Abstract
Subclinical atherosclerosis (SA) is the presence of coronary calcification in the absence of cardiovascular symptoms, and it usually progresses to atherosclerotic disease. Studies have shown an association of osteoprotegerin gene (OPG) variants with calcification process in cardiovascular diseases; however, to this day there are no studies that evaluate individuals in the asymptomatic stage of atherosclerotic disease. Therefore, the purpose of this study was to analyze the association of four genetic variants and haplotypes of the OPG gene with the development of SA, through TaqMan genotyping assays. We also aimed to identify potential response elements for transcription factors in these genetic variants. The study included 1413 asymptomatic participants (1041 were controls and 372 were individuals with SA). The rs3102735 polymorphism appeared as a protective marker (OR = 0.693; 95% CI = 0.493–0.974; pheterozygote = 0.035; OR = 0.699; 95% CI = 0.496–0.985; pcodominant 1 = 0.040) and two haplotypes were associated with SA, one as a decreased risk: GACC (OR = 0.641, 95% CI = 0.414–0.990, p = 0.045) and another as an increased risk: GACT (OR = 1.208, 95% CI = 1.020–1.431, p = 0.029). Our data suggest a lower risk of SA in rs3102735 C carriers in a representative sample of Mexican mestizo population.
1. Introduction
Subclinical atherosclerosis (SA) is defined as the presence of coronary calcification, in the absence of cardiovascular symptoms [1]. This process develops silently over the years and usually advances to a high degree when clinical symptoms appear, such as an acute coronary event. Currently, coronary artery calcification (CAC) is considered an early marker of coronary artery disease (CAD) [1,2] and the use of CAC score is one of the main approaches for a primary detection of SA in apparently healthy individuals [3,4]. Moreover, it has been reported that the calcification of coronary arteries represents a risk factor for adverse cardiovascular events [5].
The development of atherosclerotic plaques and the presence of coronary calcification is determined by a complex and still unclear interaction between traditional risk factors and genetic components [6]. The genetic approach in cardiovascular diseases has revealed polymorphisms with functional repercussions on the encoded proteins that have been associated with vascular diseases. Among these proteins, osteoprotegerin (OPG) is considered a main contributor of the vascular calcification processes [7]. The human OPG gene is located at locus 8q24.12, it contains five exons [8] and encodes for a 401-amino acid glycoprotein with a molecular weight of 60 kDa. OPG belongs to the tumor necrosis factor superfamily and contains seven domains implicated in its biological activities [7,9,10]. OPG is produced in vascular smooth muscle cells and vascular endothelial cells, among others [9,10]; it has been proposed as regulator of vascular diseases, vascular calcification and bone matrix homeostasis [11,12].
Different studies have shown an increased expression of OPG in carotid atherosclerotic lesions [13], as well as in epicardial adipose tissue of patients with coronary artery disease [14]. Moreover, elevated levels of OPG in serum have been associated with subclinical and vascular diseases [10,15,16,17,18]. To-date, few studies have investigated the genetic association of OPG gene and the presence of SA; furthermore, the results have been paradoxical [19,20]. Thus, the aim of the present study was to investigate whether the genetic variants rs2073618 (G1181C), rs3134069 (C209T), rs3134070 (T245G), rs3102735 (A163G) and haplotypes of OPG gene are associated with the risk of developing SA. Also, we performed an in silico analysis in order to identify the potential functional effects of these genetic variants.
2. Materials and Methods
2.1. Study Population
This research is a cross-sectional study performed during the baseline evaluation of individuals recruited for the Genetics of Atherosclerotic Disease study (Spanish acronym GEA). The GEA study was designed at the Instituto Nacional de Cardiología Ignacio Chávez (INCICh) to determine the genetic bases of CAD and to evaluate its association with conventional and emerging cardiovascular risk factors [21].
The study population included a total of 1413 unrelated, clinically healthy, asymptomatic participants, with no personal or family history of CAD. These participants were blood donors attending the blood bank of the INCICh or recruited by open invitations in different social services centers from 2008 to 2013, in Mexico City. The participants were characterized by biochemical determinations, medical history, demographics, anthropometry and nutritional information collected through standardized and validated questionnaires [21,22,23,24]. For this study, the exclusion criteria were: the presence of heart or renal failure, thyroid disorders, and liver and oncological diseases.
