Detection of the Lateral Thermal Spread during Bipolar Vessel Sealing in an Ex Vivo Model—Preliminary Results
Abstract
1. Introduction
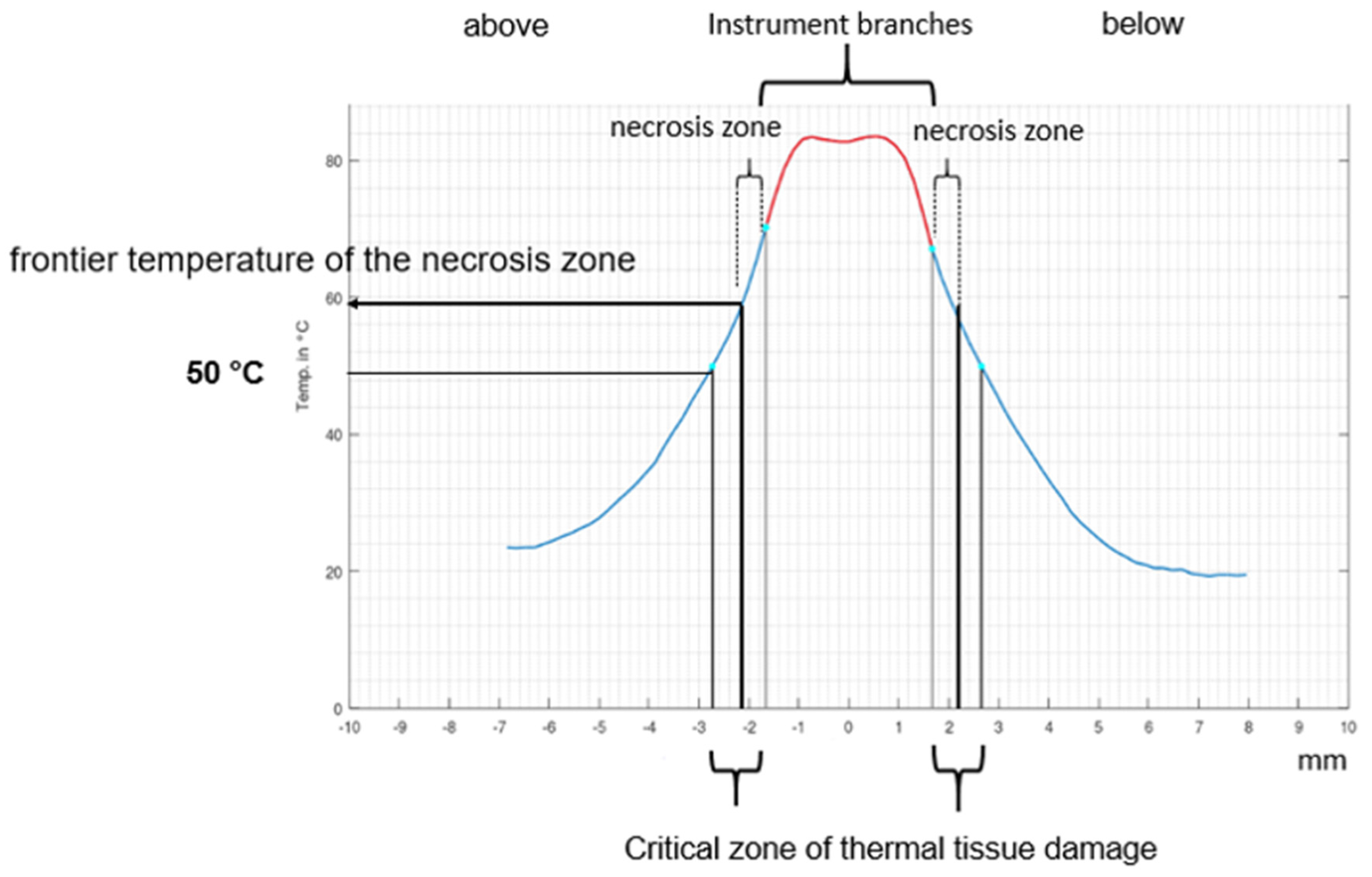
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Botteri, E.; Podda, M.; Arezzo, A.; Vettoretto, N.; Sartori, A.; Agrusa, A.; Allaix, M.E.; Anania, G.; Contul, R.B.; Caracino, V.; et al. Current status on the adoption of high energy devices in Italy: An Italian Society for Endoscopic Surgery and New Technologies (SICE) national survey. Surg. Endosc. 2021, 35, 6201–6211. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, K.; Kaya, I.; Turhal, G.; Ozturk, A.; Gursan, G.; Akyildiz, S. A comparison of electrothermal bipolar vessel sealing system and electrocautery in selective neck dissection. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3835–3838. [Google Scholar] [CrossRef] [PubMed]
- Prokopakis, E.P.; Lachanas, V.; Vardouniotis, A.S.; Velegrakis, G.A. The use of the Ligasure vessel sealing system in head and neck surgery: A report on six years of experience and a review of the literature. B-ENT 2010, 6, 19–25. [Google Scholar] [PubMed]
- Tirelli, G.; Camilot, D.; Bonini, P.; del Piero, G.C.; Biasotto, M.; Quatela, E. Harmonic Scalpel and Electrothermal Bipolar Vessel Sealing System in Head and Neck Surgery: A Prospective Study on Tissue Heating and Histological Damage on Nerves. Ann. Otol. Rhinol. Laryngol. 2015, 124, 852–858. [Google Scholar] [CrossRef]
- Mishra, N.; Samal, D.; Kar, I.B.; Sharma, G.; Baig, S.A.; Kar, R.; Birmiwal, K.G.; Sahu, G.R. Bipolar Vessel Sealing System Versus Suture Ligation in Selective Neck Dissection. J. Maxillofac. Oral Surg. 2018, 17, 495–501. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Hsin, L.-J.; Lin, W.-N.; Tsai, M.-S.; Tsai, Y.-T.; Lee, Y.-C. LigaSure small jaw versus conventional neck dissection: A systematic review and meta-analysis. J. Otolaryngol. Head Neck Surg. 2021, 50, 1–8. [Google Scholar] [CrossRef]
- Overhaus, M.; Schaefer, N.; Walgenbach, K.; Hirner, A.; Szyrach, M.N.; Tolba, R.H. Efficiency and safety of bipolar vessel and tissue sealing in visceral surgery. Minim. Invasive Ther. Allied Technol. 2012, 21, 396–401. [Google Scholar] [CrossRef]
- Smith, R.; Pasic, R. The role of vessel sealing technologies in laparoscopic surgery. Surg. Technol. Online 2008, 17, 208–212. [Google Scholar]
- Fujita, J.; Takiguchi, S.; Nishikawa, K.; Kimura, Y.; Imamura, H.; Tamura, S.; Ebisui, C.; Kishi, K.; Fujitani, K.; Kurokawa, Y.; et al. Randomized controlled trial of the LigaSure vessel sealing system versus conventional open gastrectomy for gastric cancer. Surg. Today 2014, 44, 1723–1729. [Google Scholar] [CrossRef]
