The Role of Circulating Collagen Turnover Biomarkers and Late Gadolinium Enhancement in Patients with Non-Ischemic Dilated Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Characteristics
2.2. Circulating Collagen Turnover Biomarkers
2.3. CMR Measurements
2.4. Clinical Outcome
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
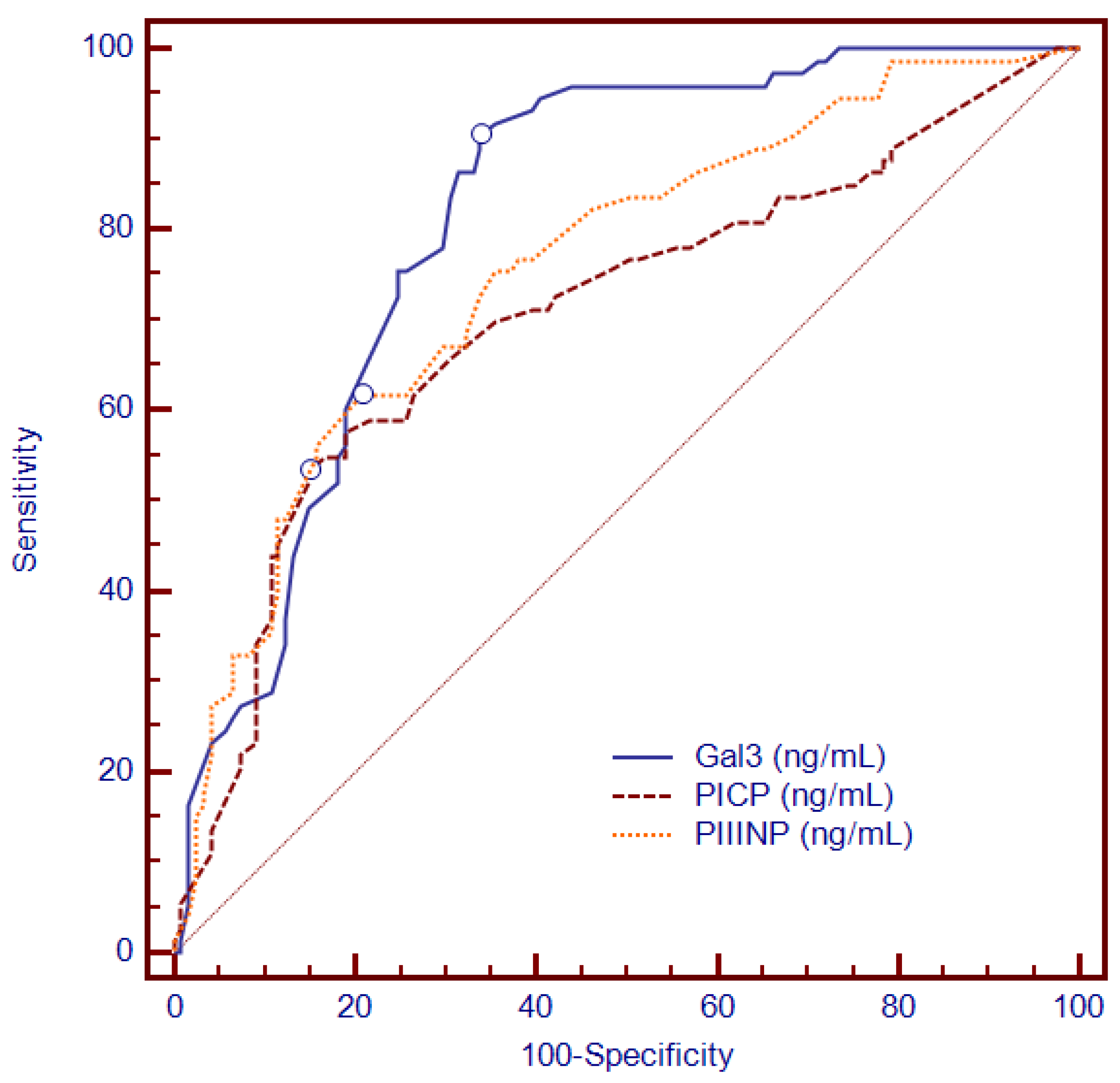
3.2. Association between Circulating Collagen Turnover Biomarkers and LGE
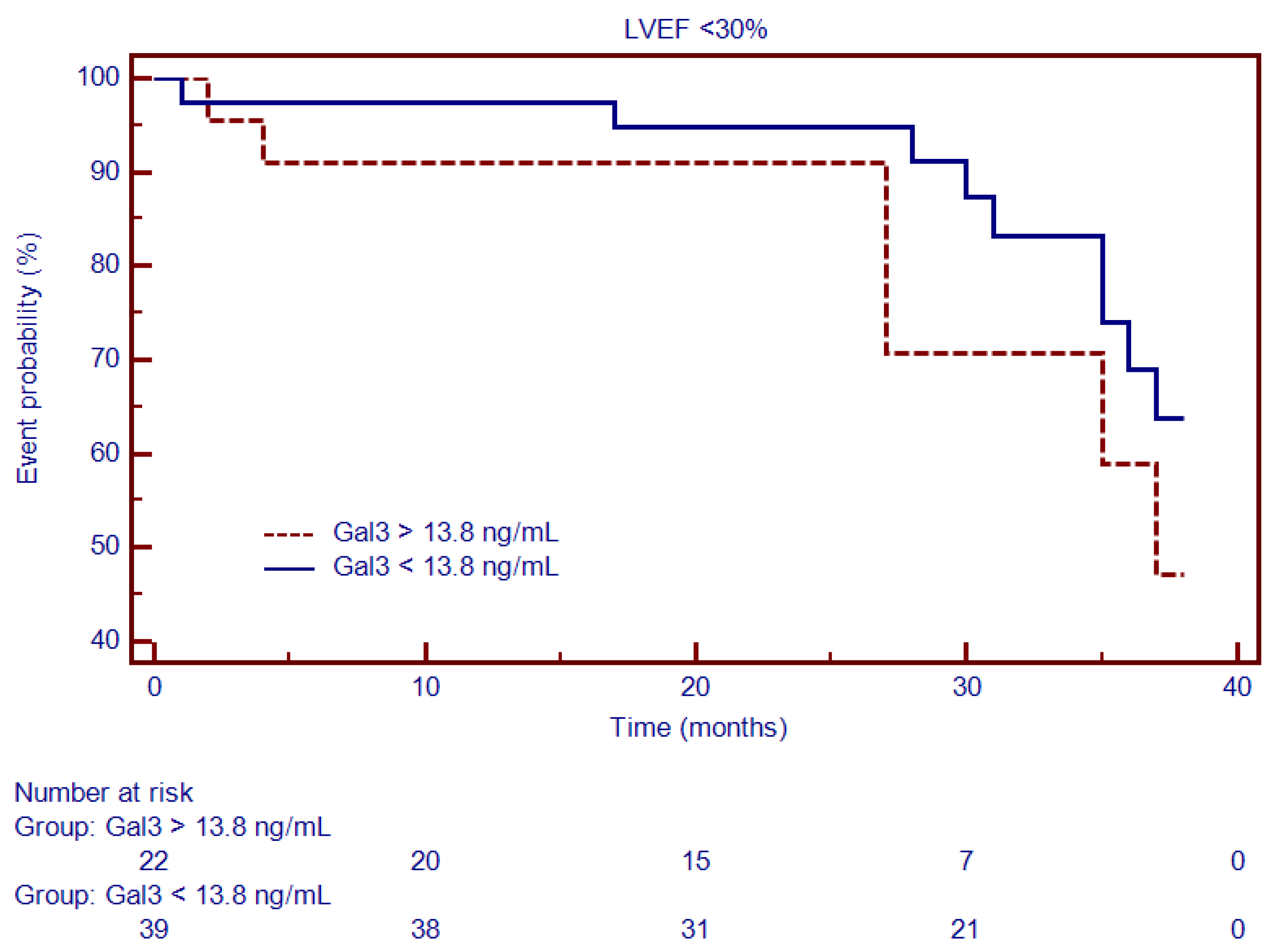
3.3. Characterization of Patients with DCM and Severely Decreased LV Function
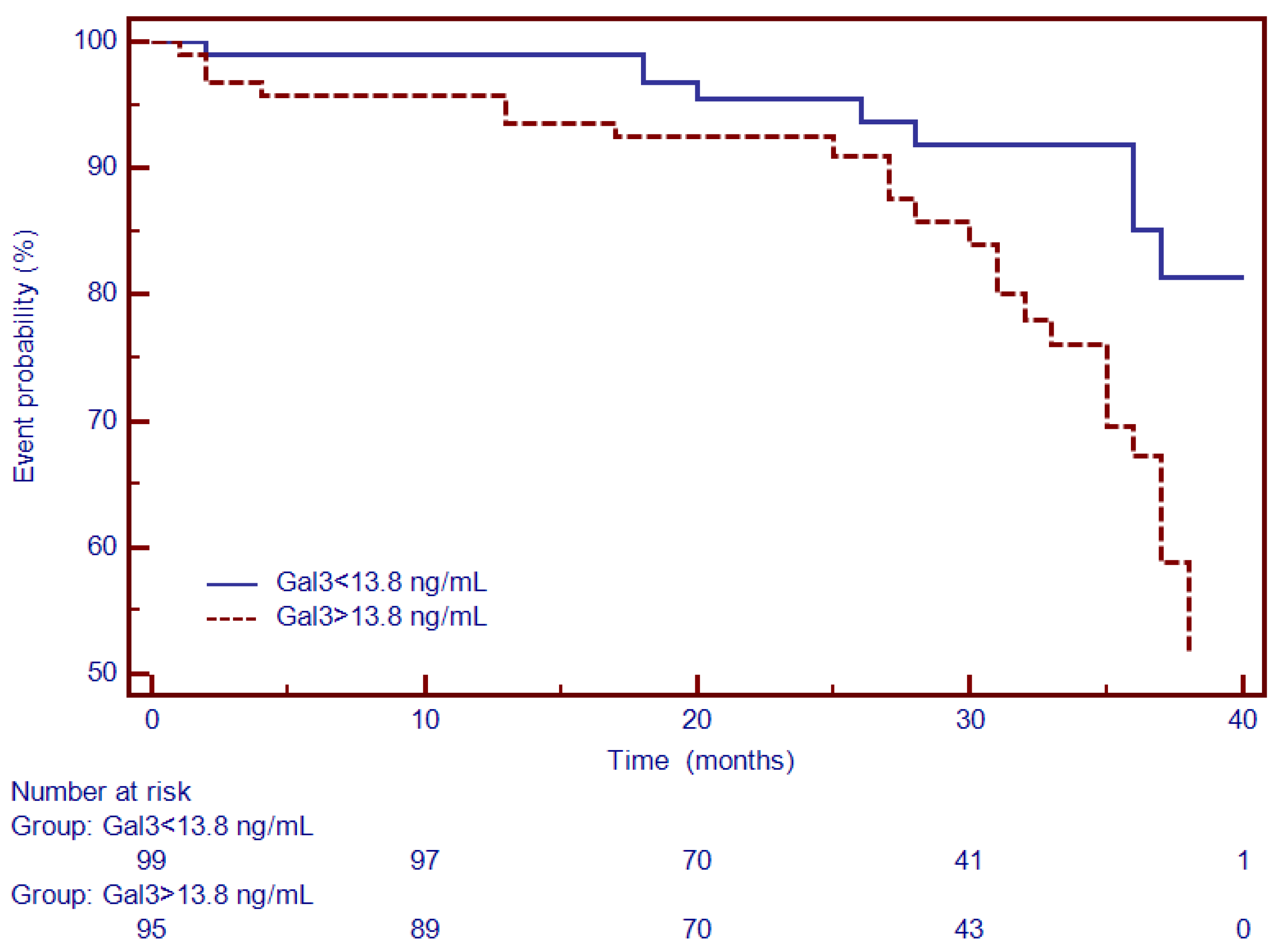
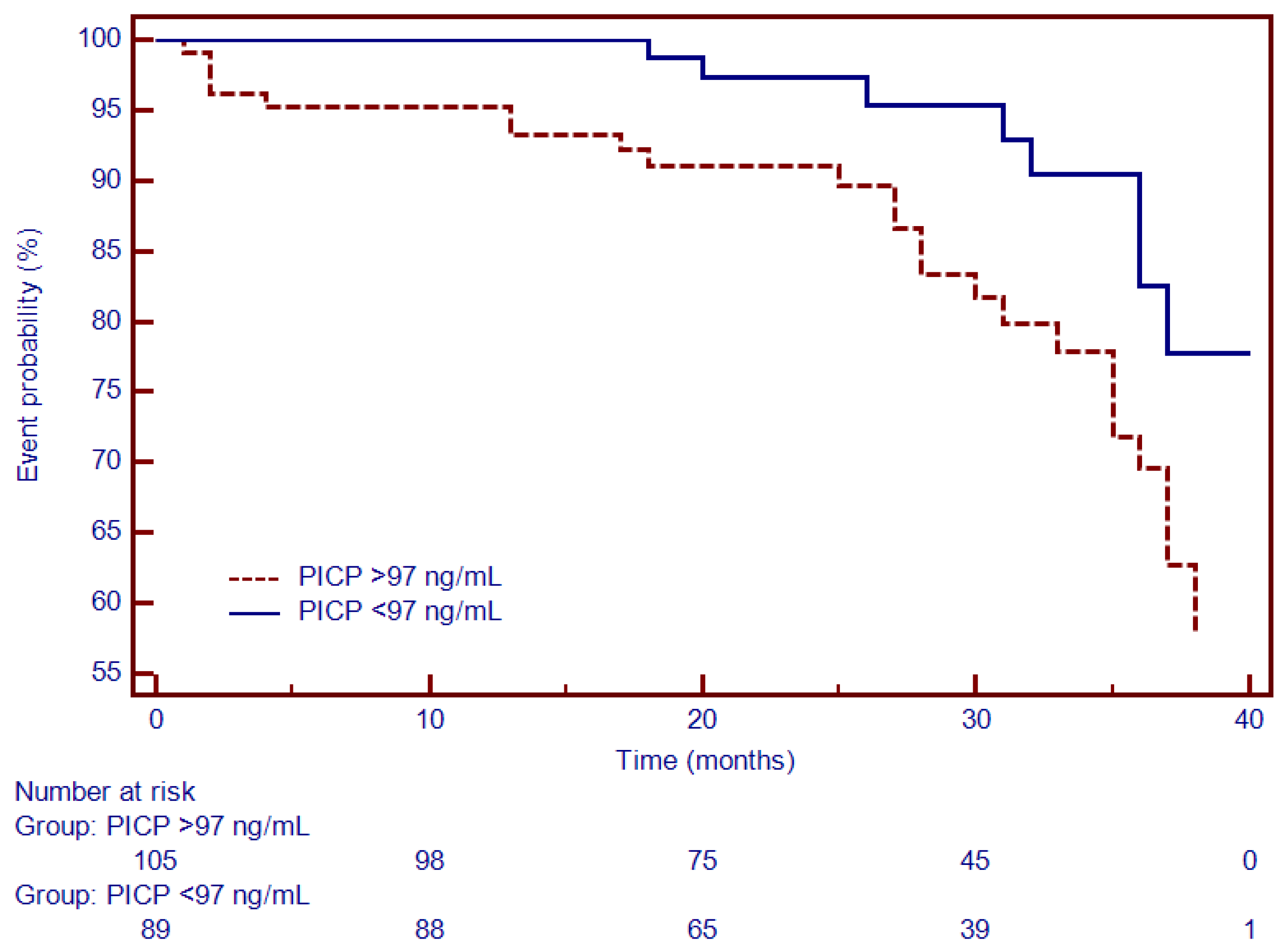
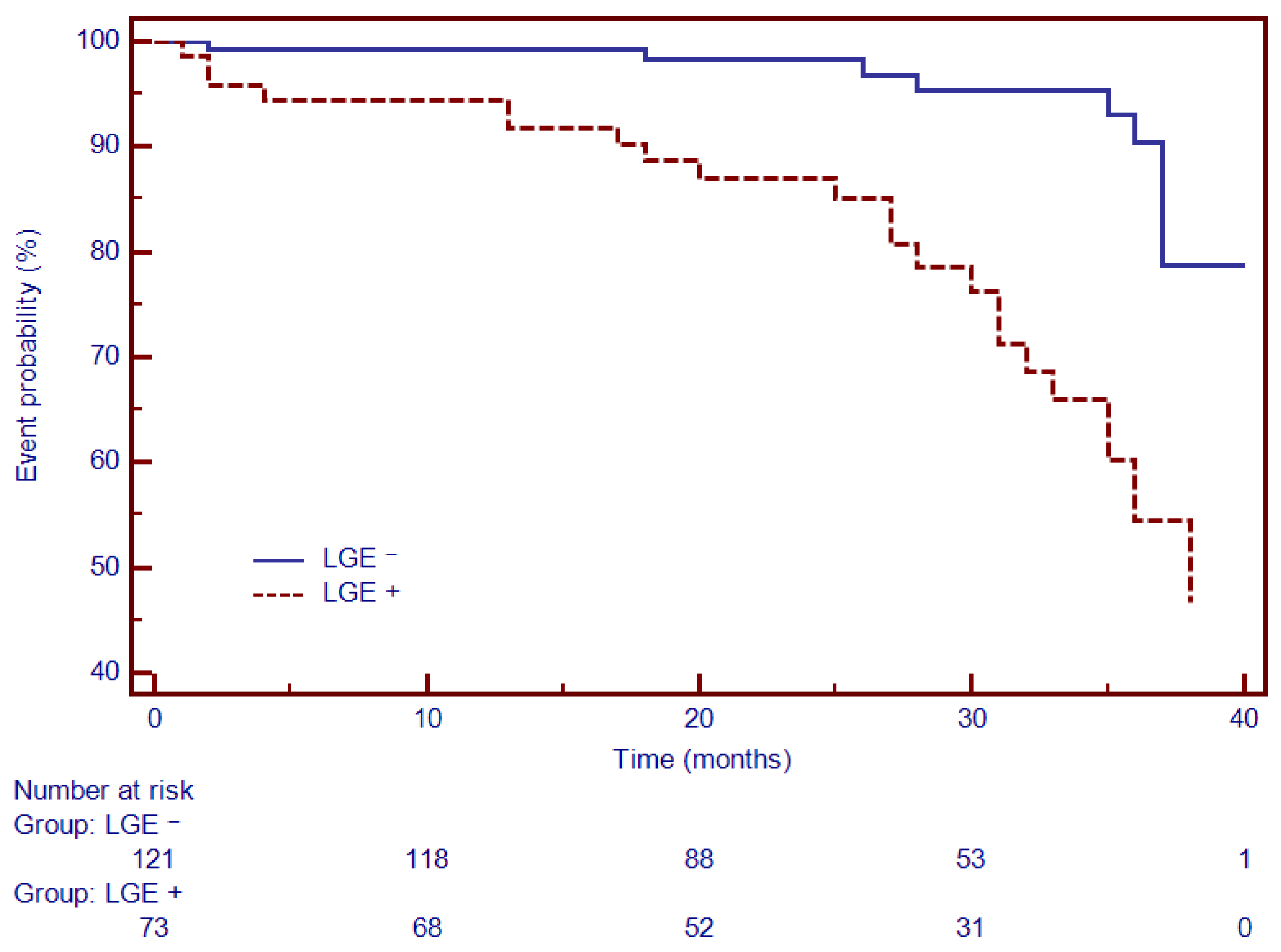
3.4. Univariate and Multivariate Cox Analysis and Time-To-Event Analysis of LGE and Circulating Collagen Turnover Biomarkers
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.H.; Ismail, N.A.; Dweck, M.R.; et al. Association of Fibrosis with Mortality and Sudden Cardiac Death in Patients with Nonischemic Dilated Cardiomyopathy. JAMA 2013, 309, 896. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.M.; Greve, A.M.; Aspelund, T.; Schelbert, E.B.; Cao, J.J.; Danielsen, R.; Þorgeirsson, G.; Sigurðsson, S.; Eiríksdóttir, G.; Harris, T.B.; et al. Prevalence and prognosis of ischaemic and non-ischaemic myocardial fibrosis in older adults. Eur. Heart J. 2019, 40, 529–538. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; De Keulenaer, G.; Bauersachs, J.; Brutsaert, D.; Cleland, J.G.; Diez, J.; Du, X.J.; Ford, P.; Heinzel, F.R.; Lipson, K.E.; et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 272–285. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Schelbert, E.B.; Díez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef]
- Weir, R.A.P.; Petrie, C.J.; Murphy, C.A.; Clements, S.; Steedman, T.; Miller, A.M.; McInnes, I.B.; Squire, I.B.; Ng, L.L.; Dargie, H.J.; et al. Galectin-3 and Cardiac Function in Survivors of Acute Myocardial Infarction. Circ. Heart Fail. 2013, 6, 492–498. [Google Scholar] [CrossRef]
