Abstract
Few studies so far have examined the impact of nutritional status on the survival of children with cancer, with the majority of them focusing on hematological malignancies. We summarized published evidence reporting the association of nutritional status at diagnosis with overall survival (OS), event-free survival (EFS), relapse, and treatment-related toxicity (TRT) in children with cancer. Published studies on children with leukemia, lymphoma, and other solid tumors have shown that both under-nourished and over-nourished children at cancer diagnosis had worse OS and EFS. Particularly, the risk of death and relapse increased by 30–50% among children with leukemia with increased body mass index at diagnosis. Likewise, the risk of TRT was higher among malnourished children with osteosarcoma and Ewing sarcoma. Nutritional status seems to play a crucial role in clinical outcomes of children with cancer, thus providing a significant modifiable prognostic tool in childhood cancer management. Future studies with adequate power and longitudinal design are needed to further evaluate the association of nutritional status with childhood cancer outcomes using a more standardized definition to measure nutritional status in this population. The use of new technologies is expected to shed further light on this understudied area and give room to person-targeted intervention strategies.
1. Introduction
Cancer is the leading cause of death in children and adolescents worldwide [1]. Every year, just over 150,000 children are diagnosed with cancer [2]. Since a large proportion of childhood cancers in low- and middle-income countries are never diagnosed, the more realistic annual number is estimated to be at least twice as high, i.e., above 360,000 children [3]. Given these caveats, the age-standardized incidence (ASR) of the disease, estimated at 140.6 per million person-years in children aged 0–14 years, is increasing, with leukemia being the most common cancer site (ASR: 46.4), followed by central nervous system (CNS) tumors (ASR: 28.2) and lymphomas (ASR: 15.2) [4]. Over the past decades, advances in childhood cancer care, including novel imaging techniques for diagnostics and risk stratification, as well as multimodal novel therapies and supportive healthcare, have led to impressive increases in five-year survival, which exceeds 80% for all cancer types and 90% for acute lymphoblastic leukemia (ALL) in many European and North American countries nowadays [5]. However, severe or life-threatening long-term consequences may occur in up to 80% of childhood cancer survivors [5,6]. Indeed, a recent report showed that mortality among cancer patients is higher than that in the general population, mainly attributed to increased cardiotoxicity and risk of second neoplasms [7]. Poor nutritional status, defined as undernutrition (body mass index (BMI) < 5th percentile) or overnutrition (BMI ≥ 85th percentile) by the World Health Organization (WHO), seems to be a poor prognostic indicator linked to treatment-related toxicities in adults with solid tumors [8]. Recent research suggests that nutritional factors may also adversely affect outcomes in children and adolescents treated for cancer [9]. However, few studies have examined the impact of nutritional status on the survival of children with cancer, with the majority of them focusing on hematological malignancies [10,11].
Undernutrition is commonly reported in children treated for cancer, with a prevalence rate estimated as high as 50% in some populations [12]. Multimodal dose-intense antineoplastic therapies, surgery, and radiotherapy may cause serious complications that synergistically enhance poor nutritional status [13]. Treating undernutrition in children with cancer will assist them in tolerating traditional and novel therapies and thus plays a crucial role in improving outcomes and quality of life [13,14,15].
Overnutrition has long been associated with treatment-related complications. Such examples include the increased risk for thrombo-hemorrhagic fatal events in children with acute promyelocytic leukemia, the increased risk for infections (i.e., urinary tract infections and infections of central lines) in patients with ALL, as well as increased nephrotoxicity and post-operative complications in patients with osteosarcoma [15,16,17,18].
We aimed to review published literature and summarize evidence reporting the association of nutritional status at diagnosis with overall survival (OS), event-free survival (EFS), relapse, and treatment-related toxicity (TRT) in children treated for different types of cancer.
