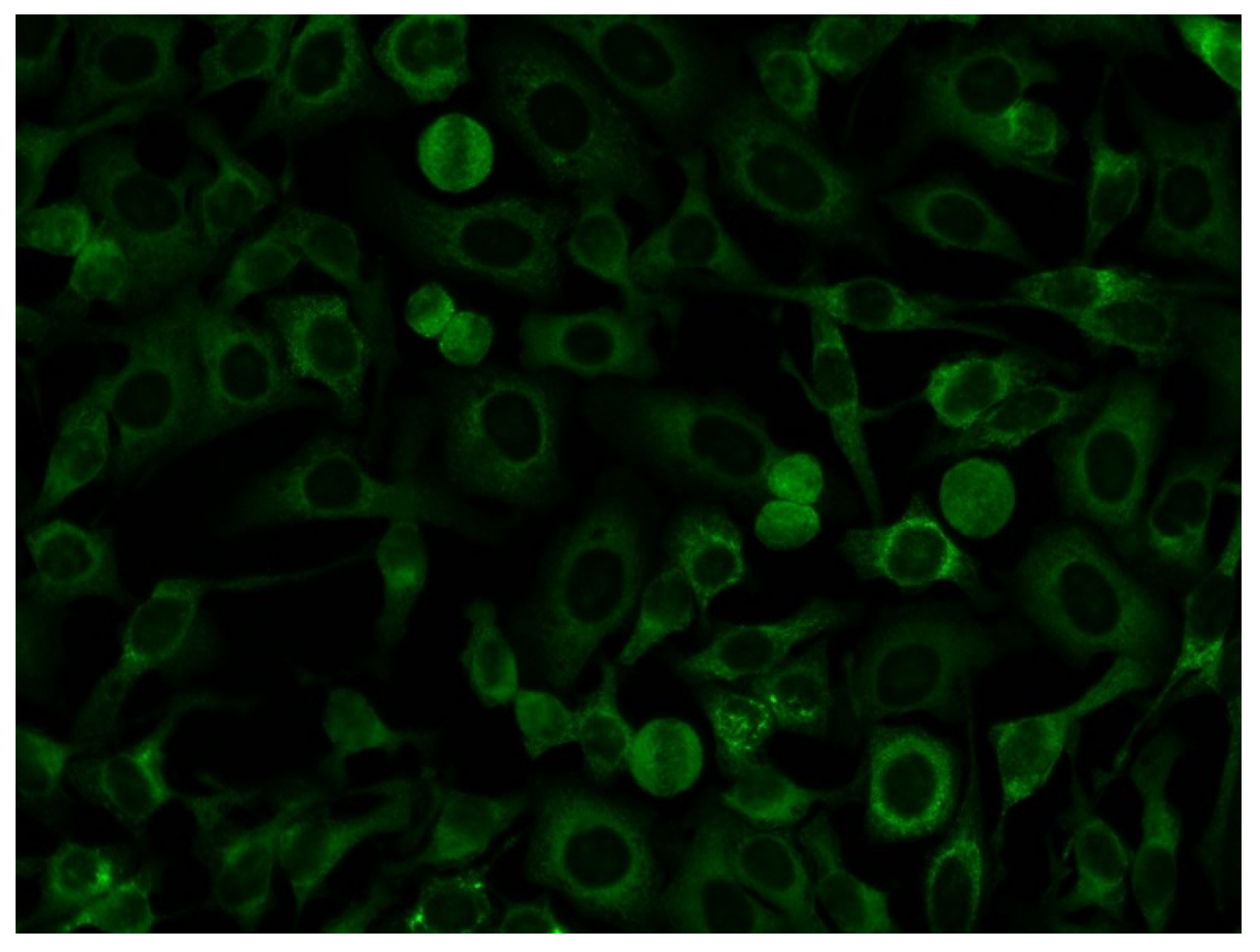
3.2. Association of Anti-OJ Antibodies and Indirect Immunofluorescence (IIF) Staining Patterns
3.3. Epitope Mapping
4. Discussion
4.1. Limitation of Current Assays for the Detection of Anti-OJ Antibodies
4.2. Linear Peptides as Antigenic Target for Anti-OJ Antibodies
4.3. Anti-OJ Antibodies and Indirect Immunofluorescence
| Antibody | Sensitivity * | Comment |
|---|---|---|
| Tansley et al. [16] | 0/14 (0%) | Poor sensitivity vs. IP |
| Mecoli et al. [22] | N/R | 2/252 samples were positive for anti-OJ by LIA, but not confirmed by IP |
| Hamaguchi et al. [18] | 0/9 (0%) | Poor sensitivity vs. IP |
| Lackner et al. [20] | N/R | All anti-OJ positive samples had diagnosis other than IIM |
| Vulsteke et al. [30] | N/R | 0/144 IIM patients positive for anti-OJ |
| Cavazzana et al. [19] | 0/2 (0%) | 2/57 IIM patients had anti-OJ by IP, but did not confirm by LIA |
| Platteel et al. [35] | N/R | 1/187 (0.5%) in IIM; 2/632 (0.3%) in controls; OR 0.2-18.5 |
| Betteridge et al. [36] | N/R | 10/1673 (0.6%) of IIM positive for anti-OJ antibodies |
| Espinosa-Ortega et al. [21] | 0/1 (0%) | 1/110 (0.9%) of IIM positive for anti-OJ antibodies |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McHugh, N.J.; Tansley, S.L. Autoantibodies in Myositis. Nat. Rev. Rheumatol. 2018, 14, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Palterer, B.; Vitiello, G.; Carraresi, A.; Giudizi, M.G.; Cammelli, D.; Parronchi, P. Bench to Bedside Review of Myositis Autoantibodies. Clin. Mol. Allergy 2018, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Bottai, M.; Tjarnlund, A.; Santoni, G.; Werth, V.P.; Pilkington, C.; de Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: A methodology report. RMD Open 2017, 3, e000507. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Tjarnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; de Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Arthritis Rheumatol. 2017, 69, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, I.E.; Tjarnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis 2017, 76, 1955–1964. [Google Scholar] [CrossRef]
- Casal-Dominguez, M.; Pinal-Fernandez, I.; Pak, K.; Huang, W.; Selva-O’Callaghan, A.; Albayda, J.; Casciola-Rosen, L.; Paik, J.J.; Tiniakou, E.; Mecoli, C.A.; et al. Performance of the 2017 European Alliance of Associations for Rheumatology/American College of Rheumatology Classification Criteria for Idiopathic Inflammatory Myopathies in Patients With Myositis-Specific Autoantibodies. Arthritis Rheumatol. 2022, 74, 508–517. [Google Scholar] [CrossRef]
- Mahler, M.; Vulsteke, J.B.; Bossuyt, X.; De, L.E.; Satoh, M. Standardisation of myositis-specific antibodies: Where are we today? Ann. Rheum. Dis 2019, 80, e132. [Google Scholar] [CrossRef]
- Mahler, M.; Malyavantham, K.; Fritzler, M.J.; Satoh, M. Comment on: The reliability of immunoassays to detect autoantibodies in patients with myositis is dependent on autoantibody specificity. Rheumatology 2021, 60, e35–e37. [Google Scholar] [CrossRef]