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.M.; Schroen, B.; André, S.; Crijns, H.J.G.M.; Gabius, H.-J.; Maessen, J.; et al. Galectin-3 Marks Activated Macrophages in Failure-Prone Hypertrophied Hearts and Contributes to Cardiac Dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- Lok, D.J.A.; Van Der Meer, P.; de la Porte, P.W.B.-A.; Lipsic, E.; Van Wijngaarden, J.; Hillege, H.L.; van Veldhuisen, D.J. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the DEAL-HF study. Clin. Res. Cardiol. 2010, 99, 323–328. [Google Scholar] [CrossRef]
- Lok, D.J.; Lok, S.I.; Bruggink-André de la Porte, P.W.; Badings, E.; Lipsic, E.; van Wijngaarden, J.; de Boer, R.A.; van Veldhuisen, D.J.; van der Meer, P. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin. Res. Cardiol. 2013, 102, 103–110. [Google Scholar] [CrossRef]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; de Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 Mediates Aldosterone-Induced Vascular Fibrosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-Y.; Rabinovich, G.A.; Liu, F.-T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef] [PubMed]
- Masci, P.G.; Doulaptsis, C.; Bertella, E.; Del Torto, A.; Symons, R.; Pontone, G.; Barison, A.; Droogné, W.; Andreini, D.; Lorenzoni, V.; et al. Incremental Prognostic Value of Myocardial Fibrosis in Patients With Non–Ischemic Cardiomyopathy Without Congestive Heart Failure. Circ. Heart Fail. 2014, 7, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ruifrok, W.P.T.; Meissner, M.; Bos, E.M.; van Goor, H.; Sanjabi, B.; van der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and Pharmacological Inhibition of Galectin-3 Prevents Cardiac Remodeling by Interfering With Myocardial Fibrogenesis. Circ. Heart Fail. 2013, 6, 107–117. [Google Scholar] [CrossRef]
- Agoston-Coldea, L.; Lupu, S.; Petrovai, D.; Mocan, T.; Mousseaux, E. Correlations between echocardiographic parameters of right ventricular dysfunction and Galectin-3 in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Med. Ultrason. 2015, 17, 486–495. [Google Scholar] [CrossRef][Green Version]
