A Comparative Study on Visual Detection of Mycobacterium tuberculosis by Closed Tube Loop-Mediated Isothermal Amplification: Shedding Light on the Use of Eriochrome Black T
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Extraction
2.2. LAMP Primers and Assays
2.3. Limit of Detection and Optimal Reaction Time
2.4. Clinical Evaluation of LAMP Assays
3. Results
3.1. Optimal Concentration
3.2. Optimal Concentrations of Mg2+
3.3. Optimal Concentrations of dNTPs
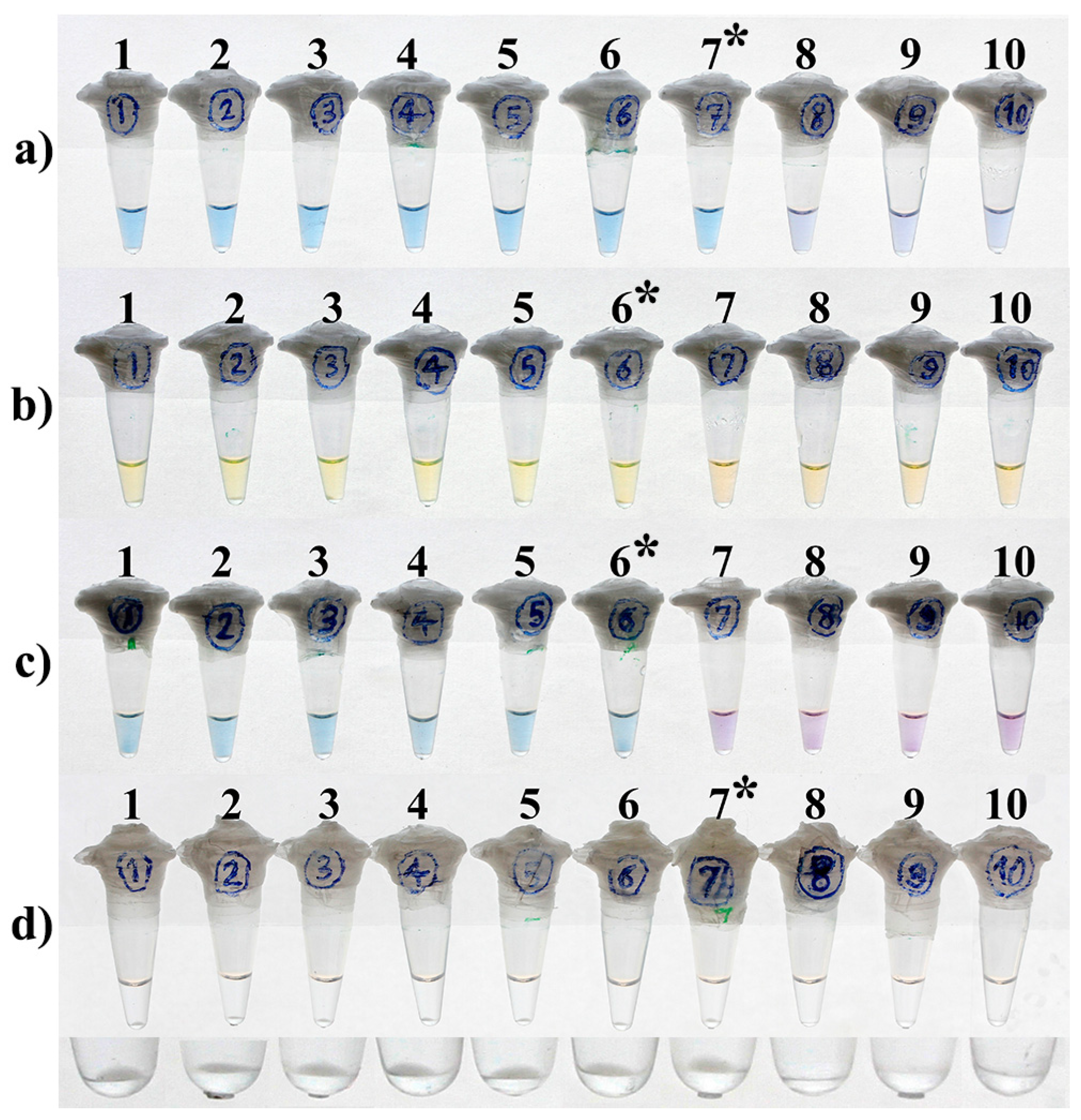
3.4. LOD and Optimal Time of the Reactions
3.5. Clinical Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicklisch, N.; Maixner, F.; Ganslmeier, R.; Friederich, S.; Dresely, V.; Meller, H.; Zink, A.; Alt, K.W. Rib lesions in skeletons from early neolithic sites in Central Germany: On the trail of tuberculosis at the onset of agriculture. Am. J. Phys. Anthropol. 2012, 149, 391–404. [Google Scholar] [CrossRef]
- Barberis, I.; Bragazzi, N.L.; Galluzzo, L.; Martini, M. The history of tuberculosis: From the first historical records to the isolation of Koch’s bacillus. J. Prev. Med. Hyg. 2017, 58, E9–E12. [Google Scholar] [PubMed]
- Cardona, P.J.; Català, M.; Prats, C. Origin of tuberculosis in the Paleolithic predicts unprecedented population growth and female resistance. Sci. Rep. 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.; Martinez, L.; Theron, G.; Wood, R.; Marais, B. Mycobacterium tuberculosis transmission in high-incidence settings-new paradigms and insights. Pathogens 2022, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- MacGregor-Fairlie, M.; Wilkinson, S.; Besra, G.S.; Goldberg Oppenheimer, P. Tuberculosis diagnostics: Overcoming ancient challenges with modern solutions. Emerg. Top. Life Sci. 2020, 4, 435–448. [Google Scholar]
- Harries, A.D.; Kumar, A.M.V. Challenges and progress with diagnosing pulmonary tuberculosis in low- and middle-income countries. Diagnostics 2018, 8, 78. [Google Scholar] [CrossRef]
- Böncüoğlu, E.; Akaslan Kara, A.; Bayram, N.; Devrim, İ.; Kiymet, E.; Çağlar, İ.; Demirağ, B.; Eraslan, C.; Bolat, E.; Ertan, Y.; et al. A diagnostic challenge is it tuberculosis? Pediatr. Infect. Dis. J. 2022, 41, e254. [Google Scholar] [CrossRef]