2. Methods
An independent and blinded literature search of the Medline database (via PubMed) was conducted from inception up to October 18 2021 using an algorithm based on relative key terms, such as “nutritional”, “undernutrition”, “obesity”, “BMI”, “prognostic”, “survival”, “relapse”, “toxicity”, “complications”, “childhood malignancies”, and “childhood cancer”. The reference lists of identified studies were searched for additional eligible articles potentially missed through the initial literature search in a procedure called “snowball” procedure. Eligible were studies that examined the association of nutritional status at diagnosis with clinical outcomes, such as OS, EFS, relapse, histological response, and TRT in children (0–14 years) with cancer. We considered both systematic reviews and meta-analyses, as well as primary publications eligible for inclusion. Other types of publications, such as letters to the editor, commentaries, and editorials, were excluded. Case reports, case series, in vitro, or animal studies were also excluded. Nutritional status was defined by each study based on body mass index (BMI), weight change, or other body composition indices that defined under- or over-nutrition. We included studies reporting on any type of pediatric cancer.
The identified articles were independently screened by two reviewers to identify those that met the pre-determined inclusion criteria. Disagreements in the selection of studies or snowball procedure were resolved by team consensus. In articles with overlapping populations, the most recent or most complete publication was considered eligible. We extracted data based on a pre-defined form, including the name of the first author, publication year, study type, childhood population characteristics (including the studied cancer sites), the definition of the nutritional status based on the study, outcomes of interest (OS, EFS, relapse, TRT) along with the accompanying effect estimates (relative risk, hazard ratio, etc.) and their corresponding 95% confidence intervals (CI) and/or p-values.
Due to the large heterogeneity in exposure and outcome definitions across the identified studies, no quantitative synthesis of the results was feasible. Thus, a narrative presentation of the eligible studies by cancer type was performed (Table 1).

Table 1.
Summary of studies reporting the association between nutritional status at diagnosis and outcomes of children with cancer *.
3. Results
The search strategy identified 4927 records, and after title, abstract, and full-text screening we included 22 publications in the present review (Figure 1). We identified 4 systematic reviews and meta-analyses and 18 primary studies. The most commonly studied cancer site was acute leukemia, reported in six publications (27%), followed by all cancer sites as a combined variable, which was reported in four primary studies (18%). The most commonly studied outcome was OS (n = 17 studies; 77%), followed by EFS (n = 10 studies; 45%), while treatment-related complications were studied as outcome in seven publications (32%).
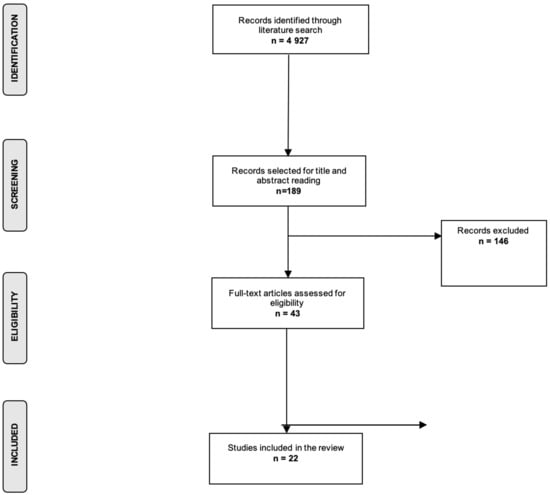
Figure 1.
Flow chart of the selection process.
