Sclerosing Microcystic Adenocarcinoma Arising from the Tongue: A Case Report and Literature Review
Abstract
:1. Introduction
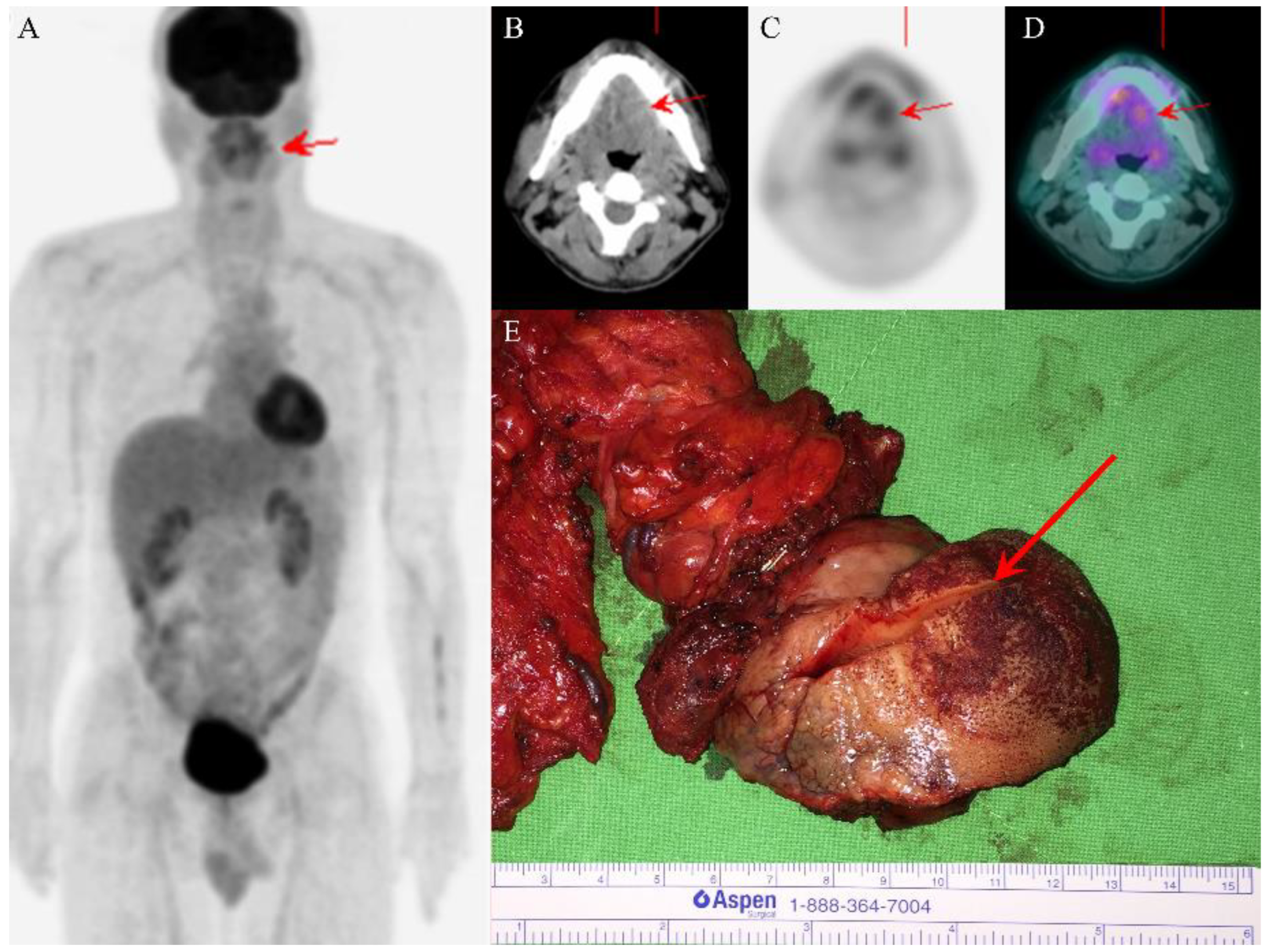
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, A.M.; Policarpio-Nicholas, M.L.; Agaimy, A.; Wick, M.R.; Mills, S.E. Sclerosing microcystic adenocarcinoma of the head and neck mucosa: A neoplasm closely resembling microcystic adnexal carcinoma. Head Neck Pathol. 2016, 10, 501–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, A.; Conn, B.I. Sclerosing microcystic adenocarcinoma of the tongue: A report of two further cases and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, e94–e102. [Google Scholar] [CrossRef] [PubMed]
- Petersson, F.; Skogvall, I.; Elmberger, G. Sclerosing sweat duct-like carcinoma of the tongue—A case report and a review of the literature. Am. J. Dermatopathol. 2009, 31, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Schipper, J.H.; Holecek, B.U.; Sievers, K.W. A tumour derived from Ebner’s glands: Microcystic adnexal carcinoma of the tongue. J. Laryngol. Otol. 1995, 109, 1211–1214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Cagaanan, A.; Hafez, G.R.; Hu, R. Sclerosing microcystic adenocarcinoma: Report of a rare case and review of literature. Head Neck Pathol. 2019, 13, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Marquez, J.; Tower, J.I.; Jacobs, D.; Chen, W.; Mehra, S.; Judson, B.L. Sequencing of sclerosing microcystic adenocarcinoma identifies mutational burden and somatic variants associated with tumorigenesis. Anticancer Res. 2020, 40, 6375–6379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, X.; Zhou, T.; Cao, H. Microcystic adnexal carcinoma: Report of rare cases. Biosci. Rep. 2020, 40, BSR20191557. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Z.L.; Goh, G.H.; Loh, K.S.; Petersson, F. Sclerosing microcystic adenocarcinoma of the parotid gland—The first recorded case with histo-cytopathologic correlation and a brief review of the literature. Ann. Diagn. Pathol. 2021, 54, 151806. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.P.; Dresser, K.A.; Kapur, P.; High, W.A.; Mahalingam, M. Microcystic adnexal carcinoma: An immunohistochemical reappraisal. Mod. Pathol. 2008, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- EI-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. (Eds.) WHO Classification of Head and Neck Tumours, 4th ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Skalova, A.; Hyrcza, M.D.; Mehrotra, R.; Wenig, B.M. Sclerosing microcystic adenocarcinoma. In WHO Classification of Tumours Editorial Board. Head and Neck Tumours: WHO Classification of Tumours Series, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2022; Volume 9, (Internet; beta version ahead of print); Available online: https://tumourclassification.iarc.who.int/chaptercontent/52/326 (accessed on 2 May 2022).