The participants underwent a chest and abdomen axial tomography using a 64-channel multi-detector helical computed tomography (CT) system (Somaton Sensation Siemens, Forcheim, Germany). After assessment of CT, CAC score was determined by the Agatston method [25]; a CAC score > 0 in the absence of coronary symptoms was defined as SA. Then, 372 individuals were identified with SA, whereas 1041 individuals with a CAC = 0 formed the control group.
The present study is in compliance with the Helsinki Declaration and was approved by the Ethics and Research Committees of the INCICh (Register number 18-1071). All enrolled participants provided a written informed consent.
2.2. Single Nucleotide Polymorphism (SNPs) Selection and Genotyping
Genomic DNA was extracted from peripheral blood using standard methods (DNA Blood Mini kit, QIAGEN, Hilden, Germany). All polymorphisms were genotyped using specific TaqMan assays on a Prism 7900HT Fast Real-Time PCR system following the supplier’s instructions (ThermoFisher Scientific, Foster City, CA, USA).
The polymorphic sites were selected by a prior review of the NCBI data base, and included polymorphisms with a minor allele frequency (MAF) >5%, previously demonstrated to be associated with cardiovascular diseases and with vascular calcification.
2.3. Bioinformatics Analysis of Prediction of Transcription Factor Bindings Sites
To identify potential response elements for transcription factors as a consequence of polymorphisms in the OPG gene promoter, the in silico analysis was performed with the bioinformatics software tools PROMO version 3.0.2, website (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3 (accessed on 12 March 2022)). This analysis was performed on flanking sequences of 25 bases upstream and 25 bases downstream for the two alleles of each polymorphism, with a dissimilarity margin less than or equal to 15% [26]. Also, SNPinfo website (https://snpinfo.niehs.nih.gov/snpinfo/snpfunc.html (accessed on 12 March 2022)) was employed and the analysis of SNPrsID corresponding to each polymorphism included the allele frequency data from all populations reported in HapMap and dbSNP [27].
2.4. Statistical Analysis
According to the data distribution, the continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range); the categorical variables were described as percentages. Either Student’s t-test or a Mann–Whitney U test was performed for the two-group comparisons of continuous variables whereas for the categorical variables the Pearson’s chi-squared test was used. In both study groups, the allele and genotype frequencies were obtained by direct count and the Hardy−Weinberg equilibrium was tested in the four polymorphisms. A logistic regression analysis was applied to determine the association of polymorphisms with subclinical atherosclerosis, under different inheritance models, adjusted by cardiovascular risk confounding variables. The construction and analysis of haplotypes were performed with the Haploview software v4.1 (Broad Institute of Massachusetts [MIT], Cambridge, MA, USA). Statistical analyses were performed using SPSS v20.0 (SPSS Inc., Chicago, IL, USA). A statistically significant value was established as p < 0.05.
3. Results
3.1. Characteristics of the Studied Groups
Clinical and metabolic characteristics as well as cardiovascular risk factors of the studied population are shown in Table 1. Statistically significant differences in individuals with SA were observed, when compared to the control group in terms of age, sex (males), body mass index, waist circumference, LDL-cholesterol, triglyceride and glucose levels (p < 0.05). Concerning cardiovascular risk factors, individuals with SA had higher frequencies of subcutaneous abdominal fat, LDL-cholesterol ≥ 130 mg/dL, and non-HDL cholesterol >160 mg/dL when compared to the control group (p < 0.05).

Table 1.
Characteristics of studied groups.
3.2. Association of OPG Gene Polymorphisms with Subclinical Atherosclerosis
Figure 1 describes the location of the evaluated polymorphisms. The sequence studied and MAF corresponding to each polymorphism are described in Table 2.
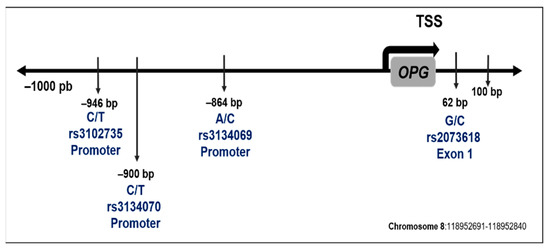
Figure 1.
Location in the OPG gene sequence of selected polymorphisms. The position is with respect to the transcription start site (TSS).

Table 2.
Description of analyzed sequence of polymorphisms studied.