- de Boer, R.A.; Lok, D.J.A.; Jaarsma, T.; van der Meer, P.; Voors, A.A.; Hillege, H.L.; van Veldhuisen, D.J. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann. Med. 2011, 43, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, G.; Del Franco, A.; Giannoni, A.; Prontera, C.; Ripoli, A.; Barison, A.; Masci, P.G.; Aquaro, G.D.; Cohen Solal, A.; Padeletti, L.; et al. Galectin-3 and myocardial fibrosis in nonischemic dilated cardiomyopathy. Int. J. Cardiol. 2015, 184, 96–100. [Google Scholar] [CrossRef]
- Raafs, A.G.; Verdonschot, J.A.J.; Henkens, M.T.; Adriaans, B.P.; Wang, P.; Derks, K.; Abdul Hamid, M.A.; Knackstedt, C.; Empel, V.P.M.; Díez, J.; et al. The combination of carboxy-terminal propeptide of procollagen type I blood levels and late gadolinium enhancement at cardiac magnetic resonance provides additional prognostic information in idiopathic dilated cardiomyopathy—A multilevel assessment of myocardial fibrosis in dilated cardiomyopathy. Eur. J. Heart Fail. 2021, 23, 933–944. [Google Scholar] [CrossRef]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Kocaoglu, M.; Pednekar, A.; Tkach, J.A.; Taylor, M.D. Quantitative assessment of velocity and flow using compressed SENSE in children and young adults with adequate acquired temporal resolution. J. Cardiovasc. Magn. Reson. 2021, 23, 113. [Google Scholar] [CrossRef]
- Arenja, N.; Andre, F.; Riffel, J.H.; Hegenbart, U.; Schönland, S.; Kristen, A.V.; Katus, H.A.; Buss, S.J. Prognostic value of novel imaging parameters derived from standard cardiovascular magnetic resonance in high risk patients with systemic light chain amyloidosis. J. Cardiovasc. Magn. Reson. 2019, 21, 53. [Google Scholar] [CrossRef]
- Liang, Y.; Li, W.; Zeng, R.; Sun, J.; Wan, K.; Xu, Y.; Cao, Y.; Zhang, Q.; Han, Y.; Chen, Y. Left Ventricular Spherical Index Is an Independent Predictor for Clinical Outcomes in Patients with Nonischemic Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2019, 12, 1578–1580. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Beek, A.; Hofman, M.; Kühl, H.; Twisk, J.; van Dockum, W.; Visser, C.; van Rossum, A. Standardizing the Definition of Hyperenhancement in the Quantitative Assessment of Infarct Size and Myocardial Viability Using Delayed Contrast-Enhanced CMR. J. Cardiovasc. Magn. Reson. 2005, 7, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Cojan-Minzat, B.O.; Zlibut, A.; Agoston-Coldea, L. Non-ischemic dilated cardiomyopathy and cardiac fibrosis. Heart Fail. Rev. 2021, 26, 1081–1101. [Google Scholar] [CrossRef] [PubMed]