| Case | Author [Reference] | Age | Sex | Location | Immunohistochemical Results | Treatment/Follow-Up (Months) | Note |
|---|---|---|---|---|---|---|---|
| 1 | Mills et al. [1] | 41 | F | Base of tongue | Negative: CD117/c-kit | Not stated/not stated | History of soft palate adenoid cystic carcinoma s/p radiotherapy 7 years prior; no lymph node involvement |
| 2 | Mills et al. [1] | 47 | F | Anterior tongue | Not available | Not stated/not stated | No lymph node involvement |
| 3 | Mills et al. [1] | 73 | M | Nasopharynx and clivus | Positive: CK cocktail, BerEP4, S100+ myoepithelial cells. Negative: CD117/c-kit, CK20, CEA | Not stated/not stated | No lymph node involvement |
| 4 | Mills et al. [1] | 54 | F | Floor of mouth | Positive: CK cocktail, SMA+ myoepithelial cells. | Not stated/not stated | No lymph node involvement |
| 5 | Mills et al. [1] | 48 | F | Floor of mouth | Positive: CK5/6 Negative: CK7 Proliferation index (Ki-67): 5% | Chemoradiotherapy/ disease free (24) | History of AML s/p stem cell transplantation 18 years prior; no lymph node involvement |
| 6 | Wood et al. [2] | 68 | F | Tongue tip | Positive: CK7, CAM5.2, p63, S100 Negative: CK20, ER, PR, TTF-1, CDX2, SMA, Calponin, CD117/c-kit Proliferation index (Ki-67): <5% | Excised with clear margins/disease free (60) | No distant metastasis |
| 7 | Wood et al. [2] | 49 | F | Right lateral tongue | Positive: CK7, p63, S100, CK5/6 Negative: CK20, ER, PR, TTF-1, CDX2, SMA, Calponin Proliferation index (Ki-67): <5% | Excised with clear margins/disease free (14) | History of mesangiocapillary glomerulonephritis and left ovarian benign mucinous cystadenoma; no lymph node involvement; no distant metastasis |
| 8 | Petersson et al. [3] | 70 | F | Left posterior tongue | Positive: CK7, LMWCK, BerEP4, HMWCK, CK5, CK18, p63, PASD Negative: CK20, TTF-1, SMA heavy chain, SMA, bcl-2, p53, p21 Proliferation index (Ki-67): 2–4% | Excised with involved margins and adjuvant radiotherapy/disease free (21) | No lymph node involvement; no distant metastasis |
| 9 | Schipper et al. [4] | 65 | M | Tongue | Positive: CAM5.2, CEA (inner layer), EMA | Radiotherapy/no change in tumor size (21) | No distant metastasis |