- Grieco, M.; Apa, D.; Spoletini, D.; Grattarola, E.; Carlini, M. Major vessel sealing in laparoscopic surgery for colorectal cancer: A single-center experience with 759 patients. World J. Surg. Oncol. 2018, 16, 101. [Google Scholar] [CrossRef]
- Slakey, D.P. Laparoscopic liver resection using a bipolar vessel-sealing device: LigaSure®. HPB 2008, 10, 253–255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solaini, L.; Arru, L.; Merigo, G.; Tomasoni, M.; Gheza, F.; Tiberio, G.A.M. Advanced Sealing and Dissecting Devices in Laparoscopic Adrenal Surgery. JSLS J. Soc. Laparoendosc. Surg. 2013, 17, 622–626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steinemann, D.C.; Lamm, S.H.; Zerz, A. Efficacy and Safety of Combined Ultrasonic and Bipolar Energy Source in Laparoscopic Surgery. J. Gastrointest. Surg. 2016, 20, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Park, D.W.; Park, S.; Kim, J.-H.; Park, S.-H.; Kim, C.-S. Lymph Node Dissection Using Bipolar Vessel-Sealing Device During Reduced Port Laparoscopic Distal Gastrectomy for Gastric Cancer: Result of a Pilot Study from a Single Institute. J. Laparoendosc. Adv. Surg. Tech. 2017, 27, 1101–1108. [Google Scholar] [CrossRef]
- Olasehinde, O.; Owojuyigbe, A.; Adeyemo, A.; Mosanya, A.; Aaron, O.; Wuraola, F.; Owoniya, T.; Owojuyigbe, T.; Alatise, O.; Adisa, A. Use of energy device in general surgical operations: Impact on peri-operative outcomes. BMC Surg. 2022, 22, 90. [Google Scholar] [CrossRef]
- Hasanov, M.; Denschlag, D.; Seemann, E.; Gitsch, G.; Woll, J.; Klar, M. Bipolar vessel-sealing devices in laparoscopic hysterectomies: A multicenter randomized controlled clinical trial. Arch. Gynecol. Obstet. 2018, 297, 409–414. [Google Scholar] [CrossRef]
- Clavé, H.; Clavé, A. Safety and Efficacy of Advanced Bipolar Vessel Sealing in Vaginal Hysterectomy: 1000 Cases. J. Minim. Invasive Gynecol. 2017, 24, 272–279. [Google Scholar] [CrossRef]
- Allam, I.S.; Makled, A.K.; Gomaa, I.A.; El Bishry, G.M.; Bayoumy, H.A.; Ali, D.F. Total laparoscopic hysterectomy, vaginal hysterectomy and total abdominal hysterectomy using electrosurgical bipolar vessel sealing technique: A randomized controlled trial. Arch. Gynecol. Obstet. 2015, 291, 1341–1345. [Google Scholar] [CrossRef]
- Karacan, T.; Ozyurek, E.; Wetherilt, L.S.; Kiyak, H.; Yilmaz, S.; Kaya, E. Safety and efficacy of using advanced electrosurgical bipolar vessel sealing during vaginal hysterectomy in morbidly obese patients: A retrospective cohort analysis. Ginekol. Polska 2017, 88, 523–529. [Google Scholar] [CrossRef]
- Gizzo, S.; Noventa, M. Electrosurgical bipolar vessel sealing for vaginal hysterectomies: Criticism of evidences from a meta-analysis. Arch. Gynecol. Obstet. 2014, 290, 1045–1046. [Google Scholar] [CrossRef]