- Halliday, B.P.; Baksi, A.J.; Gulati, A.; Ali, A.; Newsome, S.; Izgi, C.; Arzanauskaite, M.; Lota, A.; Tayal, U.; Vassiliou, V.S.; et al. Outcome in Dilated Cardiomyopathy Related to the Extent, Location, and Pattern of Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2019, 12, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Chakrabarty, A.; Shipp, N.; Molaee, P.; Madsen, P.L.; Joerg, L.; Sullivan, T.; Worthley, S.G.; De Pasquale, C.G.; Sanders, P.; et al. Effects of myocardial fibrosis and ventricular dyssynchrony on response to therapy in new-presentation idiopathic dilated cardiomyopathy: Insights from cardiovascular magnetic resonance and echocardiography. Eur. Heart J. 2012, 33, 640–648. [Google Scholar] [CrossRef]
- Nguyen, M.-N.; Ziemann, M.; Kiriazis, H.; Su, Y.; Thomas, Z.; Lu, Q.; Donner, D.G.; Zhao, W.-B.; Rafehi, H.; Sadoshima, J.; et al. Galectin-3 deficiency ameliorates fibrosis and remodeling in dilated cardiomyopathy mice with enhanced Mst1 signaling. Am. J. Physiol. Circ. Physiol. 2019, 316, H45–H60. [Google Scholar] [CrossRef]
- López, B.; González, A.; Ravassa, S.; Beaumont, J.; Moreno, M.U.; San José, G.; Querejeta, R.; Díez, J. Circulating Biomarkers of Myocardial Fibrosis. J. Am. Coll. Cardiol. 2015, 65, 2449–2456. [Google Scholar] [CrossRef]
- Aoki, T.; Fukumoto, Y.; Sugimura, K.; Oikawa, M.; Satoh, K.; Nakano, M.; Nakayama, M.; Shimokawa, H. Prognostic Impact of Myocardial Interstitial Fibrosis in Non-Ischemic Heart Failure. Circ. J. 2011, 75, 2605–2613. [Google Scholar] [CrossRef]
- Vigliano, C.A.; Cabeza Meckert, P.M.; Diez, M.; Favaloro, L.E.; Cortés, C.; Fazzi, L.; Favaloro, R.R.; Laguens, R.P. Cardiomyocyte Hypertrophy, Oncosis, and Autophagic Vacuolization Predict Mortality in Idiopathic Dilated Cardiomyopathy With Advanced Heart Failure. J. Am. Coll. Cardiol. 2011, 57, 1523–1531. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Rossignol, P.; Pizard, A.; Machu, J.-L.; Collier, T.; Girerd, N.; Huby, A.-C.; Gonzalez, A.; Diez, J.; López, B.; et al. Potential spironolactone effects on collagen metabolism biomarkers in patients with uncontrolled blood pressure. Heart 2019, 105, 307–314. [Google Scholar] [CrossRef]