- Parsons, L.M.; Somoskövi, Á.; Gutierrez, C.; Lee, E.; Paramasivan, C.; Abimiku, A.l.; Spector, S.; Roscigno, G.; Nkengasong, J. Laboratory diagnosis of tuberculosis in resource-poor countries: Challenges and opportunities. Clin. Microbiol. Rev. 2011, 24, 314–350. [Google Scholar] [CrossRef]
- Kivihya-Ndugga, L.; van Cleeff, M.; Juma, E.; Kimwomi, J.; Githui, W.; Oskam, L.; Schuitema, A.; van Soolingen, D.; Nganga, L.; Kibuga, D. Comparison of PCR with the routine procedure for diagnosis of tuberculosis in a population with high prevalences of tuberculosis and human immunodeficiency virus. J. Clin. Microbiol. 2004, 42, 1012–1015. [Google Scholar] [CrossRef][Green Version]
- Gillespie, S.H. Mycobacterium tuberculosis. In Principles and Practice of Clinical Bacteriology, 2nd ed.; Gillespie, S.H., Hawkey, P.M., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 159–169. [Google Scholar]
- Sankar, S.; Ramamurthy, M.; Nandagopal, B.; Sridharan, G. An appraisal of PCR-based technology in the detection of Mycobacterium tuberculosis. Mol. Diagn. Ther. 2011, 15, 1–11. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Lu, J.-J.; Chang, C.-Y.; Chou, W.-P.; Hsieh, J.C.-H.; Lin, C.-R.; Wu, M.-H. Development of a high sensitivity TaqMan-based PCR assay for the specific detection of Mycobacterium tuberculosis complex in both pulmonary and extrapulmonary specimens. Sci. Rep. 2019, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Mor, P.; Dahiya, B.; Sharma, S.; Sheoran, A.; Parshad, S.; Malhotra, P.; Gulati, P.; Mehta, P.K. Diagnosis of peritoneal tuberculosis by real-time immuno-PCR assay based on detection of a cocktail of Mycobacterium tuberculosis CFP-10 and HspX proteins. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. Morb. Mortal. Wkly. Rep. 2009, 58, 7–10. [Google Scholar]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The Use of Loop-Mediated Isothermal Amplification (TB-LAMP) for the Diagnosis of Pulmonary Tuberculosis: Policy Guidance; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Aryan, E.; Makvandi, M.; Farajzadeh, A.; Huygen, K.; Alvandi, A.