The expected and observed frequencies of the four polymorphisms in the whole sample of this study were in Hardy-Weinberg equilibrium. The genotype distributions are described in Figure 2. A different distribution of the OPG polymorphism rs3102735 was observed in individuals with SA when compared to the control group. Individuals with SA showed decreased frequencies of the C allele rs3102735 when compared to controls (OR = 0.693; 95% CI = 0.493–0.974; pheterozygote = 0.035; OR = 0.699; 95% CI = 0.496–0.985; pcodominant 1 = 0.040). Conversely, similar distributions of the OPG polymorphisms rs3134070, rs3134069 and rs2073618 were found in individuals with SA and controls. These analyses were adjusted by age, sex, body mass index, diabetes mellitus, subcutaneous abdominal fat, smoking habits, concentrations of apolipoprotein AI, LDL-cholesterol, calcium and phosphorus serum concentrations, as well as alkaline phosphatase, alanine transaminase and aspartate transaminase activities.
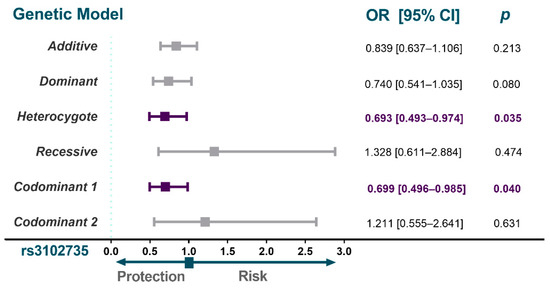
Figure 2.
Association analysis of OPG gene polymorphisms with SA. The models shown were adjusted by sex, age, BMI, smoking habits, diabetes mellitus, LDL-cholesterol, subcutaneous abdominal fat, alkaline phosphatase, alanine transaminase and aspartate transaminase activities, apolipoprotein AI concentrations and phosphorus and calcium serum concentration. SA, subclinical atherosclerosis; MAF, minor allele frequency; OR, odds ratio; CI, confidence interval.
3.3. Haplotype Association Analysis of OPG Gene Polymorphisms
Two haplotypes (GACT and GACC) had different frequencies between groups (Figure 3); individuals with SA showed an increased frequency of the GACT haplotype when compared to the control group (OR = 1.208, 95% CI = 1.020–1.431, p = 0.029). While the GACC haplotype showed a decreased frequency in individuals with SA when compared to the control group (OR = 0.641, 95% CI = 0.414–0.990, p = 0.045).
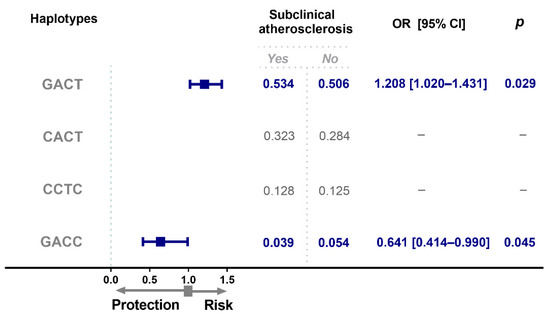
Figure 3.
OPG haplotype frequencies in individuals with subclinical atherosclerosis. Haplotype analysis was performed based on the order and position of the polymorphisms in the chromosome. (rs2073618, rs3134069, rs3134070, rs3102735). OR, odds ratio; CI, confidence interval.
3.4. In Silico Analysis of Transcription Factor Binding Sites to OPG Polymorphisms
The in silico analysis of response elements of transcription factors showed that rs3102735 modifies a DNA binding site; the substitution of cytosine for thymine causes the loss of the specific binding site for the enhancer-binding protein β (C/EBP-β) and the enhancer-binding protein α (C/EBP-α) factors.
3.5. Analysis of CAC Score between C Allele Carriers and Non-C Carriers
To explore whether the C allele of the rs3102735 polymorphism had any impact on coronary calcification, we compared the CAC score of patients with SA, grouped as C carriers and non-C carriers; this analysis showed a higher CAC score in non-C carriers than in C carriers, but this difference did not reach statistical significance (CAC = 34.30 [6.35–86.85], CAC = 24.60 [4.30–93.50], p > 0.05, respectively).