- Mahler, M.; Betteridge, Z.; Bentow, C.; Richards, M.; Seaman, A.; Chinoy, H.; McHugh, N. Comparison of Three Immunoassays for the Detection of Myositis Specific Antibodies. Front. Immunol. 2019, 10, 848. [Google Scholar] [CrossRef]
- Sato, S.; Kuwana, M.; Hirakata, M. Clinical characteristics of Japanese patients with anti-OJ (anti-isoleucyl-tRNA synthetase) autoantibodies. Rheumatology 2007, 46, 842–845. [Google Scholar] [CrossRef]
- Mahler, M.; Miller, F.W.; Fritzler, M.J. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: A comprehensive review. Autoimmun. Rev. 2014, 13, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.; Cavagna, L.; González-Gay, M.A. New Criteria Needed for Antisynthetase Syndrome. JAMA Neurol. 2018, 75, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Targoff, I.N. Autoantibodies to aminoacyl-transfer RNA synthetases for isoleucine and glycine. Two additional synthetases are antigenic in myositis. J. Immunol. 1990, 144, 1737–1743. [Google Scholar] [CrossRef]
- Targoff, I.N.; Trieu, E.P.; Miller, F.W. Reaction of anti-OJ autoantibodies with components of the multi-enzyme complex of aminoacyl-tRNA synthetases in addition to isoleucyl-tRNA synthetase. J. Clin. Investig. 1993, 91, 2556–2564. [Google Scholar] [CrossRef]
- Vulsteke, J.B.; Satoh, M.; Malyavantham, K.; Bossuyt, X.; De Langhe, E.; Mahler, M. Anti-OJ autoantibodies: Rare or underdetected? Autoimmun. Rev. 2019, 18, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Tansley, S.L.; Li, D.; Betteridge, Z.E.; McHugh, N.J. The reliability of immunoassays to detect autoantibodies in patients with myositis is dependent on autoantibody specificity. Rheumatology 2020, 59, 2109–2114. [Google Scholar] [CrossRef] [PubMed]
- Tansley, S.L.; McHugh, N.J. Comment on: The reliability of immunoassays to detect autoantibodies in patients with myositis is dependent on autoantibody specificity: Reply. Rheumatology 2020, 59, 2177–2178. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Kuwana, M.; Takehara, K. Comparison of anti-OJ antibody detection assays between an immunoprecipitation assay and line blot assay. Mod. Rheumatol. 2017, 27, 551–552. [Google Scholar] [CrossRef]
- Cavazzana, I.; Fredi, M.; Ceribelli, A.; Mordenti, C.; Ferrari, F.; Carabellese, N.; Tincani, A.; Satoh, M.; Franceschini, F. Testing for myositis specific autoantibodies: Comparison between line blot and immunoprecipitation assays in 57 myositis sera. J. Immunol. Methods 2016, 433, 1–5. [Google Scholar] [CrossRef]
- Lackner, A.; Tiefenthaler, V.; Mirzayeva, J.; Posch, F.; Rossmann, C.; Kastrati, K.; Radner, H.; Demel, U.; Gretler, J.; Stotz, M.; et al. The use and diagnostic value of testing myositis-specific and myositis-associated autoantibodies by line immuno-assay: A retrospective study. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720x20975907. [Google Scholar] [CrossRef]