-H.; Gouya, M.-M.; Sadrizadeh, A.; Romano, M. Clinical value of IS6110-based loop-mediated isothermal amplification for detection of Mycobacterium tuberculosis complex in respiratory specimens. J. Infect. 2013, 66, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.P.; Othman, S.; Lau, Y.L.; Radu, S.; Chee, H.Y. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018, 124, 626–643. [Google Scholar] [CrossRef] [PubMed]
- Soroka, M.; Wasowicz, B.; Rymaszewska, A. Loop-mediated isothermal amplification (LAMP): The better sibling of PCR? Cells 2021, 10, 1931. [Google Scholar] [CrossRef] [PubMed]
- Njiru, Z.K. Loop-mediated isothermal amplification technology: Towards point of care diagnostics. PLoS Negl. Trop. Dis. 2012, 6, e1572. [Google Scholar] [CrossRef]
- Park, J.W. Principles and applications of loop-mediated isothermal amplification to point-of-care tests. Biosensors 2022, 12, 857. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.; Mage, P.L.; Csordas, A.T.; Eisenstein, M.; Soh, H.T. Simultaneous elimination of carryover contamination and detection of DNA with uracil-DNA-glycosylase-supplemented loop-mediated isothermal amplification (UDG-LAMP). Chem. Commun. 2014, 50, 3747–3749. [Google Scholar] [CrossRef] [PubMed]
- Aryan, E.; Makvandi, M.; Farajzadeh, A.; Huygen, K.; Bifani, P.; Mousavi, S.L.; Fateh, A.; Jelodar, A.; Gouya, M.M.; Romano, M. A novel and more sensitive loop-mediated isothermal amplification assay targeting IS6110 for detection of Mycobacterium tuberculosis complex. Microbiol. Res. 2010, 165, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Quoc, N.B.; Phuong, N.D.N.; Chau, N.N.B.; Linh, D.T.P. Closed tube loop-mediated isothermal amplification assay for rapid detection of hepatitis B virus in human blood. Heliyon 2018, 4, e00561. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Honda, E.; Ogura, A.; Nomoto, A.; Hanaki, K.-I. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques 2009, 46, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Bioch. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef]