| 10 | Zhang et al. [5] | 55 | F | Floor of mouth | Positive: AE1/3, p63, CK5/6, CK7, EMA, p63, p40, S100 Negative: androgen receptor, SOX10, CD34, beta-catenin, CD68, IgG, IgG4, CD117/c-kit | Excised with involved margins and adjuvant radiotherapy/disease free (10) | Multiple sclerosis and a family history of BRCA gene mutation; no distant metastasis |
| 11 | Jiang et al. [6] | 41 | F | Right tongue tip | Positive: CK7 (inner layer), p40 and p63 (outer layer) Negative: SOX10, CD117/c-kit, S100, mammoglobin, GATA3 | Excised with clear margins/not stated | History of psoriatic arthritis with immunosuppressive therapy; no lymph node involvement |
| 12 | Zhang et al. [7] | 51 | M | Left tongue | Positive: CK5/6, CK8/18, EMA, CK7, p63, S100, CD10, SMA Negative: CK20, calponin, myb, her2, bcl2, p53, CD117/c-kit, cd43 Proliferation index (Ki-67): <5% | Excision/disease free (38) | No lymph node involvement; no distant metastasis |
| 13 | Tan et al. [8] | 73 | M | Left parotid gland | Positive: EMA, CK7, SOX10, p63, S100, PAS (for eosinophilic secretions) Negative: CD117 Proliferation index (Ki-67): 5% | Excised with involved deep resection margin/disease free (4) | History of nasopharyngeal carcinoma s/p radiotherapy 23 years prior; concurrent tonsillar SCC; no lymph node involvement |
| 14 | Current case | 48 | F | Left tongue | Positive: CK7, p40, p63 Negative: CD117/c-kit Proliferation index (Ki-67): <5% | Excised with involved margins/disease free (30) | History of right tongue SCC s/p partial glossectomy 9 years prior; no lymph node involvement; no distant metastasis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-Y.; Hwang, T.-Z.; Jin, Y.-T.; Chen, C.-C. Sclerosing Microcystic Adenocarcinoma Arising from the Tongue: A Case Report and Literature Review. Diagnostics 2022, 12, 1288. https://doi.org/10.3390/diagnostics12051288
Lee Y-Y, Hwang T-Z, Jin Y-T, Chen C-C. Sclerosing Microcystic Adenocarcinoma Arising from the Tongue: A Case Report and Literature Review. Diagnostics. 2022; 12(5):1288. https://doi.org/10.3390/diagnostics12051288
Chicago/Turabian StyleLee, Yi-Ying, Tzer-Zen Hwang, Ying-Tai Jin, and Chien-Chin Chen. 2022. "Sclerosing Microcystic Adenocarcinoma Arising from the Tongue: A Case Report and Literature Review" Diagnostics 12, no. 5: 1288. https://doi.org/10.3390/diagnostics12051288
APA StyleLee, Y.-Y., Hwang, T.-Z., Jin, Y.-T., & Chen, C.-C. (2022). Sclerosing Microcystic Adenocarcinoma Arising from the Tongue: A Case Report and Literature Review. Diagnostics, 12(5), 1288. https://doi.org/10.3390/diagnostics12051288