- Giraudet, G.; Lucot, J.; Sanz, F.; Rubod, C.; Collinet, P.; Cosson, M. Outpatient vaginal hysterectomy: Comparison of conventional suture ligature versus electrosurgical bipolar vessel sealing. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Kangas, R.; Veräjänkorva, E.; Koskivuo, I. Ligasure impact™ might reduce blood loss, complications, and re-operation occurrence after abdominoplasty in massive-weight-loss patients: A Comparative Study. Scand. J. Surg. 2020, 109, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.; MacKenzie, H.; Hanna, G.B. Non-vascular experimental and clinical applications of advanced bipolar radiofrequency thermofusion technology in the thorax and abdomen: A systematic review. Surg. Endosc. 2015, 29, 1659–1678. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Huang, K.G. Energy devices in gynecological laparoscopy—Archaic to modern era. Gynecol. Minim. Invasive Ther. 2017, 6, 147–151. [Google Scholar] [CrossRef]
- Lauroy, A.; Verhaeghe, C.; Vidal, F.; Parant, O.; Legendre, G.; Guerby, P. Perioperative outcomes using Liga Sure compared with conventional technique in peripartum hysterectomy. Arch. Gynecol. Obstet. 2020, 301, 229–234. [Google Scholar] [CrossRef]
- Kraemer, B.; Tsaousidis, C.; Kruck, S.; Schenk, M.; Scharpf, M.; Kommoss, S.; Brucker, S.; Nuessle, D.; Enderle, M.D.; Biber, U. Safety and effectiveness of a novel generator algorithm for bipolar vessel sealing: A randomised controlled chronic animal study. BMC Surg. 2019, 19, 160. [Google Scholar] [CrossRef]
- Shibao, K.; Joden, F.; Adachi, Y.; Kohi, S.; Kudou, Y.; Kikuchi, Y.; Matayoshi, N.; Sato, N.; Murayama, R.; Hirata, K. Repeated partial tissue bite with inadequate cooling time for an energy device may cause thermal injury. Surg. Endosc. 2021, 35, 3189–3198. [Google Scholar] [CrossRef]
- Chikamoto, A.; Kaida, T.; Arima, K.; Higashi, T.; Taki, K.; Ida, S.; Okabe, H.; Nitta, H.; Hayashi, H.; Hashimoto, D.; et al. Heat injury to the inferior vena cava by bipolar tissue sealer. Surg. Endosc. 2016, 30, 1519–1522. [Google Scholar] [CrossRef]
- Koyanagi, K.; Kato, F.; Nakanishi, K.; Ozawa, S. Lateral thermal spread and recurrent laryngeal nerve paralysis after minimally invasive esophagectomy in bipolar vessel sealing and ultrasonic energy devices: A comparative study. Esophagus 2018, 15, 249–255. [Google Scholar] [CrossRef]
- Suzuki, T.; Hattori, R.; Minagawa, T.; Uehara, T.; Ogawa, T.; Ishizuka, O. Intestinal Injury by Heat Conduction from Surgical Sealing Devices. JSLS J. Soc. Laparoendosc. Surg. 2019, 23, 23. [Google Scholar] [CrossRef]