- Boehme, C.C.; Nabeta, P.; Henostroza, G.; Raqib, R.; Rahim, Z.; Gerhardt, M.; Sanga, E.; Hoelscher, M.; Notomi, T.; Hase, T. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J. Clin. Microbiol. 2007, 45, 1936–1940. [Google Scholar] [CrossRef]
- Aryan, E.; Neshani, A.; Sadeghian, H.; Safdari, H. Visual detection of Mycobacterium tuberculosis by loop-mediated isothermal amplification. Eur. Respir. J. 2016, 48, OA1509. [Google Scholar]
- Zhang, Y.; Ren, G.; Buss, J.; Barry, A.J.; Patton, G.C.; Tanner, N.A. Enhancing colorimetric loop-mediated isothermal amplification speed and sensitivity with guanidine chloride. Biotechniques 2020, 69, 178–185. [Google Scholar] [CrossRef]
- Wang, D.G. Visual detection of Mycobacterium tuberculosis complex with loop-mediated isothermal amplification and Eriochrome Black T. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2014; Volume 618, pp. 264–267. [Google Scholar]
- Pandey, B.D.; Poudel, A.; Yoda, T.; Tamaru, A.; Oda, N.; Fukushima, Y.; Lekhak, B.; Risal, B.; Acharya, B.; Sapkota, B. Development of an in-house loop-mediated isothermal amplification (LAMP) assay for detection of Mycobacterium tuberculosis and evaluation in sputum samples of Nepalese patients. J. Med. Microbiol. 2008, 57, 439–443. [Google Scholar] [CrossRef]
- van Soolingen, D.; Haas, P.E.W.; Kremer, K. Restriction fragment length polymorphism typing of mycobacteria. In Mycobacterium Tuberculosis Protocols; Parish, T., Stoker, N.G., Eds.; Humana Press: Totowa, NJ, USA, 2001; pp. 179–180. [Google Scholar]
- Petroff, S. A new and rapid method for the isolation and cultivation of tubercle bacilli directly from the sputum and feces. J. Experiment. Med. 1915, 21, 38–42. [Google Scholar] [CrossRef]
- Afghani, B.; Stutman, H.R. Polymerase chain reaction for diagnosis of M. tuberculosis: Comparison of simple boiling and a conventional method for DNA extraction. Biochem. Mol. Med. 1996, 57, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Domesle, K.J.; Ge, B. Loop-mediated isothermal amplification for Salmonella detection in food and feed: Current applications and future directions. Foodborne Pathog. Dis. 2018, 15, 309–331. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Cheng, S.; Chu, Y.; Wu, H.; Zou, B.; Huang, H.; Xi, T.; Zhou, G. A closed-tube detection of loop-mediated isothermal amplification (LAMP) products using a wax-sealed fluorescent intercalator. J. Nanosci. Nanotechnol. 2013, 13, 3999–4005. [Google Scholar] [CrossRef] [PubMed]
- Diribe, O.; North, S.; Sawyer, J.; Roberts, L.; Fitzpatrick, N.; La Ragione, R. Design and application of a loop-mediated isothermal amplification assay for the rapid detection of Staphylococcus pseudintermedius. J. Vet. Diagn. Invest. 2014, 26, 42–48. [Google Scholar] [CrossRef]
- Wastling, S.L.; Picozzi, K.; Kakembo, A.S.; Welburn, S.C. LAMP for human African trypanosomiasis: A comparative study of detection formats. PLoS Negl. Trop. Dis. 2010, 4, e865. [Google Scholar] [CrossRef]
- Yang, B.; Wang, X.; Li, H.; Li, G.; Cao, Z.; Cheng, X. Comparison of loop-mediated isothermal amplification and real-time PCR for the diagnosis of tuberculous pleurisy. Lett. Appl. Microbiol. 2011, 53, 525–531. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Cui, Y.; Zhao, J.; Cui, Z.; Li, Q.; Qu, K. Electrochemical behavior of calcein and the interaction between calcein and DNA. Electroanalysis 2012, 24, 1878–1886. [Google Scholar] [CrossRef]
- Wu, D.; Kang, J.; Li, B.; Sun, D. Evaluation of the RT-LAMP and LAMP methods for detection of Mycobacterium tuberculosis. J. Clin. Lab. Anal. 2018, 32, e22326. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yoo, I.S.; An, J.H.; Kim, S. A novel paper-plastic hybrid device for the simultaneous loop-mediated isothermal amplification and detection of DNA. Mater. Lett. 2018, 214, 243–246. [Google Scholar] [CrossRef]
- Oh, S.J.; Seo, T.S. Combination of a centrifugal microfluidic device with a solution-loading cartridge for fully automatic molecular diagnostics. Analyst 2019, 144, 5766–5774. [Google Scholar] [CrossRef]
- Caliendo, A.M.; Gilbert, D.N.; Ginocchio, C.C.; Hanson, K.E.; May, L.; Quinn, T.C.; Tenover, F.C.; Alland, D.; Blaschke, A.J.; Bonomo, R.A.; et al. Better tests, better care: Improved diagnostics for infectious diseases. Clin. Infect. Dis. 2013, 57, S139–S170. [Google Scholar] [CrossRef] [PubMed]