4. Discussion
In the present study, we assessed four polymorphisms of the OPG gene in Mexican individuals, in order to explore their possible association with SA. In only one polymorphism (rs3102735) a dissimilar distribution was observed in individuals with SA when compared to the control group. Currently, there are only a few studies involving the role of OPG gene polymorphisms with the presence of SA. Our data concerning the frequency of the rs3102735 polymorphism suggest a potential usefulness as a genetic marker of SA.
Nevertheless, the participation of rs3102735 as a susceptibility marker is still under debate. Even if this polymorphism has been shown to be associated with the incidence of diseases other than cardiovascular diseases [8,28,29], a lack of association with the incidence of symptomatic CAD [30] or large artery atherosclerosis stroke [28] has been reported. The topography of atherosclerotic lesions as well as the stage of the disease may be at the basis of these discrepancies.
Similarly, the analysis of OPG polymorphisms performed by Soufi et al. and Rhee et al., included rs3134069 and rs3102735 polymorphisms, and did not demonstrate any association with CAD in Caucasian and Korean populations, respectively [29,31]. Discrepancies concerning the association between OPG polymorphisms and cardiovascular diseases may be related to several circumstances, such as the number of patients included in every study and the technique used for genotyping and the number of SNPs evaluated. (a) We determined four SNPs and we used CT to evaluate the CAC score, whereas the report by Alkady et al. [32] was based in the intima–media thickness by echography as a subrogate of atherosclerosis and they only analyzed one polymorphism. Considering that OPG is involved in the calcification process in tissues, the lack of association in the study by Alkady et al. [32] is not surprising, whereas we defined SA by the presence of coronary calcification. (b) The report by Soufi et al. included just 468 male patients with and without CAD, as compared to the 1413 participants in our study. In addition, some genetic determinations were made by RFLP, whereas we used TaqMan genotyping assays in our study. (c) The study by Rhee et al. also included a small number of patients in comparison to our report. (d) There is a potential ethnic contribution to take into account, since all these studies were performed in populations whose genetic backgrounds differ from that of the Mexican mestizo population [33,34,35,36].
Furthermore, the known physiological role of OPG allows an acceptable interpretation of the contribution of the protein to SA [37]. During atherosclerosis progression, OPG expression decreases in blood vessels concomitantly with the increase in the receptor activator of NF-κB (RANK) ligand (RANKL) [38]. The interaction of RANKL and RANK activates the NF-κB pathway with the consequent transcription of pro-calcifying genes in VSMC, such as bone morphogenetic protein-4 [37]. OPG is a decoy receptor that binds to RANKL, thus preventing the interaction between RANK and RANKL [39], thus inhibiting the osteogenic differentiation of VSMC. Consequently, it is likely that OPG plays an anti-calcifying role that is consistent with increased levels of OPG during certain stages of the coronary heart disease, where OPG may be over-expressed to compensate for the deposition of calcium in the subendothelial space [14,37]. This idea is supported by the high serum levels of OPG in several vascular diseases and carotid intima–media thickness observed in patients with pathologies related to cardiovascular risk factors [40,41,42,43]. To gain more insight about the possible impact of the rs3102735 polymorphism, we performed an in silico analysis to establish whether there is a response element that could be affected by the substitution of T for C in the promoter sequence of the OPG gene. This approach suggests that the presence of the C allele, corresponding to MAF of the rs3102735, introduces a C/EBP response element that may enhance the expression of the OPG gene. C/EBPs are a group of transcription factors that belong to a superfamily formed by CREB, AP-1 and ATF that fulfill functions such as immune response and energy metabolism [44]. Therefore, the presence of the C allele of the rs3102735 may have a functional role in the expression of the OPG gene.
To further explore the potential impact of this polymorphism on coronary calcification, we compared patients with SA who were C carries to patients with SA who were non-C carriers. Even if the CAC score tended to higher values in non-C carriers, the difference did not reach statistical significance.
Furthermore, it is important to consider some limitations in our study: (a) our results provide a genetic approach to the potential contribution of osteoprotegerin but we did not establish whether the polymorphisms determine the protein expression; (b) age and gender are commonly associated with osteoprotegerin plasma levels, and our data cannot discard a potential interaction between OPG polymorphisms and these two characteristics; (c) additional studies are necessary to validate our findings with different ethnicities; (d) functional studies are required to clarify the role of OPG polymorphisms in the subclinical manifestation of atherosclerosis.
