Abstract
(1) Background: High bleeding risk is associated with adverse outcomes in ACS patients. We aimed to evaluate temporal trends in treatment and outcomes of ACS patients according to bleeding risk. (2) Methods: Included were ACS patients enrolled in ACSIS surveys. Patients were divided into three groups according to enrolment period: early (2002–2004), mid (2006–2010) and recent (2012–2018). Each group was further stratified into three subgroups according to CRUSADE bleeding risk score. The primary endpoints were 30-day MACE and 1-year all-cause mortality. (3) Results: Included were 13,058 ACS patients. High bleeding risk patients were less frequently treated with guideline-based medications and coronary revascularization. They also had higher rates of 30-day MACE and 1-year all-cause mortality regardless of the enrollment period. Among patients enrolled in early period, 30-day MACE rates were 10.8%, 17.5% and 24.3% (p < 0.001) and 1-year all-cause mortality rates were 2%, 7.7% and 23.6% (p < 0.001) in the low, moderate and high bleeding risk groups, respectively. Among patients enrolled in mid period, 30-day MACE rates were 7.7%, 13.4% and 23.5% (p < 0.001) and 1-year all-cause mortality rates were 1.5%, 7.2% and 22.1% (p < 0.001) in low, moderate and high bleeding risk groups, respectively. For patients enrolled in recent period, 30-day MACE rates were 5.7%, 8.6% and 16.2%, (p < 0.001) and 1-year all-cause mortality rates were 2.1%, 6% and 22.4%, (p < 0.001) in low, moderate and high bleeding risk groups, respectively. These differences remained significant following a multivariate analysis. (4) Conclusions: The percentage of patients at high bleeding risk has decreased over the last years. Despite recent improvements in the treatment of ACS patients, high bleeding risk remains a strong predictor of adverse outcomes.
1. Introduction
High bleeding risk is associated with a higher rate of adverse outcomes including short- and long-term mortality in patients with ACS [1,2,3,4,5]. Nevertheless, despite their high cardiovascular risk, ACS patients at high bleeding risk are more commonly selected for conservative management rather than an invasive strategy and are less frequently treated with guideline-based medical therapy [1]. In recent decades, the treatment of patients with ACS has improved dramatically with the introduction of new anti-platelet agents, potent lipid-lowering medications and major advances in both percutaneous and surgical coronary revascularizations [6,7,8,9]. This improved treatment has been associated with a subsequent reduction in mortality and cardiovascular complications among different populations of ACS patients [10,11,12]. Whether the improvement in treatment has resulted in a better outcome for ACS patients at high bleeding risk has not been evaluated yet. The current study was aimed to evaluate temporal trends in the prevalence, treatment and outcomes of ACS patients at high bleeding risk.
2. Materials and Methods
Study population: The Acute Coronary Syndromes Israeli Survey (ACSIS) is carried out for 2 months every 2–3 years in all intensive coronary care units and cardiology departments in Israel. The study population consisted of patients presenting with ST-elevation and non-ST-elevation myocardial infarction or unstable angina pectoris that were included in the ACSIS Surveys during 2000–2018. Study physicians recorded all clinical and demographic data on pre-specified forms for consecutive participants. The diagnosis of ACS was based on clinical, electrocardiographic and biochemical criteria, and patients were managed at the discretion of each medical center.
Bleeding risk assessment: The CRUSADE bleeding risk score (heart rate, systolic blood pressure, hematocrit, creatinine clearance, sex, signs of heart failure at admission and history of vascular disease or diabetes mellitus) was used for bleeding risk assessment [13]. It was calculated for each individual patient at the presentation to the hospital and patients were stratified into three groups of low, intermediate and high bleeding risk (CRUSADE score 1–30, 31–40 and 41–96, respectively). We compared baseline characteristics, treatment and clinical outcomes of ACS patients according to bleeding risk in the entire ACSIS population and in a separate analysis for patients enrolled in early (2002, 2004), mid (2006, 2010) and late (2013, 2015, 2018) surveys.
Outcomes: The primary endpoints of the study were 1-year all-cause mortality and 30-day major adverse cardiovascular events (MACE). MACE was the composite of all-cause mortality, myocardial infarction and cerebrovascular accident (CVA). Mortality rates were determined for all participants from hospital charts and by matching the identification numbers of the patients with the Israeli National Population Registry.