- Xu, Y.; Li, W.; Wan, K.; Liang, Y.; Jiang, X.; Wang, J.; Mui, D.; Li, Y.; Tang, S.; Guo, J.; et al. Myocardial Tissue Reverse Remodeling After Guideline-Directed Medical Therapy in Idiopathic Dilated Cardiomyopathy. Circ. Heart Fail. 2021, 14, e007944. [Google Scholar] [CrossRef] [PubMed]



| Data | All Patients (n = 194) | LGE− (n = 121) | LGE+ (n = 73) | p |
|---|---|---|---|---|
| Clinical features | ||||
| Age, mean (SD), years | 48.7 (14.3) | 47.9 (14.7) | 50.0 (13.6) | NS |
| Masculine gender, n (%) | 144 (74.2) | 88 (72.7) | 56 (76.7) | NS |
| BMI, Kg/m2 | 27.4 (4.7) | 27.2 (4.5) | 27.6 (5.1) | NS |
| HR, mean (SD), bpm | 73 (16.0) | 70 (14.2) | 76 (17.9) | NS |
| SBP, mean (SD), mmHg | 134 (19.1) | 135 (18.5) | 131 (19.7) | NS |
| AHT, n (%) | 102 (52.5) | 72 (59.5) | 30 (41.0) | <0.05 |
| Diabetes mellitus, n (%) | 63 (32.5) | 43 (35.5) | 20 (27.4) | <0.05 |
| Dyslipidemia, n (%) | 111 (57.2) | 70 (57.8) | 41 (56.2) | NS |
| Smokers, n (%) | 65 (33.5) | 41 (33.8) | 24 (32.8) | NS |
| NYHA I/II/III class | 30/97/37 | 20/59/23 | 10/38/14 | <0.05 |
| Medication | ||||
| Betablockers, n (%) | 149 (76.8) | 93 (76.8) | 56 (76.7) | NS |
| ACEI or ARB2, n (%) | 147 (75.7) | 92 (76.0) | 56 (76.7) | NS |
| Calcium channel blockers, n (%) | 32 (16.5) | 20 (16.5) | 12 (16.4) | NS |
| Diuretics, n (%) | 118 (60.8) | 73 (60.3) | 45 (61.4) | NS |
| Biomarkers | ||||
| NT-proBNP, median (IQR), ng/L | 16,900 (8700–39,500) | 16,200 (8700–36,200) | 17,200 (10,600–39,500) | <0.001 |
| CPP, median (IQR), ng/mL | 12.7 (1.8–87) | 8.2 (1.8–68.2) | 17.1 (4.3–87) | <0.001 |
| PICP, median (IQR), ng/mL | 97 (23–347) | 74 (23–344) | 156 (38–347) | <0.001 |
| PIIINP, median (IQR), ng/mL | 4.1 (1.7–8.7) | 3.5 (1.7–7.1) | 5.1 (2.1–8.7) | <0.001 |
| Gal3, median (IQR), ng/mL | 13.8 (2.2–26.6) | 9.1 (2.2–23.6) | 17.7 (6.1–26.6) | <0.001 |