We recognize that our study was unpowered for subgroup comparisons; therefore, a large population study may be necessary to explore the potential influence of the C allele on CAC score. Moreover, the possibility that a neighboring gene co-segregates with the OPG gene, influencing the protective role described in this study, should not be discarded.
Concerning the haplotype analysis, the GACT haplotype was associated with an increased risk of having SA, whereas the GACC haplotype was associated with a decreased risk of presenting with subclinical disease. As expected, the main difference between the haplotype of protection and that of risk was the C allele of the rs3102735polymorphism. Considering that our data represent the first evidence in SA, additional studies are needed to validate the functional role of this genetic variant and its possible clinical impact in the progression of atherosclerosis in asymptomatic individuals. This study contributes to the knowledge in the field of molecular cardiology with the clinical interest of developing future panels of genetic markers of ischemic disease, in asymptomatic individuals, for use in preventing its progression.
5. Conclusions
Our data showed a lower genetic risk of SA in C allele carries of OPG (rs3102735) polymorphism. In our research, it was possible to distinguish one haplotype of risk and another haplotype of protection against SA. These results represent a first OPG genetic evidence in the field. However, additional studies that evaluate different populations are necessary to establish if these genetic markers are useful for a more accurate early screening of individuals at risk of asymptomatic atherosclerosis.
Author Contributions
Conceptualization, J.M.R.-P. and N.P.-H.; methodology, B.G.C.-S., B.R.-R. and V.M.B.-C.; formal analysis, R.P.-S.; resources, J.M.R.-P. and N.P.-H.; visualization and supervision, J.M.R.-P., N.P.-H. and J.R.-S.; project administration, J.M.R.-P.; funding acquisition, J.M.R.-P.; data curation, R.P.-S. and B.G.C.-S.; writing—original draft preparation, J.M.R.-P., N.P.-H., G.V.-A. and Ó.P.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded using the budget allocated for research in the Genomics Laboratory of the Molecular Biology Department of the Instituto Nacional de Cardiología Ignacio Chávez in Mexico City with funding number: 18-1071.
Institutional Review Board Statement
The study was conducted according with the Declaration of Helsinki, and approved by the Ethics and Research Committee of the Instituto Nacional de Cardiología Ignacio Chávez (protocol code 18-1071 and date of approval 28 September 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data described in this article can be requested from the corresponding author.
Acknowledgments
Part of the experiments were performed by Benny Giovanni Cazarín-Santos to obtain a Doctorate degree (Research in Medicine) by the Superior Medicine School of the Instituto Politécnico Nacional in Mexico City and the Consejo Nacional de Ciencia y Tecnología provided a scholarship with number (CVU 819447). The authors are grateful to Silvestre Ramírez-Fuentes and Marva Arellano-González for their assistance with the DNA extraction. The Instituto National de Cardiología Ignacio Chávez covered the costs of publication in open access.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Posadas-Romero, C.; López-Bautista, F. Prevalencia y extensión de la calcificación arterial coronaria en población mexicana asintomática cardiovascular: Estudio Genética de la Enfermedad Aterosclerosa. Arch. Cardiol. Mex. 2017, 87, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Valerio, J.; Rodas-Díaz, M.A. Aortic valve calcification prevalence and association with coronary risk factors and atherosclerosis in Mexican population. Arch. Cardiol. Mex. 2017, 87, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, A.; Santangelo, G. Cardiovascular Calcification as a Marker of Increased Cardiovascular Risk and a Surrogate for Subclinical Atherosclerosis: Role of Echocardiography. J. Clin. Med. 2021, 10, 1668. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, P.; Dasseni, N. Cardiac calcification as a marker of subclinical atherosclerosis and predictor of cardiovascular events: A review of the evidence. Eur. J. Prev. Cardiol. 2019, 26, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Su, S.A. Emerging roles of fibroblasts in cardiovascular calcification. J. Cell. Mol. Med. 2021, 25, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Miller, M. An emerging paradigm in atherosclerosis: Focus on subclinical disease. Postgrad. Med. 2009, 121, 49–59. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A. The Role of Osteoprotegerin in Vascular Calcification and Bone Metabolism: The Basis for Developing New Therapeutics. Calcif. Tissue Int. 2019, 105, 239–251. [Google Scholar] [CrossRef]