- Tahir, M.; Gilkison, W. Ureteric Injury due to the Use of LigaSure. Case Rep. Urol. 2013, 2013, 989524. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Durrant, J.; Smeak, D.; Newman, J. The Thermal Safety Profile of a New Bipolar Vessel Sealing Device for Thyroid Surgery. Surg. Technol. Online 2021, 39, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.A.; Cresswell, A.B.; Frank, T.G.; Cuschieri, A. Real-time thermography during energized vessel sealing and dissection. Surg. Endosc. 2003, 17, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.W., 3rd; Talley, D.K. Laser-tissue interactions. Optom. Clin. 1995, 4, 17–31. [Google Scholar] [PubMed]
- Jacques, S.L. Laser-Tissue Interactions: Photochemical, Photothermal, and Photomechanical. Surg. Clin. N. Am. 1992, 72, 531–558. [Google Scholar] [CrossRef]
- Murray, A.; Mitchell, D.C.; Wood, R.F. Lasers in surgery. Br. J. Surg. 1992, 79, 21–26. [Google Scholar] [CrossRef]
- Peavy, G.M. Lasers and laser-tissue interaction. Vet. Clin. N. Am. Small Anim. Pract. 2002, 32, 517–534. [Google Scholar] [CrossRef]
- Reinisch, L. Laser Physics And Tissue Interactions. Otolaryngol. Clin. N. Am. 1996, 29, 893–914. [Google Scholar] [CrossRef]
- Eberli, D.; Hefermehl, L.J.; Müller, A.; Sulser, T.; Knönagel, H. Thermal spread of vessel-sealing devices evaluated in a clinically relevant in vitro model. Urol. Int. 2011, 86, 476–482. [Google Scholar] [CrossRef]
- Goudie, E.; Oliveira, R.; Thiffault, V.; Jouquan, A.; Hadjeres, R.; Berdugo, J.; Ferraro, P.; Liberman, M. Heat production during pulmonary artery sealing with energy vessel-sealing devices in a swine model. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 847–852. [Google Scholar] [CrossRef]


| marSeal® | Critical Zone above (µm) | Necrosis Zone (µm) | Frontier Temperature (°C) | Rilate |
|---|---|---|---|---|
| 1 | 2070 | 421.9 | 57.5 | 20.3 |
| 2 | 2830 | 442.6 | 66.5 | 15.6 |
| 3 | 2630 | 439.7 | 66.0 | 16.7 |
| 4 | 2570 | 535.3 | 68.7 | 20.8 |
| 5 | 2660 | 461.5 | 64.7 | 17.3 |
| 6 | 1900 | 285.7 | 63.5 | 15.0 |
| 7 | 1360 | 327.8 | 59.4 | 24.1 |
| 8 | 1620 | 385.1 | 63.0 | 23.7 |
| 9 | 2500 | 316.2 | 71.3 | 12.6 |
| 10 | 2630 | 373.5 | 67.5 | 14.2 |
| 11 | 1830 | 486.8 | 68.3 | 26.6 |
| 12 | 2050 | 521.7 | 58.2 | 25.4 |
| 13 | 3310 | 492.8 | 64.7 | 15.7 |
| 14 | 2290 | 374.6 | 70.1 | 16.3 |
| 15 | 2470 | 322.8 | 64.6 | 13.1 |
| mean | 2315 | 412.5 | 64.93 | 17.8 |
| SD | 509.2 | 79.0 | 4.1 | 4.6 |
| Critical Zone below (µm) | Necrosis Zone (µm) | Frontier Temperature (°C) | Rilate | |
| 1 | 1990 | 416.2 | 59.6 | 20.9 |
| 2 | 1580 | 497.6 | 65.1 | 31.5 |
| 3 | 1540 | 385.9 | 63.1 | 25.0 |
| 4 | 1590 | 562.2 | 60.7 | 35.4 |
| 5 | 1220 | 606.8 | 57.9 | 49.7 |
| 6 | 1590 | 384.4 | 61.3 | 24.1 |
| 7 | 2030 | 522.7 | 66.8 | 25.7 |
| 8 | 1430 | 494.5 | 62.1 | 34.5 |
| 9 | 1980 | 332.6 | 67.5 | 16.8 |
| 10 | 1660 | 331.7 | 62.6 | 19.9 |