Statistical analysis: Patients’ characteristics were presented as n (%) for categorical variables, and as mean (sd) or median (IQR) for normal/non-normal distributed continuous variables. The cohort was divided into three groups according to enrolment periods (early, mid and recent). Each group was further divided into three subgroups according to the CRUSADE bleeding risk score (low, intermediate and high risk). Baseline characteristics, treatment and clinical outcomes were compared between patients in the different bleeding risk groups within each time period. Chi-square test was used for comparison of categorical variables. Analysis of variance with one degree of freedom was performed for comparison of normally distributed continuous variables. The Kendall rank correlation was performed for non-normal distribution. Kaplan–Meier curves were used to present 1-year survival rates. The comparison of outcomes between different bleeding risk categories within the same enrolment period was performed using a pairwise log rank test with Holm’s p-value adjustment. Multivariate adjustment was further conducted using Cox proportional hazard models. Included in the adjustment were relevant baseline demographic and clinical characteristics not already included in the CRUSADE score. All analyses were performed using R (R-studio, V.4.0.3, Vienna, Austria).
3. Results
The 13,058 ACS patients had a median age of 63 years and included 77.9% men. Of them, 3702 were enrolled at the early period, 5248 at mid and 4108 at the recent periods. Each time period was further divided into three subgroups according to CRUSADE bleeding risk. The percentage of patients at high bleeding risk decreased in recent surveys. Baseline characteristics according to enrolment period and bleeding risk are presented in Table 1. At all enrolment periods, patients at high bleeding risk were older, more frequently women and more commonly presented with cardiovascular risk factors and a history of cardiovascular disease. Accordingly, patients with higher bleeding risk were more commonly treated with cardiovascular medication prior to hospitalization.

Table 1.
Baseline characteristics according to bleeding risk.
In hospital treatment according to bleeding risk: In hospital treatment characteristics according to enrolment period and bleeding risk are presented in Table 2. Regardless of enrollment period, ACS patients at high bleeding risk were less frequently treated with guideline-based medical therapy, including anti-platelet agents, statins and ACE-inhibitors/ARBs. Among high bleeding risk patients enrolled in recent surveys, anti-platelet therapy less commonly included ticagrelor or prasugrel rather than clopidogrel. Moreover, referral rates for an invasive strategy with coronary angiography and subsequent coronary angioplasty during hospitalization were lower in the high bleeding risk groups regardless of enrolment period. Referral for surgical revascularization during hospital admission decreased in recent compared to early enrolment periods, regardless of bleeding risk.

Table 2.
In hospital treatment characteristics according to enrolment period and bleeding risk.
Outcome of ACS patients according to bleeding risk: Kaplan–Meier curves comparing 1-year mortality according to bleeding risk for each enrolment period are presented in Figure 1. High bleeding risk was associated with increased mortality rates regardless of enrolment period. We further conducted a multivariate analysis for 1-year all-cause mortality for the study groups. An analysis using the low bleeding risk as a reference demonstrated significantly higher 1-year mortality rates among patients at high bleeding risk in all enrolment periods. Among patients enrolled in early period, odds ratio for 1-year all-cause mortality were 2.88, 95%CI 1.8–4.4, p < 0.001 and 6.35, 95%CI 4.2–9.5, p < 0.001 in moderate and high bleeding risk compared to low bleeding risk group, respectively. For patients enrolled in mid period, odds ratio for 1-year all-cause mortality were 3.65, 95%CI 2.4–5.5, p < 0.001 and 8.6, 95%CI 5.8–12.7, p < 0.001 in moderate and high bleeding risk compared to low bleeding risk group, respectively. For patients enrolled in the recent period, the odds ratio for 1-year all-cause mortality was 2.2, 95%CI 1.4–3.5, p = 0.001 and 5.8, 95%CI 3.9–8.6, p < 0.001 in moderate and high bleeding risk compared to low bleeding risk group, respectively.