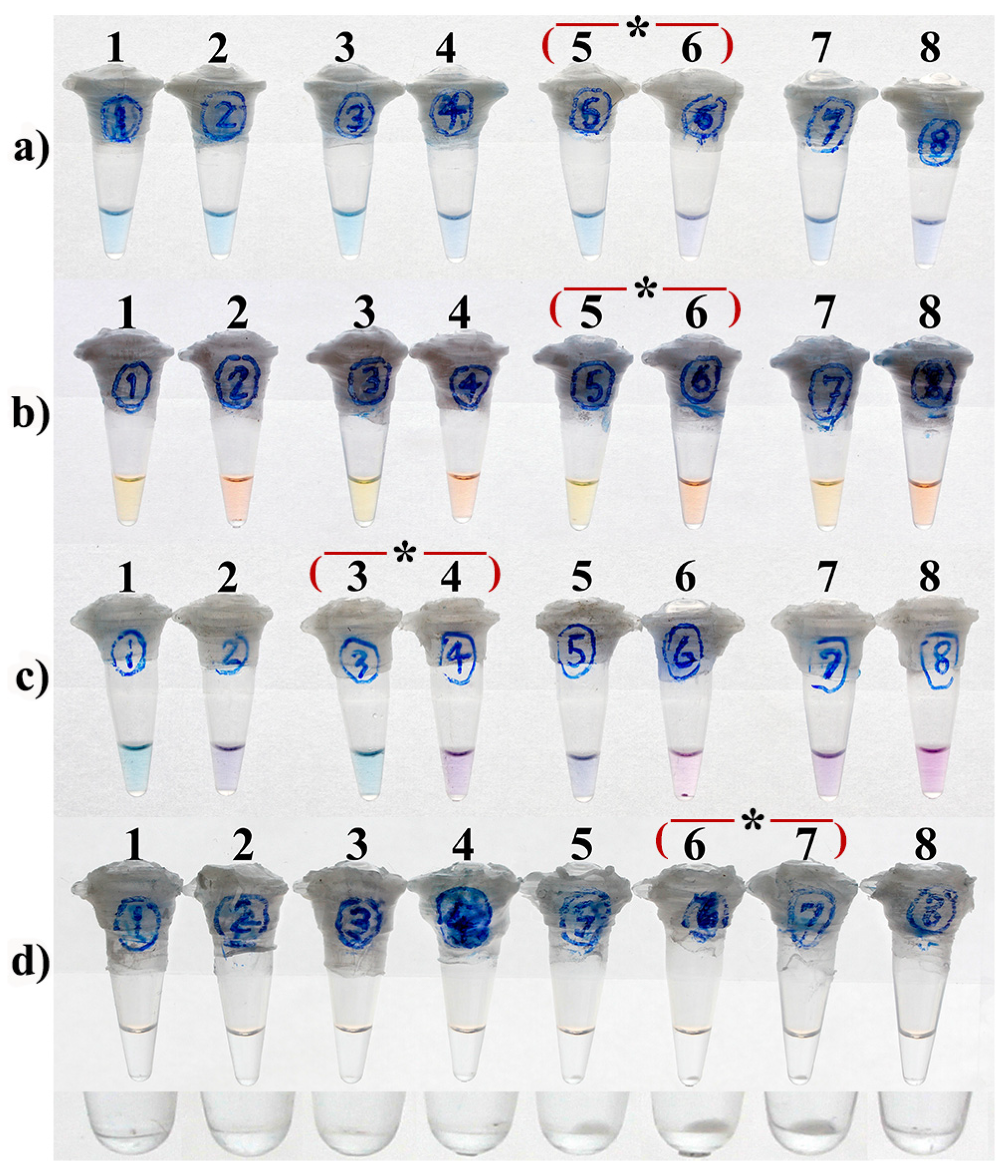
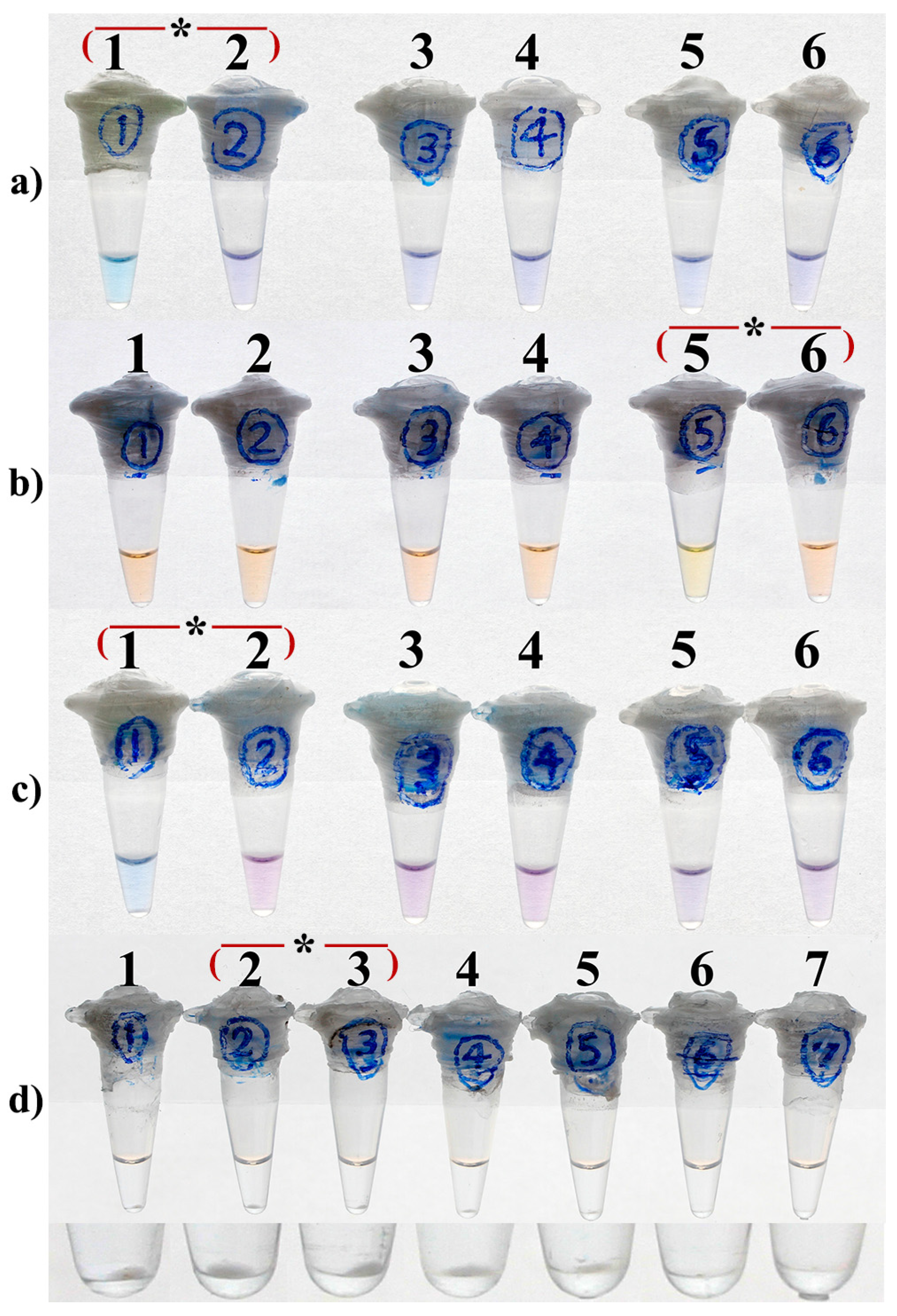
| LAMP Reaction Time (min) | EBT Concentrations (µM) | ||||
|---|---|---|---|---|---|
| 40 | 60 | 80 | 100 | 120 | |
| 15 | − | − | − | − | − |
| 30 | + | + | − | − | − |
| 45 | + | + | − | − | − |
| 60 | + | + | − | − | − |
| 75 | + | + | − | − | − |
| 90 | + | + | − | − | − |
| 105 | + | + | − | − | − |
| 120 | + | + | − | − | − |
| 135 | + | + | − | − | − |
| 150 | + | + | + | − | − |
| 180 | + | + | + | − | − |
| Reaction Time a | Method | Mtb Purified DNA/Reaction (25 µL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 ng | 10 ng | 1 ng | 100 pg | 10 pg | 1 pg | 100 fg | 10 fg | 1 fg | 0 | ||
| 15 min | HNB-LAMP | − | − | − | − | − | − | − | − | − | − |
| Calcein-LAMP | + | + | + | + | + | + | − | − | − | − | |
| EBT-LAMP | − | − | − | − | − | − | − | − | − | − | |
| Turbidity-LAMP | + | + | + | + | + | + | + | − | − | − | |
| 30 min | HNB-LAMP | + | + | + | + | + | + | + | − | − | − |
| Calcein-LAMP | + | + | + | + | + | + | − | − | − | − | |
| EBT-LAMP | + | + | + | + | + | + | − | − | − | − | |
| Turbidity-LAMP | + | + | + | + | + | + | + | − | − | − | |
| 45 min | HNB-LAMP | + | + | + | + | + | + | + | − | − | − |
| Calcein-LAMP | + | + | + | + | + | + | − | − | − | − | |
| EBT-LAMP | + | + | + | + | + | + | − | − | − | − | |
| Turbidity-LAMP | + | + | + | + | + | + | + | − | − | − | |
| LAMP Monitoring Methods a | ||||
|---|---|---|---|---|
| T-LAMP | C-LAMP | H-LAMP | E-LAMP | |
| Principle | Turbidimetry | Colorimetry | Colorimetry | Colorimetry |
| Mechanism | Mg2PPi precipitation results in the formation of white turbidity in the reaction | Sequestering of Mn2+ from calcein results in the color change of this metal ion indicator dye | Sequestering of Mg2+ from hydroxynaphtol Blue leads to a color change of the indicator | Sequestering of Mg2+ from eriochrome black T leads to a color change of the indicator |
| LAMP results (negative/positive) | Clear/Turbid | Orange/Yellow | Violet/Sky blue | Purple/Sky blue |