3.1. Hematological Malignancies
A previous meta-analysis of 11 articles found poorer EFS in children with ALL who had higher BMI (≥85th percentile; relative risk [RR]: 1.35; 95% CI: 1.20, 1.51; n = 6 studies; I2: 34%, p = 0.18) compared to those with lower BMI at diagnosis (Table 1). In addition, children with higher BMI at diagnosis had significantly higher mortality (RR: 1.31; 95% CI: 1.09, 1.58; n = 4 studies; I2: 49%, p = 0.12) [19]. Between-study heterogeneity was non-significant in all meta-analyses, whereas no evidence of publication bias was shown. Children with acute myeloid leukemia (AML) and higher BMI at diagnosis were also associated with poorer EFS (RR: 1.36; 95% CI: 1.16, 1.60; I2: 0%, p = 0.99) and OS (RR: 1.56; 95% CI: 1.32, 1.86; I2: 0%, p = 0.66) compared to children with lower BMI [19]. Similarly, two other meta-analyses showed significant associations between obesity and childhood leukemia survival outcomes. In particular, a meta-analysis including 11 studies found a statistically significant association between obesity, as defined by high BMI at diagnosis, and poorer OS (hazard ratio [HR]: 1.30, 95% CI: 1.16, 1.46; n = 7 studies; I2: 71%; p = 0.002); however, statistically significant between-study heterogeneity was noted in this meta-analysis. High BMI at diagnosis was also associated with poorer EFS (HR: 1.46, 95% CI: 1.29, 1.64; n = 7 studies; I2: 39%, p = 0.13) among children with acute leukemia [20]. A more recent meta-analysis (2018) showed an increased mortality rate (HR: 1.79, 95% CI: 1.03, 3.10; n = 3 studies; I2: 0%, p = 0.37), as well as increased risk for relapse (HR: 1.28, 95% CI: 1.04, 1.57; n = 2 studies; I2: 0%, p = 0.59) for children older than 10 years with ALL. However, these meta-analyses included a limited number of studies (n = 2–3) [21]. A statistically significant association between obesity and increased mortality for AML was also shown (HR: 1.64, 95% CI: 1.32, 2.04; n = 3 studies; I2: 0%, p = 0.73), whereas no effect sizes were reported for AML relapse [21]. A recent study from Brazil assessed the impact of nutritional status on outcomes of children (n = 148) undergoing allogeneic hematopoietic stem cell transplantation (HSCT). Severe malnutrition was linked to a statistically significantly increased risk of acute graft versus host disease (HR: 1.68, 95% CI: 1.02, 2.74), as well as increased mortality (HR: 3.63, 95% CI: 1.76, 7.46), poorer progression-free survival (HR: 2.12, 95% CI: 1.25, 3.60), and poorer OS (HR: 3.27, 95% CI: 1.90, 5.64) [22]. A meta-analysis including 24 studies (18 on adolescents and 6 on children) examined the association between BMI and clinical outcomes after HSCT in patients with hematological malignancies. Compared to normal, lower BMI before or during transplantation was significantly linked to poorer OS (RRpre-HSCT stage: 1.17, 95% CI: 1.08, 1.27; n = 11 studies; I2: 0%, p = 0.67/RRHSCT stage: 1.34, 95% CI: 1.01, 1.78; n = 5 studies; I2: 60%, p = 0.04), as well as to poorer EFS (RRpre-HSCT stage: 1.29, 95% CI: 0.96, 1.72; n = 5 studies; I2: 18%, p = 0.30/RRHSCT stage: RR: 1.53, 95% CI: 1.09, 2.06; n = 2 studies; I2: 31%, p = 0.23). Again, the small number of included studies in some meta-analyses should be acknowledged. By contrast, there was no impact of high BMI on clinical outcomes among these patients at any transplantation stage [23].
A recent study in Pakistan showed that OS in children with Hodgkin lymphoma significantly decreased in cases of moderate (79%) and severe malnutrition (75%) compared to no malnutrition (96%; p = 0.006) [24]. By contrast, a 2021 study including 191 children with leukemia and lymphoma showed no impact of malnutrition on clinical outcomes [25].
The small sample sizes and the retrospective design of the identified studies should be acknowledged when interpreting these results. However, overall evidence from published studies suggests that poor nutritional status, especially over nutrition at diagnosis, may be a significant predictor of poor outcomes among children with leukemia, particularly ALL. Further research is needed to investigate the association with other leukemia subtypes, especially the rarer AML subtype, as well as the potential association with lymphomas, where evidence remains inconclusive.