| eGFR, mean (SD), mL/min/1.73 m2 | 87.1 (21.2) | 87.7 (20.4) | 86.1 (22.6) | NS |
| CMR | ||||
| LVEDV indexed, median (SD), mL/m2 | 131.1 (34.5) | 124.2 (29.7) | 142.4 (39.1) | <0.001 |
| LVESV indexed, median (SD), mL/m2 | 86.8 (33.7) | 78.1 (28.4) | 101.2 (36.9) | <0.001 |
| LVM indexed, median (SD), g/m2 | 86.1 (20.5) | 83.3 (19.4) | 90.5 (21.6) | <0.01 |
| LVEF, median (SD), % | 35.2 (9.6) | 38.2 (7.8) | 30.3 (9.3) | <0.001 |
| LAV indexed, median (SD), mL/m2 | 55.5 (21.4) | 53.1 (20.4) | 60.6 (22.2) | <0.05 |
| LV-LAS, median (SD), % | −9.7 (5.3) | −10.5 (5.1) | −8.5 (5.4) | <0.001 |
| LVSI, median (SD) | 0.41 (0.13) | 0.38 (0.15) | 0.44 (0.12) | <0.001 |
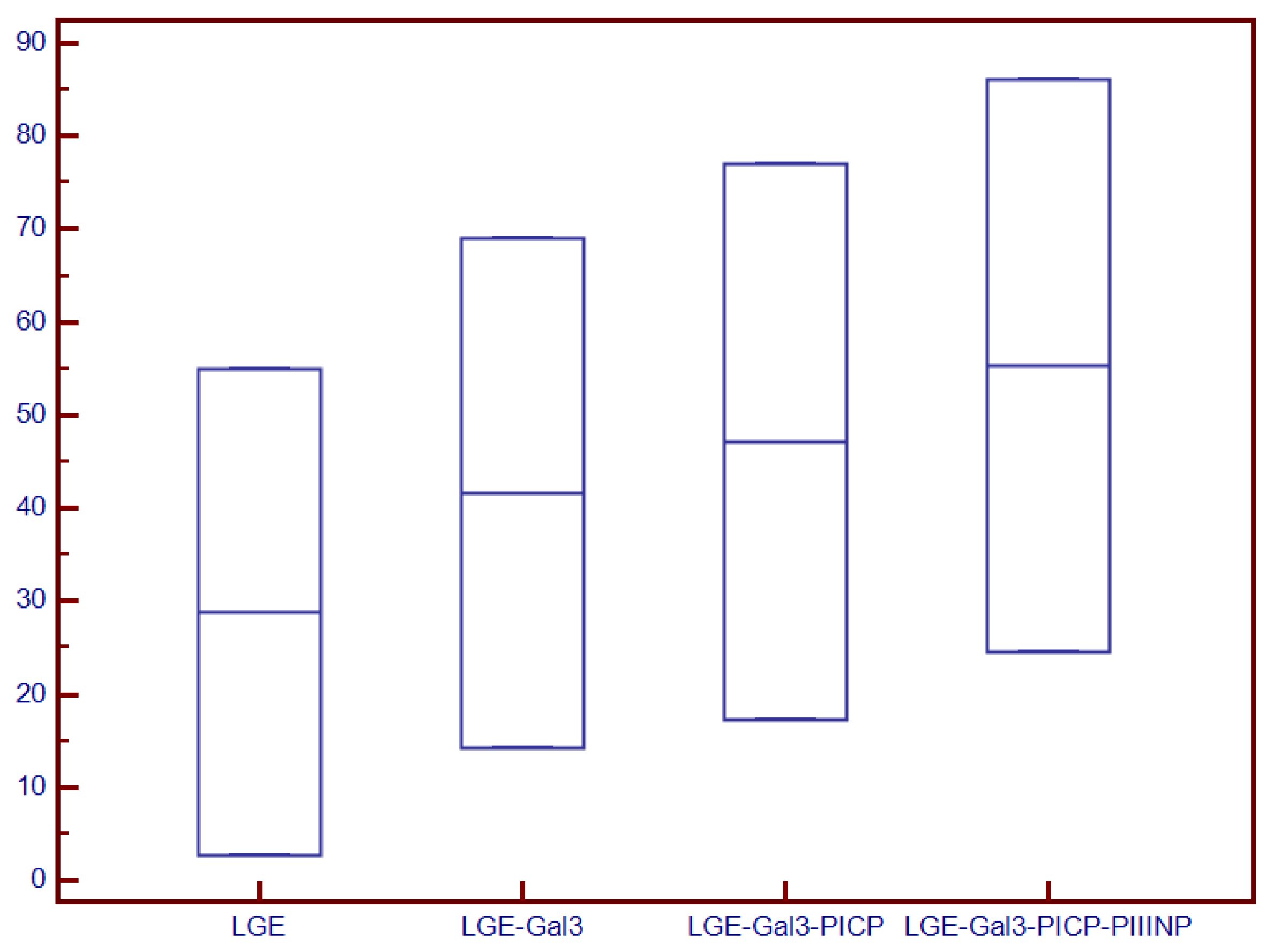
| LGE mass, median (IQR), g | - | - | 31.2 (1–89) | N/A |
| LGE mass/LVM, median (IQR), % | - | - | 18.4 (0.6–56) | N/A |
| Data | All Patients (n = 194) | LVEF 31–45% (n = 131) | LVEF < 30% (n = 61) | p |
|---|---|---|---|---|
| NT-proBNP, median (IQR), ng/L | 16,900 (8700–39,500) | 16,200 (8700–36,200) | 17,500 (10,500–39,500) | <0.01 |
| CPP, median (IQR), ng/mL | 12.7 (1.8–87) | 9.5 (1.8–68.2) | 17.5 (3.2–87) | <0.001 |
| PICP, median (IQR), ng/mL | 97 (23–347) | 79 (23–344) | 147 (32–347) | <0.001 |
| PIIINP, median (IQR), ng/mL | 4.1 (1.7–8.7) | 3.9 (1.7–8.7) | 4.5 (1.9–8.7) | <0.001 |
| Gal3, median (IQR), ng/mL | 13.8 (2.2–26.6) | 9.6 (2.2–26.6) | 17.7 (3.1–23.6) | <0.001 |
| eGFR, mean (SD), mL/min/1.73 m2 | 87.1 (21.2) | 87.7 (20.4) | 86.1 (22.6) | NS |
| LVEDV indexed, median (SD), mL/m2 | 131.1 (34.5) | 117.4 (21.6) | 160.7 (38.8) | <0.001 |
| LVESV indexed, median (SD), mL/m2 | 86.8 (33.7) | 69.9 (15.7) | 124.2 (32.3) | <0.001 |
| LVM indexed, median (SD), g/m2 | 86.1 (20.5) | 80.9 (17.7) | 97.1 (21.9) | <0.01 |
| LAV indexed, median (SD), mL/m2 | 55.5 (21.4) | 51.7 (19.5) | 63.7 (22.8) | <0.01 |
| LAS, median (SD), % | −9.7 (5.3) | −11.6 (5.1) | −5.7 (2.5) | <0.001 |
| LVSI, median (SD) | 0.41 (0.13) | 0.37 (0.09) | 0.46 (0.13) | <0.001 |
| LGE mass, median (IQR), g | 14.2 (0.9–88) | 6.4 (0.9–71.1) | 31.2 (1–88) | <0.001 |
| LGE mass/LVM, median (IQR), % | 8.8 (0.6–64.2) | 4.5 (0.6–44.7) | 18.4 (16.9–64.2) | N/A |