| Visual inspection of results | Yes | Yes | Yes | Yes |
| Performing in closed system | Yes | Yes | Yes | Yes |
| Potential for subjective error in reading the result | High | High | High | Low |
| LOD b | 100 fg | 1 pg | 100 fg | 1 pg |
| Diagnostic sensitivity (%) | 100 | 93.3 | 93.3 | 93.3 |
| Additional cost c | None | ++ | ++++ | + |
| O.C. of each indicator/reaction d | - | 25 µM | 4.5 mM | 60 µM |
| Time-to-positivity of LAMP (min.) | 15 | 15 | 30 | 30 |
| Inhibitory effect on LAMP reaction | None | Yes, a 10-fold reduction in LOD at optimal concentration | No inhibitory effect at optimal concentration | Yes, a 10-fold reduction in LOD at optimal concentration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neshani, A.; Zare, H.; Sadeghian, H.; Safdari, H.; Riahi-Zanjani, B.; Aryan, E. A Comparative Study on Visual Detection of Mycobacterium tuberculosis by Closed Tube Loop-Mediated Isothermal Amplification: Shedding Light on the Use of Eriochrome Black T. Diagnostics 2023, 13, 155. https://doi.org/10.3390/diagnostics13010155
Neshani A, Zare H, Sadeghian H, Safdari H, Riahi-Zanjani B, Aryan E. A Comparative Study on Visual Detection of Mycobacterium tuberculosis by Closed Tube Loop-Mediated Isothermal Amplification: Shedding Light on the Use of Eriochrome Black T. Diagnostics. 2023; 13(1):155. https://doi.org/10.3390/diagnostics13010155
Chicago/Turabian StyleNeshani, Alireza, Hosna Zare, Hamid Sadeghian, Hadi Safdari, Bamdad Riahi-Zanjani, and Ehsan Aryan. 2023. "A Comparative Study on Visual Detection of Mycobacterium tuberculosis by Closed Tube Loop-Mediated Isothermal Amplification: Shedding Light on the Use of Eriochrome Black T" Diagnostics 13, no. 1: 155. https://doi.org/10.3390/diagnostics13010155
APA StyleNeshani, A., Zare, H., Sadeghian, H., Safdari, H., Riahi-Zanjani, B., & Aryan, E. (2023). A Comparative Study on Visual Detection of Mycobacterium tuberculosis by Closed Tube Loop-Mediated Isothermal Amplification: Shedding Light on the Use of Eriochrome Black T. Diagnostics, 13(1), 155. https://doi.org/10.3390/diagnostics13010155