| 11 | 2480 | 407.1 | 70.5 | 16.4 |
| 12 | 1330 | 514.4 | 59.9 | 38.6 |
| 13 | 1520 | 373.2 | 64.6 | 24.5 |
| 14 | 2010 | 295.7 | 65.8 | 14.7 |
| 15 | 1550 | 275.6 | 63.8 | 17.8 |
| mean | 1700 | 426.7 | 63.42 | 25.1 |
| SD | 331.3 | 100.7 | 3.38 | 9.8 |
| BiCision® | Critical Zone above (µm) | Necrosis Zone (µm) | Frontier Temperature (°C) | Rilate |
| 1 | 1600 | 886.6 | 56.5 | 55.4 |
| 2 | 1480 | 733.8 | 56.0 | 49.6 |
| 3 | 2750 | 956.8 | 65.2 | 34.8 |
| 4 | 1950 | 654.8 | 58.7 | 33.6 |
| 5 | 1760 | 479.1 | 57.9 | 27.2 |
| 6 | 1650 | 518.4 | 57.3 | 31.4 |
| 7 | 2600 | 497.9 | 64.0 | 19.2 |
| 8 | 1560 | 654.8 | 60.6 | 42.0 |
| 9 | 2270 | 768.1 | 60.9 | 33.8 |
| 10 | 2340 | 597.0 | 61.7 | 25.5 |
| 11 | 1730 | 499.5 | 59.3 | 28.9 |
| 12 | 2970 | 567.0 | 64.6 | 19.0 |
| 13 | 1400 | 582.9 | 55.4 | 41.6 |
| 14 | 3090 | 811.1 | 70.6 | 26.2 |
| 15 | 1330 | 431.5 | 57.6 | 33.4 |
| mean | 2032 | 642.6 | 60.42 | 31.62 |
| SD | 592.4 | 158.2 | 4.2 | 10.3 |
| Critical Zone below (µm) | Necrosis Zone (µm) | Frontier Temperature (°C) | Rilate | |
| 1 | 1270 | 694.7 | 52 | 54.7 |
| 2 | 1440 | 688.3 | 56.6 | 47.8 |
| 3 | 2010 | 973.6 | 51.2 | 48.4 |
| 4 | 730 | 618.2 | 52.1 | 84.7 |
| 5 | 720 | 535.4 | 52 | 74.4 |
| 6 | 1530 | 542.8 | 59.8 | 34.5 |
| 7 | 1490 | 596.4 | 64.7 | 40.0 |
| 8 | 820 | 603.1 | 54.2 | 73.5 |
| 9 | 1280 | 696.9 | 54.7 | 54.4 |
| 10 | 920 | 556.8 | 53.8 | 60.5 |
| 11 | 820 | 592.8 | 53.3 | 72.3 |
| 12 | 890 | 588.5 | 53 | 66.1 |
| 13 | 870 | 748.8 | 51.3 | 86.1 |
| 14 | 1550 | 682.3 | 62.4 | 44.0 |
| 15 | 1390 | 561.3 | 58.1 | 40.4 |
| mean | 1182 | 645.3 | 55.28 | 54.59 |
| SD | 386.9 | 111.9 | 4.1 | 16.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirschbaum, A.; Jonas, J.; Surowiec, T.M.; Pehl, A.; Mirow, N. Detection of the Lateral Thermal Spread during Bipolar Vessel Sealing in an Ex Vivo Model—Preliminary Results. Diagnostics 2022, 12, 1217. https://doi.org/10.3390/diagnostics12051217
Kirschbaum A, Jonas J, Surowiec TM, Pehl A, Mirow N. Detection of the Lateral Thermal Spread during Bipolar Vessel Sealing in an Ex Vivo Model—Preliminary Results. Diagnostics. 2022; 12(5):1217. https://doi.org/10.3390/diagnostics12051217
Chicago/Turabian StyleKirschbaum, Andreas, Jan Jonas, Thomas M. Surowiec, Anika Pehl, and Nikolas Mirow. 2022. "Detection of the Lateral Thermal Spread during Bipolar Vessel Sealing in an Ex Vivo Model—Preliminary Results" Diagnostics 12, no. 5: 1217. https://doi.org/10.3390/diagnostics12051217
APA StyleKirschbaum, A., Jonas, J., Surowiec, T. M., Pehl, A., & Mirow, N. (2022). Detection of the Lateral Thermal Spread during Bipolar Vessel Sealing in an Ex Vivo Model—Preliminary Results. Diagnostics, 12(5), 1217. https://doi.org/10.3390/diagnostics12051217