- Espinosa-Ortega, F.; Holmqvist, M.; Alexanderson, H.; Storfors, H.; Mimori, T.; Lundberg, I.E.; Ronnelid, J. Comparison of autoantibody specificities tested by a line blot assay and immunoprecipitation-based algorithm in patients with idiopathic inflammatory myopathies. Ann. Rheum. Dis 2019, 78, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Mecoli, C.A.; Albayda, J.; Tiniakou, E.; Paik, J.J.; Zahid, U.; Danoff, S.K.; Casciola-Rosen, L.; Casal-Dominguez, M.; Pak, K.; Pinal-Fernandez, I.; et al. Myositis Autoantibodies: A Comparison of Results From the Oklahoma Medical Research Foundation Myositis Panel to the Euroimmun Research Line Blot. Arthritis Rheumatol. 2020, 72, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Fritzler, M.J.; Choi, M.Y.; Mahler, M. The Antinuclear Antibody Test in the Diagnosis of Antisynthetase Syndrome and Other Autoimmune Myopathies. J. Rheumatol. 2018, 45, 444–445. [Google Scholar] [CrossRef]
- Aggarwal, R.; Dhillon, N.; Fertig, N.; Koontz, D.; Qi, Z.; Oddis, C.V. A Negative Antinuclear Antibody Does Not Indicate Autoantibody Negativity in Myositis: Role of Anticytoplasmic Antibody as a Screening Test for Antisynthetase Syndrome. J. Rheumatol. 2017, 44, 223–229. [Google Scholar] [CrossRef]
- Aggarwal, R.; Oddis, C.V. Drs. Aggarwal and Oddis reply. J. Rheumatol. 2018, 45, 446. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.; Garcia-De La Torre, I.; Gonzalez-Bello, Y.C.; Vazquez-Del, M.M.; Andrade-Ortega, L.; Medrano-Ramirez, G.; Navarro-Zarza, J.E.; Maradiaga-Cecena, M.; Loyo, E.; Rojo-Mejia, A.; et al. Autoantibodies to Mi-2 alpha and Mi-2 beta in patients with idiopathic inflammatory myopathy. Rheumatology 2019, 58, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana, I.; Richards, M.; Bentow, C.; Seaman, A.; Fredi, M.; Giudizi, M.G.; Palterer, B.; Pratesi, F.; Migliorini, P.; Franceschini, F.; et al. Evaluation of a novel particle-based assay for detection of autoantibodies in idiopathic inflammatory myopathies. J. Immunol. Methods 2019, 474, 112661. [Google Scholar] [CrossRef]
- Mahler, M.; Malyavantham, K.; Seaman, A.; Bentow, C.; Anunciacion-Llunell, A.; Sanz-Martínez, M.T.; Viñas-Gimenez, L.; Selva-O’Callaghan, A. Profiling of Myositis Specific Antibodies and Composite Scores as an Aid in the Differential Diagnosis of Autoimmune Myopathies. Diagnostics 2021, 11, 2246. [Google Scholar] [CrossRef]
- Muro, Y.; Yamano, Y.; Yoshida, K.; Oto, Y.; Nakajima, K.; Mitsuma, T.; Kikuchi, S.; Matsumae, A.; Ogawa-Momohara, M.; Takeichi, T.; et al. Immune recognition of lysyl-tRNA synthetase and isoleucyl-tRNA synthetase by anti-OJ antibody-positive sera. J. Autoimmun. 2021, 122, 102680. [Google Scholar] [CrossRef]
- Vulsteke, J.B.; De Langhe, E.; Claeys, K.G.; Dillaerts, D.; Poesen, K.; Lenaerts, J.; Westhovens, R.; Van Damme, P.; Blockmans, D.; De Haes, P.; et al. Detection of myositis-specific antibodies. Ann. Rheum. Dis. 2019, 78, e7. [Google Scholar] [CrossRef]