- Abdi, S.; Binbaz, R.A. Association of RANKL and OPG Gene Polymorphism in Arab Women with and without Osteoporosis. Genes 2021, 12, 200. [Google Scholar] [CrossRef]
- Dutka, M.; Bobiński, R. Osteoprotegerin and RANKL-RANK-OPG-TRAIL signalling axis in heart failure and other cardiovascular diseases. Heart Fail. Rev. 2021, 1–17. [Google Scholar] [CrossRef]
- Kiani, A.N.; Aukrust, P. Serum osteoprotegrin (OPG) in subclinical atherosclerosis in systemic lupus erythematosus. Lupus 2017, 26, 865–870. [Google Scholar] [CrossRef]
- Dekker, M.; Waissi, F. High levels of osteoprotegerin are associated with coronary artery calcification in patients suspected of a chronic coronary syndrome. Sci. Rep. 2021, 11, 18946. [Google Scholar] [CrossRef] [PubMed]
- Makarović, S.; Makarović, Z. Osteoprotegerin and Vascular Calcification: Clinical and Prognostic Relevance. Coll. Antropol. 2015, 39, 461–468. [Google Scholar] [PubMed]
- Higgins, C.L.; Isbilir, S. Distribution of alkaline phosphatase, osteopontin, RANK ligand and osteoprotegerin in calcified human carotid atheroma. Protein J. 2015, 34, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Luna-Luna, M.; Cruz-Robles, D. Differential expression of osteopontin, and osteoprotegerin mRNA in epicardial adipose tissue between patients with severe coronary artery disease and aortic valvular stenosis: Association with HDL subclasses. Lipids Health Dis. 2017, 16, 156. [Google Scholar] [CrossRef]
- Cottin, Y.; Issa, R. Association between Serum Osteoprotegerin Levels and Severity of Coronary Artery Disease in Patients with Acute Myocardial Infarction. J. Clin. Med. 2021, 10, 4326. [Google Scholar] [CrossRef]
- Strobescu-Ciobanu, C.; Giuşcă, S.E. Osteopontin and osteoprotegerin in atherosclerotic plaque—Are they significant markers of plaque vulnerability? Rom. J. Morphol. Embryol. 2020, 61, 793–801. [Google Scholar] [CrossRef]
- Pérez de Ciriza, C.; Moreno, M. Circulating osteoprotegerin is increased in the metabolic syndrome and associates with subclinical atherosclerosis and coronary arterial calcification. Clin. Biochem. 2014, 47, 272–278. [Google Scholar] [CrossRef]
- Hakimi, M.; Hyhlik-Dürr, A. The expression of glycophorin A and osteoprotegerin is locally increased in carotid atherosclerotic lesions of symptomatic compared to asymptomatic patients. Int. J. Mol. Med. 2013, 32, 331–338. [Google Scholar] [CrossRef][Green Version]
- Miramontes-González, J.P.; Usategui-Martín, R. VEGFR2 and OPG genes modify the risk of subclinical coronary atherosclerosis in patients with familial hypercholesterolemia. Atherosclerosis 2019, 285, 17–22. [Google Scholar] [CrossRef]
- Pleskovič, A.; Ramuš, S.M. Polymorphism rs2073618 of the osteoprotegerin gene as a potential marker of subclinical carotid atherosclerosis in Caucasians with type 2 diabetes mellitus. Vasa 2017, 46, 355–362. [Google Scholar] [CrossRef][Green Version]
- Villarreal-Molina, T.; Posadas-Romero, C. The ABCA1 gene R230C variant is associated with decreased risk of premature coronary artery disease: The genetics of atherosclerotic disease (GEA) study. PLoS ONE 2012, 7, e49285. [Google Scholar] [CrossRef] [PubMed]
- Posadas-Sánchez, R.; López-Uribe Á, R. Association of the I148M/PNPLA3 (rs738409) polymorphism with premature coronary artery disease, fatty liver, and insulin resistance in type 2 diabetic patients and healthy controls. The GEA study. Immunobiology 2017, 222, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Medina-Urrutia, A.; Posadas-Romero, C. Role of adiponectin and free fatty acids on the association between abdominal visceral fat and insulin resistance. Cardiovasc. Diabetol. 2015, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Avila, M.; Romieu, I. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998, 40, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Mautner, G.C.; Mautner, S.L. Coronary artery calcification: Assessment with electron beam CT and histomorphometric correlation. Radiology 1994, 192, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Messeguer, X.; Escudero, R. PROMO: Detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 2002, 18, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Taylor, J.A. SNPinfo: Integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 2009, 37, W600–W605. [Google Scholar] [CrossRef]