| Data | All Patients (n = 194) | MACEs− (n = 161) | MACEs+ (n = 33) | p |
|---|---|---|---|---|
| Clinical features | ||||
| Age, mean (SD), years | 48.7 (14.3) | 48.5 (13.6) | 49.3 (17.7) | NS |
| Masculine gender, n (%) | 144 (74.2) | 121 (84.0) | 23 (26.0) | NS |
| BMI, Kg/m2 | 27.4 (4.7) | 27.6 (4.7) | 26.1 (4.4) | NS |
| HR, mean (SD), bpm | 73 (16.0) | 72 (15.4) | 75 (18.2) | NS |
| SBP, mean (SD), mmHg | 134 (19.1) | 135 (19.2) | 131 (17.9) | NS |
| AHT, n (%) | 102 (52.5) | 87 (85.2) | 15 (14.8) | <0.001 |
| Diabetes mellitus, n (%) | 63 (32.5) | 52 (82.5) | 11 (17.5) | <0.001 |
| Dyslipidemia, n (%) | 111 (57.2) | 91 (81.9) | 20 (18.1) | <0.001 |
| Smokers, n (%) | 65 (33.5) | 57 (87.7) | 8 (12.3) | <0.001 |
| NYHA I/II/III class | 30/97/37 | 21/91/31 | 9/6/6 | <0.05 |
| Medication | ||||
| Betablockers, n (%) | 149 (76.8) | 124 (83.2) | 25 (16.8) | <0.001 |
| ACEI or ARB2, n (%) | 147 (75.7) | 125 (85.0) | 22 (15.0) | <0.001 |
| Calcium channel blockers, n (%) | 32 (16.5) | 22 (68.7) | 8 (31.3) | <0.001 |
| Diuretics, n (%) | 118 (60.8) | 93 (78.8) | 25 (21.2) | <0.001 |
| Biomarkers | ||||
| NT-proBNP, median (IQR), ng/L | 16,900 (8700–39,500) | 14,000 (8700–36,600) | 19,300 (10,200–39,500) | <0.001 |
| CPP, median (IQR), ng/mL | 12.7 (1.8–87) | 9.9 (1.8–87) | 16.2 (3.1–82.9) | <0.001 |
| PICP, median (IQR), ng/mL | 97 (23–347) | 92 (23–347) | 118 (32–338) | <0.001 |
| PIIINP, median (IQR), ng/mL | 4.1 (1.7–8.7) | 4.0 (1.7–8.3) | 4.5 (2.1–8.7) | <0.01 |
| Gal3, median (IQR), ng/mL | 13.8 (2.2–26.6) | 11 (2.2–26.6) | 17.2 (3.0–24.0) | 0.001 |
| eGFR, mean (SD), mL/min/1.73 m2 | 87.1 (21.2) | 86.1 (19.7) | 89.6 (25.8) | NS |
| CMR | ||||
| LVEDV indexed, median (SD), mL/m2 | 131.1 (34.5) | 130.4 (35.0) | 134.7 (32.7) | NS |
| LVESV indexed, median (SD), mL/m2 | 86.8 (33.7) | 85.4 (33.9) | 93.7 (32.4) | NS |
| LVM indexed, median (SD), g/m2 | 86.1 (20.5) | 85.9 (20.5) | 86.4 (20.6) | NS |
| LVEF, median (SD), % | 35.2 (9.6) | 35.9 (9.2) | 31.7 (9.1) | <0.01 |
| LAV indexed, median (SD), mL/m2 | 55.5 (21.4) | 54.2 (21.7) | 61.7 (18.4) | NS |
| LV-LAS, median (SD), % | −9.7 (5.3) | −10.2 (5.5) | −7.8 (3.5) | <0.01 |