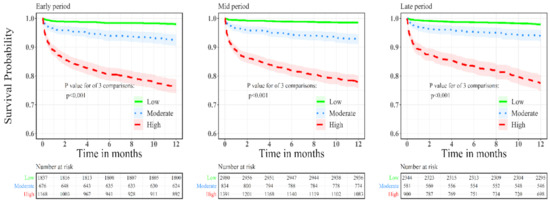
Figure 1.
Kaplan–Meier curves for 1-year all-cause mortality according to bleeding risk for patients enrolled in early, mid and recent surveys.
High bleeding risk was associated with increased risk of 30-day MACE regardless of enrolment period (Figure 2). We further conducted a multi-variant analysis for 30-day MACE for the study groups. An analysis using the low bleeding risk as a reference demonstrated significantly higher 30-day MACE rates among patients at high bleeding risk in all enrolment periods. Among patients enrolled in early period, the odds ratio for 30-day MACE was 1.45, 95%CI 1.1–1.8, p = 0.01 and 1.8, 95%CI 1.3–2.3, p < 0.001 in moderate and high bleeding risk compared to low bleeding risk group, respectively. For patients enrolled in mid period, the odds ratio for 30-day MACE was 1.48, 95%CI 1.1–1.9, p = 0.004 and 2.57, 95%CI 1.9–3.3, p < 0.001 in moderate and high bleeding risk compared to low bleeding risk group, respectively. For patients enrolled in the recent period, the odds ratio for 30-day MACE was 1.47, 95%CI 1.02–2.1, p = 0.04 and 2.38, 95%CI 1.6–3.3, p < 0.001 in moderate and high bleeding risk compared to low bleeding risk group, respectively.
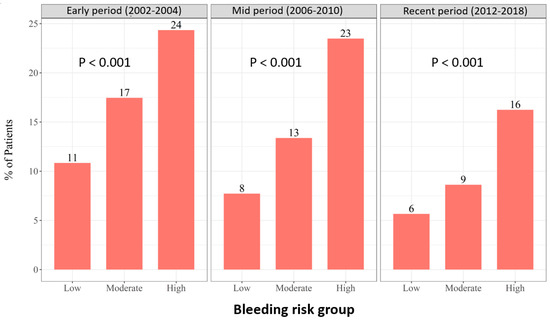
Figure 2.
The 30-day MACE according to bleeding risk and enrolment period.
4. Discussion
The current study demonstrated a significant improvement in the treatment of ACS patients over the past two decades. ACS patients included in recent surveys were more frequently selected for an invasive strategy with coronary angiography and subsequent revascularization and were more commonly treated with guideline-based medical therapy. Nevertheless, even in the recent period, high bleeding risk remained a strong predictor for adverse cardiovascular outcomes and mortality. Despite their increased cardiovascular risk, ACS patients at high bleeding risk were more commonly selected for conservative management rather than an invasive strategy and were less frequently treated with guideline-based medical therapy regardless of enrolment period.
Several risk scores have been suggested for the assessment of bleeding risk in patients with ACS including the CRUSADE, ACUITY and the Academic Research Consortium for High Bleeding Risk (ARC-HBR) bleeding risk score [1]. The CRUSADE bleeding score was initially developed for NSTE-ACS patients [13] and was subsequently also validated for patients with STEMI [14]. A meta-analysis comparing different bleeding scores performance including 18,155 ACS patients from 17 studies demonstrated that the CRUSADE score was the most widely used score, and also performed better especially in patients selected for invasive strategy [15]. These findings were further supported by additional studies [16,17]. Accordingly, the CRUSADE score has been recommended by international clinical guidelines for bleeding risk assessment in ACS [1].
The association between high bleeding risk and adverse outcomes including in-hospital and 1-year mortality of patients with ACS has been demonstrated in several studies [2,3,4,5]. However, the current study is, to the best of our knowledge, the first to assess temporal trends in the treatment and outcome of ACS patients according to their bleeding risk. The association between bleeding risk and outcome is not completely understood and appears to be multifactorial. First, both major and minor bleeding events have been well demonstrated to be associated with poor outcomes in ACS patients including short- and long-term mortality [18,19]. Second, concerns regarding major bleeding events may influence both patient’s and physician’s treatment decisions, including the preference of a non-invasive strategy in order to minimize the need for anticoagulation and potent anti-platelet therapy. Indeed, we found that ACS patients at high bleeding risk were more commonly selected for a conservative rather than an invasive strategy. Moreover, patients at high bleeding risk were less frequently treated with guideline-based medical therapy. While the lower rate of treatment with antiplatelet drugs may be explained by the increased bleeding risk, patients at high bleeding risk were also less frequently treated with statins and ACE inhibitors. Finally, the presence of high bleeding risk is also associated with an older age and baseline cardiovascular comorbidities including diabetes, heart failure, previous MI, stroke and chronic kidney disease, which are all independently associated with poor outcomes in patients with ACS.