3.2. Ewing Sarcoma
A USA cohort study including data from the University of California, San Francisco (UCSF) and Stanford University Medical Centers (n = 142 patients with Ewing sarcoma) showed non-statistically significant correlations between BMI at diagnosis and TRT (p = 0.43), specifically grade 3 and grade 4 non-hematologic toxicities during follow-up [26] (Table 1). With regards to clinical outcomes, in a study from Israel, abnormal BMI, defined as high or low BMI combined into one category, was statistically significantly associated with poor histologic response, namely tumor necrosis < 90% (odds ratio [OR]: 4.33, 95% CI: 1.12, 19.14), as well as worse OS (HR: 2.76, 95% CI: 1.19, 9.99) [27], whereas no correlations were found with EFS in this population (n = 50 patients). Lastly, regarding TRT, a study from Canada (n = 71 patients) reported significant correlations between low BMI at diagnosis and cardiotoxicity among children receiving anthracycline chemotherapy for Ewing sarcoma (p = 0.03), though this association was non-significant in multivariate logistic regression models (p = 0.35) after adjusting for potential covariates [28]. Given the limited sample sizes and the retrospective design of the identified studies, further research is needed to allow firm conclusions to be drawn regarding the association of nutritional status with Ewing sarcoma outcomes.
3.3. Osteosarcoma
Two longitudinal cohort studies derived from the Children’s Oncology Group (COG) examined the impact of nutritional status on TRT and clinical outcomes in patients with osteosarcoma [29,30] (Table 1). Regarding TRT, the first study (n = 498) showed that high BMI was significantly associated with an increased risk of arterial thrombosis in the post-operative period (OR: 9.40, p = 0.03) [29]. The second study showed that children with high BMI had increased odds of developing grade III–IV nephrotoxicity (OR = 2.70, 95% CI: 1.20, 6.40). Regarding clinical outcomes, children in the high BMI group had a significantly poorer 5-year OS of 70% compared to children with normal BMI (OS: 80%; HR: 1.60, 95% CI: 1.14, 2.24). There was also a trend towards worse EFS at 3 years from diagnosis in children with high BMI compared to those with normal BMI (66% versus 75%; HR: 1.30, 95% CI: 0.90, 1.80), whereas there was no impact of low BMI on OS and EFS among these patients [30].
3.4. Rhabdomyosarcoma
Two studies based on prospective data obtained from the COG assessed the association between nutritional status and rhabdomyosarcoma outcomes [31,32] (Table 1). In the first study, nutritional status was not a prognostic indicator for infections or survival. However, patients with more than 10% weight loss at 24 weeks of follow-up had a significantly increased number of hospitalization days (OR: 1.24, 95% CI: 1.00, 1.54) [32] and a trend towards a higher risk of grade 3 and grade 4 toxicity (OR: 1.16, 95% CI: 0.99, 1.35) at 42 weeks of follow-up. Children with low BMI (<10th percentile) did not have significantly worse OS compared to those with normal BMI at baseline (HR: 1.70, 95% CI: 0.98, 2.96; p = 0.0596). The second USA study on 570 children with intermediate-risk rhabdomyosarcoma examined the impact of tumor volume and patient weight on EFS based on a partitioning algorithm and accounting for age and greatest tumor dimension. The results of the algorithm showed that tumor volume (≥20 cm3), histology, and patient weight (≥50 kg) were statistically significantly associated with twofold worse EFS (HR: 2.12, 95% CI: 1.12, 4.02) [31].