- Infantino, M.; Tampoia, M.; Fabris, M.; Alessio, M.G.; Previtali, G.; Pesce, G.; Deleonardi, G.; Porcelli, B.; Musso, M.; Grossi, V.; et al. Combining immunofluorescence with immunoblot assay improves the specificity of autoantibody testing for myositis. Rheumatology 2019, 58, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Minoru, S.M.; Fritzler, M.J. Comment on: Concordance between myositis autoantibodies and anti-nuclear antibody patterns in a real-world, Australian cohort. Rheumatology 2022, 61, e290–e291. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Fritzler, M.J. PM1-Alpha ELISA: The assay of choice for the detection of anti-PM/Scl autoantibodies? Autoimmun. Rev. 2009, 8, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.; Andrade, L.E.C.; Carballo, O.G.; Conrad, K.; Francescantonio, P.L.C.; Fritzler, M.J.; de la Torre, I.G.; Herold, M.; Klotz, W.; de Melo Cruvinel, W.; et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: The International Consensus on ANA patterns (ICAP) perspective. Ann. Rheum. Dis. 2019, 78, 879–889. [Google Scholar] [CrossRef]
- Platteel, A.; Wevers, B.; Lim, J.; Bakker, J.; Bontkes, H.; Curvers, J.; Damoiseaux, J.; Heron, M.; de Kort, G.; Limper, M.; et al. Frequencies and clinical associations of myositis-related antibodies in The Netherlands: A one-year survey of all Dutch patients. J. Transl. Autoimmun. 2019, 3, 100013. [Google Scholar] [CrossRef]
- Betteridge, Z.; Tansley, S.; Shaddick, G.; Chinoy, H.; Cooper, R.G.; New, R.P.; Lilleker, J.B.; Vencovsky, J.; Chazarain, L.; Danko, K.; et al. Frequency, mutual exclusivity and clinical associations of myositis autoantibodies in a combined European cohort of idiopathic inflammatory myopathy patients. J. Autoimmun. 2019, 101, 48–55. [Google Scholar] [CrossRef]

| Selection Method | IARS | KARS | Combined * |
|---|---|---|---|
| LIA (n = 39) | 7/39 (17.9%) | 3/39 (7.7%) | 14/39 (35.9%) |
| IP (n = 15) | 4/15 (26.7%) | 6/15 (40.0%) | 12/15 (80.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritzler, M.J.; Bentow, C.; Satoh, M.; McHugh, N.; Ghirardello, A.; Mahler, M. Deciphering the Autoantibody Response to the OJ Antigenic Complex. Diagnostics 2023, 13, 156. https://doi.org/10.3390/diagnostics13010156
Fritzler MJ, Bentow C, Satoh M, McHugh N, Ghirardello A, Mahler M. Deciphering the Autoantibody Response to the OJ Antigenic Complex. Diagnostics. 2023; 13(1):156. https://doi.org/10.3390/diagnostics13010156
Chicago/Turabian StyleFritzler, Marvin J., Chelsea Bentow, Minoru Satoh, Neil McHugh, Anna Ghirardello, and Michael Mahler. 2023. "Deciphering the Autoantibody Response to the OJ Antigenic Complex" Diagnostics 13, no. 1: 156. https://doi.org/10.3390/diagnostics13010156
APA StyleFritzler, M. J., Bentow, C., Satoh, M., McHugh, N., Ghirardello, A., & Mahler, M. (2023). Deciphering the Autoantibody Response to the OJ Antigenic Complex. Diagnostics, 13(1), 156. https://doi.org/10.3390/diagnostics13010156