In recent years several strategies to reduce bleeding in ACS patients have been developed, including the preference of the radial approach, avoidance of pre-loading with anti-platelet therapy, gastric protection with proton pump inhibitors and the option to shorten dual anti-platelet treatment duration in patients at high bleeding risk. These strategies have resulted in better treatment and outcomes in the general population of ACS patients. Indeed, we demonstrated higher rates of referral for an invasive strategy and treatment with guideline-based medical therapy in recent surveys regardless of bleeding risk. Nevertheless, even with contemporary therapy, patients at high bleeding risk remain highly susceptible to adverse cardiovascular outcomes.
Our study has several limitations that warrant consideration. First, our database did not include data regarding post-discharge bleeding events and the very low rates of in-hospital major bleeding events precluded a meaningful analysis. Second, since the specific cause of death was not available, the primary endpoint of our study was all-cause rather than cardiovascular mortality. Finally, the ACSIS is a large national survey and therefore our findings should be extrapolated to other countries with caution.
5. Conclusions
The percentage of patients at high bleeding risk decreased in recent surveys. Despite the improvement in the treatment of ACS patients in recent years, high bleeding risk remains a strong predictor of poor clinical outcomes.
Author Contributions
Conceptualization, D.P. and Z.A.; methodology, T.O. (Tal Ovdat); validation, T.O. (Tal Ovdat); formal analysis, T.O. (Tal Ovdat) and T.O. (Tsafrir Or); investigation, Z.A. and D.P.; resources, A.A.; data curation A.O. and M.S.; writing—original draft preparation, Z.A.; writing—review and editing, D.P. and A.A.; visualization, M.G.; supervision, D.P.; project administration, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patient(s) to publish this paper.
Data Availability Statement
Not applicable.
Acknowledgments
The author wishes to acknowledge the contribution of Dmitriy Katz, Tamar Shitrit and Rami Khashab.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Cordero, A.; Rodriguez-Manero, M.; García-Acuña, J.M.; López-Palop, R.; Cid, B.; Carrillo, P.; Agra-Bermejo, R.; González-Salvado, V.; Iglesias-Alvarez, D.; Bertomeu-Martínez, V.; et al. Additive value of the CRUSADE score to the GRACE score for mortality risk prediction in patients with acute coronary syndromes. Int. J. Cardiol. 2017, 245, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Lee, C.H.; Chen, C.C.; Chang, S.H.; Wang, C.Y.; Hsieh, I.C. Predictive performance of HAS-BLED risk score for long-term survival in patients with non-ST elevated myocardial infarction without atrial fibrillation. J. Cardiol. 2017, 69, 136–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mehran, R.; Pocock, S.J.; Nikolsky, E.; Clayton, T.; Dangas, G.D.; Kirtane, A.J.; Parise, H.; Fahy, M.; Manoukian, S.V.; Feit, F.; et al. A risk score to predict bleeding in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 2010, 55, 2556–2566. [Google Scholar] [CrossRef] [PubMed]
- Natsuaki, M.; Morimoto, T.; Yamaji, K.; Watanabe, H.; Yoshikawa, Y.; Shiomi, H.; Nakagawa, Y.; Furukawa, Y.; Kadota, K.; Ando, K.; et al. Prediction of Thrombotic and Bleeding Events After Percutaneous Coronary Intervention: CREDO-Kyoto Thrombotic and Bleeding Risk Scores. J. Am. Heart Assoc. 2018, 7, e008708. [Google Scholar] [CrossRef] [PubMed]
- Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; Murphy, S.A.; Riesmeyer, J.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Puymirat, E.; Taldir, G.; Aissaoui, N.; Lemesle, G.; Lorgis, L.; Cuisset, T.; Bourlard, P.; Maillier, B.; Ducrocq, G.; Ferrieres, J.; et al. Use of invasive strategy in non-ST-segment elevation myocardial infarction is a major determinant of improved long-term survival: FAST-MI (French Registry of Acute Coronary Syndrome). JACC Cardiovasc. Interv. 2012, 5, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Bueno, H.; Rossello, X.; Pocock, S.J.; Van de Werf, F.; Chin, C.T.; Danchin, N.; Lee, S.W.; Medina, J.; Huo, Y. In-Hospital Coronary Revascularization Rates and Post-Discharge Mortality Risk in Non-ST-Segment Elevation Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2019, 74, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Shuvy, M.; Beeri, G.; Klein, E.; Cohen, T.; Shlomo, N.; Minha, S.; Pereg, D. Accuracy of the Global Registry of Acute Coronary Events (GRACE) Risk Score in Contemporary Treatment of Patients With Acute Coronary Syndrome. Can. J. Cardiol. 2018, 34, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Shuvy, M.; Chen, S.; Vorobeichik, D.; Krashin, E.; Shlomo, N.; Goldenberg, I.; Pereg, D. Temporal trends in management and outcomes of patients with acute coronary syndrome according to renal function. Int. J. Cardiol. 2017, 232, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Subherwal, S.; Bach, R.G.; Chen, A.Y.; Gage, B.F.; Rao, S.V.; Newby, L.K.; Wang, T.Y.; Gibler, W.B.; Ohman, E.M.; Roe, M.T.; et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: The CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009, 119, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Solé, A.; Sánchez-Elvira, G.; Sánchez-Salado, J.C.; Lorente-Tordera, V.; Salazar-Mendiguchía, J.; Sánchez-Prieto, R.; Romaguera-Torres, R.; Ferreiro-Gutiérrez, J.L.; Gómez-Hospital, J.A.; Cequier-Fillat, A. CRUSADE bleeding risk score validation for ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Thromb. Res. 2013, 132, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.K.M.; Mehta, O.H.; Liao, Y.B.; Wang, M.T.M.; Stewart, R.; White, H. Meta-Analysis of Bleeding Scores Performance for Acute Coronary Syndrome. Heart Lung Circ. 2020, 29, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Abu-Assi, E.; Raposeiras-Roubin, S.; Lear, P.; Cabanas-Grandío, P.; Girondo, M.; Rodríguez-Cordero, M.; Pereira-López, E.; Romaní, S.G.; González-Cambeiro, C.; Alvarez-Alvarez, B.; et al. Comparing the predictive validity of three contemporary bleeding risk scores in acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Castini, D.; Centola, M.; Ferrante, G.; Cazzaniga, S.; Persampieri, S.; Lucreziotti, S.; Salerno-Uriarte, D.; Sponzilli, C.; Carugo, S. Comparison of CRUSADE and ACUITY-HORIZONS Bleeding Risk Scores in Patients With Acute Coronary Syndromes. Heart Lung Circ. 2019, 28, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Kikkert, W.J.; Delewi, R.; Ouweneel, D.M.; van Nes, S.H.; Vis, M.M.; Baan JJr Koch, K.T.; Dangas, G.D.; Mehran, R.; de Winter, R.J.; Peters, R.J.; et al. Prognostic value of access site and nonaccess site bleeding after percutaneous coronary intervention: A cohort study in ST-segment elevation myocardial infarction and comprehensive meta-analysis. JACC Cardiovasc. Interv. 2014, 7, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Kikkert, W.J.; van Geloven, N.; van der Laan, M.H.; Vis, M.M.; Baan, J., Jr.; Koch, K.T.; Peters, R.J.; de Winter, R.J.; Piek, J.J.; Tijssen, J.G.; et al. The prognostic value of bleeding academic research consortium (BARC)-defined bleeding complications in ST-segment elevation myocardial infarction: A comparison with the TIMI (Thrombolysis In Myocardial Infarction), GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries), and ISTH (International Society on Thrombosis and Haemostasis) bleeding classifications. J. Am. Coll. Cardiol. 2014, 63, 1866–1875. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).