3.5. Other Cancer Sites
Two studies reported non-statistically significant associations between nutritional status and OS in children with neuroblastoma and Wilms tumor [33,34] (Table 1). A study from Turkey evaluated the impact of nutritional status on the survival of children with all types of cancer. Though malnutrition was a significant complication in this population, with a prevalence of 30% at diagnosis increased to 38% three months later, there was no impact of malnutrition on survival outcomes [35]. Likewise, a study on 139 children with Ewing sarcoma and osteosarcoma reported high proportions of malnutrition 2 years after treatment initiation (43% of osteosarcoma and 25% of Ewing sarcoma) [36]; again, malnutrition had non-significant effects on survival outcomes. A more recent study assessed the prognostic effect of sarcopenia, defined by the BMI-z score, the prognostic nutritional index (PNI), and the total psoas muscle area (tPMA), on the survival of children with bone and soft tissue sarcomas [37]. This study showed that the decrease in PNI (p = 0.03) and tPMA of more than 25% (p = 0.04) were associated with worse one-year OS; yet, more research is needed to evaluate the prognostic significance of tPMA in children with sarcomas. A study on pediatric cancer patients in Hungary showed that undernutrition at diagnosis, defined by the BMI Z-score and the ideal body weight percent (IBW%), was associated with worse 5-year OS only in patients with solid tumors [38]. Lastly, a study from the Netherlands, including 269 children with all types of cancer, found a significant impact of malnutrition on OS (HR: 3.63, 95% CI: 1.52–8.70), whereas increased weight loss (>5%) was also linked to increased risk of febrile neutropenia and subsequent bacteremia during the first year following diagnosis (OR: 3.05, 95% CI: 1.27, 7.30) [39]. Similar findings have been reported by a recent study in Italy which showed that weight loss of more than 5% at 3 months following diagnosis was associated with poorer OS (HR: 2.75, 95% CI: 1.12, 6.79) and a higher risk of infections requiring hospitalization (HR: 7.72, 95% CI: 2.27, 26.2) [40].
4. Discussion
The present review identified 22 published studies reporting associations between nutritional status and survival outcomes in children diagnosed with cancer. Despite the large heterogeneity in studied exposures and outcomes, the present findings suggest that malnutrition is a significant predictor of outcomes in childhood cancer. In particular, published studies on children with acute leukemia, lymphoma, and other solid tumors have shown that both under-nourished and over-nourished children at cancer diagnosis had worse OS and EFS. Of note is that the risk of death and relapse increased by 30–50% among children with leukemia with increased BMI at diagnosis. Likewise, the risk of TRT was higher among malnourished children with osteosarcoma and Ewing sarcoma.
Over the last decades, epidemiological and clinical research has aimed to identify prognostic indicators of childhood cancer with the ultimate goal of optimal risk stratification and targeted therapeutic interventions. Several sociodemographic and clinical factors have been reported to affect the prognosis of childhood cancer, such as age, socioeconomic status, disease subtype, cytogenetic and molecular markers, laboratory markers, predisposing syndromes, tumor size, presence of metastases, etc. [41,42]. However, very few environmental factors have been investigated as potential predictors of outcomes in children with cancer [43,44]. Among these factors, nutritional status is a modifiable marker that has long been postulated to affect survival in both adult and pediatric malignancies [45]. Potential underlying mechanisms that may underlie the association between nutritional status and health outcomes include its impact on body composition makeup, modification of tumor microenvironment, and potential alteration of chemotherapy pharmacokinetics [46]. In particular, changes in lean tissue and fat mass can alter the distribution of chemotherapeutic agents, modify their metabolism, and subsequently affect their clearance, especially the clearance of hydrophilic and/or lipophilic drugs from systemic circulation.
4.1. Undernutrition and Cancer Outcomes
Undernutrition has been associated with treatment-related adverse effects and survival among adult patients with cancer [46]. Several underlying biological mechanisms have been proposed to explain this association. Weight loss and decreased muscle and fat mass can be induced by cancer progression, and they both result in a negative balance between synthetic and degradative protein pathways. Moreover, weight and muscle loss are responsible for the activation of inflammatory response and apoptosis of myocytes, as well as for the decreased muscle regeneration capacity [47]. In addition, muscle loss may decrease the excretion of anabolic hormones, such as testosterone and insulin-like growth factor (IGF)-1 [48]. Such phenomena may alter drug pharmacokinetics and thus contribute to the increased risk of dose-response toxicity, which is often observed in cancer patients with low muscle and lean tissue mass [48]. Though evidence in children with cancer is limited, previous studies have shown significant skeletal muscle mass wasting with a concurrent fat mass increase in children with ALL following treatment initiation [49]. Muscle wasting has been related to reductions in chemotherapeutic doses, delays of treatment, and/or premature treatment cessation [50]. Similar findings have been reported by studies on children with several types of solid cancer, concluding that body composition markers seem to be crucial prognostic indicators among pediatric oncology patients [49,51].