| LVSI, median (SD) | 0.41 (0.13) | 0.38 (0.11) | 0.47 (0.13) | <0.001 |
| LGE mass, median (IQR), g | 14.3 (0–89) | 11.2 (0–86) | 29.9 (23–89) | <0.001 |
| LGE mass/LVM, median (IQR), % | 8.4 (0–56) | 6.6 (0–52.8) | 19.4 (1.2–56) | <0.001 |
| Parameters | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR Unadjusted (95% CI) | p | HR Adjusted (95% CI) | p | |
| LGE | 4.06 (1.94–8.52) | 0.0001 | 4.91 (2.06–11.6) | 0.008 |
| Gal3 | 2.67 (1.32–5.28) | 0.008 | 1.11 (1.03–1.19) | 0.04 |
| PICP | 1.04 (1.01–1.07) | 0.001 | 1.00 (0.98–1.07) | NS |
| PIIINP | 1.09 (1.02–1.11) | 0.001 | 1.00 (0.98–1.03) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revnic, R.; Cojan-Minzat, B.O.; Zlibut, A.; Orzan, R.-I.; Agoston, R.; Muresan, I.D.; Horvat, D.; Cionca, C.; Chis, B.; Agoston-Coldea, L. The Role of Circulating Collagen Turnover Biomarkers and Late Gadolinium Enhancement in Patients with Non-Ischemic Dilated Cardiomyopathy. Diagnostics 2022, 12, 1435. https://doi.org/10.3390/diagnostics12061435
Revnic R, Cojan-Minzat BO, Zlibut A, Orzan R-I, Agoston R, Muresan ID, Horvat D, Cionca C, Chis B, Agoston-Coldea L. The Role of Circulating Collagen Turnover Biomarkers and Late Gadolinium Enhancement in Patients with Non-Ischemic Dilated Cardiomyopathy. Diagnostics. 2022; 12(6):1435. https://doi.org/10.3390/diagnostics12061435
Chicago/Turabian StyleRevnic, Radu, Bianca Olivia Cojan-Minzat, Alexandru Zlibut, Rares-Ilie Orzan, Renata Agoston, Ioana Danuta Muresan, Dalma Horvat, Carmen Cionca, Bogdan Chis, and Lucia Agoston-Coldea. 2022. "The Role of Circulating Collagen Turnover Biomarkers and Late Gadolinium Enhancement in Patients with Non-Ischemic Dilated Cardiomyopathy" Diagnostics 12, no. 6: 1435. https://doi.org/10.3390/diagnostics12061435
APA StyleRevnic, R., Cojan-Minzat, B. O., Zlibut, A., Orzan, R.-I., Agoston, R., Muresan, I. D., Horvat, D., Cionca, C., Chis, B., & Agoston-Coldea, L. (2022). The Role of Circulating Collagen Turnover Biomarkers and Late Gadolinium Enhancement in Patients with Non-Ischemic Dilated Cardiomyopathy. Diagnostics, 12(6), 1435. https://doi.org/10.3390/diagnostics12061435