- Zhao, H.; Cao, Y. The association between OPG rs3102735 gene polymorphism, microembolic signal and stroke severity in acute ischemic stroke patients. Gene 2017, 613, 25–29. [Google Scholar] [CrossRef]
- Soufi, M.; Schoppet, M. Osteoprotegerin gene polymorphisms in men with coronary artery disease. J. Clin. Endocrinol. Metab. 2004, 89, 3764–3768. [Google Scholar] [CrossRef][Green Version]
- Pérez-Hernández, N.; Posadas-Sánchez, R. Genetic Variants and Haplotypes in OPG Gene Are Associated with Premature Coronary Artery Disease and Traditional Cardiovascular Risk Factors in Mexican Population: The GEA Study. DNA Cell Biol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef]
- Rhee, E.J.; Oh, K.W. The relationship between four single nucleotide polymorphisms in the promoter region of the osteoprotegerin gene and aortic calcification or coronary artery disease in Koreans. Clin. Endocrinol. 2006, 64, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Alkady, E.A.; Selim, Z.I. Association of serum osteoprotegerin and osteoprotegerin gene polymorphism with subclinical carotid artery atherosclerosis and disease activity in rheumatoid arthritis patients. Egypt. Rheumatol. 2020, 42, 183–188. [Google Scholar] [CrossRef]
- Barquera, R.; Hernández-Zaragoza, D.I. The immunogenetic diversity of the HLA system in Mexico correlates with underlying population genetic structure. Hum. Immunol 2020, 81, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Del Angel-Pablo, A.D.; Juárez-Martín, A.I. HLA Allele and Haplotype Frequencies in Three Urban Mexican Populations: Genetic Diversity for the Approach of Genomic Medicine. Diagnostics 2020, 10, 47. [Google Scholar] [CrossRef]
- Salazar-Flores, J.; Dondiego-Aldape, R. Population structure and paternal admixture landscape on present-day Mexican-Mestizos revealed by Y-STR haplotypes. Am. J. Hum. Biol. 2010, 22, 401–409. [Google Scholar] [CrossRef]
- Juárez-Cedillo, T.; Zuñiga, J. Genetic admixture and diversity estimations in the Mexican Mestizo population from Mexico City using 15 STR polymorphic markers. Forensic Sci. Int. Genet. 2008, 2, e37–e39. [Google Scholar] [CrossRef]
- Luna-Luna, M.; Zentella-Dehesa, A. Epicardial Adipose Tissue in the Progression and Calcification of the Coronary Artery Disease. In Biochemistry of Cardiovascular Dysfunction in Obesity; Springer: Berlin/Heidelberg, Germany, 2020; pp. 195–213. [Google Scholar]
- Min, H.; Morony, S. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J. Exp. Med. 2000, 192, 463–474. [Google Scholar] [CrossRef]
- Schoppet, M.; Preissner, K.T. RANK ligand and osteoprotegerin: Paracrine regulators of bone metabolism and vascular function. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 549–553. [Google Scholar] [CrossRef]
- Gunes, M.; Temizkan, S. Serum osteoprotegerin levels, endothelial function and carotid intima-media thickness in type 2 diabetic patients. J. Diabetes Complicat. 2021, 35, 108073. [Google Scholar] [CrossRef]
- Krzanowski, M.; Krzanowska, K. Elevated Circulating Osteoprotegerin Levels in the Plasma of Hemodialyzed Patients With Severe Artery Calcification. Ther. Apher. Dial. 2018, 22, 519–529. [Google Scholar] [CrossRef]
- Gaudio, A.; Privitera, F. Relationships between osteoprotegerin, receptor activator of the nuclear factor kB ligand serum levels and carotid intima-media thickness in patients with type 2 diabetes mellitus. Panminerva Med. 2014, 56, 221–225. [Google Scholar] [PubMed]
- Akinci, B.; Demir, T. Serum osteoprotegerin is associated with carotid intima media thickness in women with previous gestational diabetes. Diabetes Res. Clin. Pract. 2008, 82, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kalvakolanu, D.V.; Roy, S.K. CCAAT/enhancer binding proteins and interferon signaling pathways. J. Interferon Cytokine Res. 2005, 25, 757–769. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).