4.2. Overnutrition and Cancer Outcomes
With regard to overnutrition at diagnosis and survival from childhood cancer, several potential mechanisms have been proposed. There is clinical evidence suggesting that obese patients may have altered pharmacokinetics in the metabolism of chemotherapeutic agents, although prospective trials have not confirmed such variations [52,53]. Overnutrition may also affect both the tumor microenvironment and the microenvironment of the host [54]. In particular, the adipose tissue microenvironment physiology in obese patients is characterized by increased inflammation, as well as disruptions in vascularity and fibrosis which may contribute to tumor progression [54]. Moreover, obesity is associated with hyperinsulinemia and peripheral insulin resistance, which result in reduced growth hormone (GH) secretion and subsequent activation of the IGF-I system as a response to the decreased GH [55]. The association of the IGF-II axis with obesity remains less clear. Low IGF-II levels have been reported in obese children, especially in those with insulin resistance and increased inflammatory markers, such as IL-6 and TNF-a [56]. The overexpression of the IGF-I and -II axis in obese individuals seems to also play a crucial pathogenetic role in the first (initiating) “molecular hit” that contributes not only to leukemia development but also to the disease clinical course [57,58]. Indeed, numerous epidemiologic studies have supported this hypothesis, showing that both children of high birthweight and patients with diabetes have high expression of the IGF-I and II systems and are at increased risk of developing leukemia [18,59]. In addition, these metabolic and chronic inflammatory changes in obese cancer patients may result in the activation of multiple molecular cancer pathways, which subsequently increase the risk of disease resistance and progression [60].
4.3. Nutritional Interventions
Apart from the direct effect of cancer itself on nutritional status, several factors can also affect both appetite and food intake among cancer patients. Such factors include some common complications of cancer treatment, such as nausea, vomiting, mucositis (oral, esophageal, and bowel), diarrhea, and/or constipation [61]. Thus, targeted interventions to improve the nutritional status of children with cancer remain challenging. Dietary support and physical exercise recommendations both aim to maintain and improve the normal physical growth of patients before and during treatment [62]. A recent study evaluated the nutritional status following the implementation of a nutritional algorithm in children with cancer from low- and middle-income countries [63]. This study showed an increase in the mid-upper-arm circumference in children enrolled in the algorithm protocol compared to those receiving the standard nutritional care provided by their institution (p = 0.02); however, a non-significant difference in weight change was noted between the two comparison groups (p = 0.15) [63]. Two other trials assessed the prognostic effect of ω-3 fatty acids and black seed oil on methotrexate-induced toxicity in children with ALL showing a decrease in the risk of hepatotoxicity during the maintenance treatment phase [64,65]. Other interventions that have been explored include the use of glutamine in order to sustain the integrity of the mucosal cell and gut barrier, which is often damaged in chemotherapy-related mucositis [66]. Though evidence is scarce in pediatric patients, one study has shown a significant decrease in the use of parenteral feeding in children with cancer after the exogeneous administration of glutamine without any adverse effects; yet the incidence of mucositis was not reduced in the intervention group [66]. Novel alternative avenues to be explored include the potential beneficial effect of antioxidants or a ketogenic diet on clinical outcomes of children with cancer; however, further research is warranted in this understudied area [67].
Overall, treatment of under- and over-nutrition in cancer patients is not easy on clinical grounds. Interventions should be proactive, aiming to prevent the nutritional depletion before it becomes clinically apparent [68]. Parents and guardians of patients are important components in supporting the nutritional status of children with cancer throughout therapy [69]. Educational and dietician’s support is also crucial in optimizing the health care support of little patients with cancer [70]. Currently, several approaches are being implemented targeting the family-based nutrition and cooking education of parents and guardians of children with cancer [71,72,73]. Among such interventions, web-based dietary and cooking intervention approaches have gained great attention among parents and young patients [74,75,76]. Preliminary results have shown that early interventions resulted in better nutrition practices, i.e., lower sodium consumption [73]. However, obstacles to the participation of families have been noted by several studies, mainly due to treatment-related complications affecting the child’s health, requirement of parents’ presence at the hospital, as well as time, financial and other logistics restraints [71,72]. Future research is thus needed to address these barriers and encourage nutrition and cooking education in a family-based manner.
4.4. Methodological Considerations
The limited power of published studies is an important methodological issue when evaluating the potential association between malnutrition and survival of pediatric cancer. Even in the case of meta-analysis, the limited number of included studies (less than 10 studies) in the majority of meta-analyses performed should be acknowledged. Moreover, most studies focused on the most frequent cancer site, acute leukemia, whereas evidence on the remaining cancer sites is scarce, thus not allowing firm conclusions to be drawn. In addition, the large heterogeneity in exposure and outcome definition should be acknowledged. The majority of identified studies considered nutritional status by measuring BMI. Other nutritional indices were weight change, BMI z-score, prognostic nutritional index (PNI), and total psoas muscle area (tPMA). Of note is that recent research argues for the use of BMI-related measures to evaluate the lean mass in cancer patients. By contrast, recent studies have proposed other mainly imaging-based measures, such as cross-sectional computed tomography, which seem to assess sarcopenia and obesity in this population more accurately [50,77,78]. However, only a few studies have evaluated the impact of such measures on the clinical outcomes of adult patients with cancer [77,79,80]. Moreover, the prognostic significance of imaging-based measures in children with cancer needs to be further explored, also addressing the potential harms, i.e., those related to exposure to radiation [80]. Additionally, while in developing countries, “underweight” is certainly occurring in the normal population at a certain high percentage, “overweight” is basically the norm in highly developed countries. For example, the USA population shows an overweight prevalence of >40% [81]. So, maybe the occurrence of underweight and overweight children with cancer reflects the situation in the respective countries and is hence a “larger problem”. Regarding the outcome assessment, the identified studies assessed the impact of nutritional status on various clinical endpoints, such as OS, EFS, TRT, or specific treatment-related toxicities (cardiotoxicity, nephrotoxicity, etc.). Such heterogeneity did not allow the quantitative synthesis of the results in the context of a meta-analysis. Lastly, the retrospective design of several studies is another limitation, which increases the risk of misclassification bias; indeed, misclassification might be differential in retrospective studies, leading to recall bias, namely reports of higher exposures in children with the disease.
5. Conclusions
Despite the large heterogeneity of published literature and several other limitations reported herein, nutritional status seems to play a crucial role in the survival and clinical outcomes of children with cancer, thus providing a significant modifiable prognostic tool in childhood cancer management. Interventions targeting the optimal nutrition and normal development of children with cancer are thus necessary. Future studies with adequate power and longitudinal design are still needed to further evaluate the association of nutritional status with childhood cancer outcomes using a more standardized definition to measure nutritional status in this population. The use of new technologies to longitudinally and accurately assess the nutritional status on a large scale from diagnosis onwards is expected to shed further light on this understudied area and to give room to individualized person-targeted intervention strategies.
Author Contributions
M.A.K. and E.E.N. conceived and designed the study. M.A.K., G.M. and E.E.N. acquired and collected the data. M.A.K. analyzed the data. M.A.K. drafted the initial version of the manuscript. G.M., C.F.T., A.K., X.T., K.K.T., L.G.S., J.S., T.S., E.T.P. and E.E.N. drafted and critically revised the manuscript for important intellectual content and gave final approval of the version to be published. E.E.N. is the guarantor. All authors have read and agreed to the published version of the manuscript.
Funding
The present study is co-financed by Greece and the European Union (European Social Fund-ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the project “Reinforcement of Postdoctoral Researchers—2nd Cycle” (MIS-5033021), implemented by the State Scholarships Foundation (ΙΚΥ). None of the funders had any influence on the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. All authors had access to the data in the study and had final responsibility for the decision to submit for publication. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Institutional Review Board Statement
Not required.
Informed Consent Statement
The lead author (M.A.K.) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Data Availability Statement
No additional data are available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; IICC-3 Contributors; et al. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- American Cancer Society’s (ACS) publications. Cancer Facts & Figures 2020 and Cancer Facts & Figures 2016, and the ACS Website (January 2020). 2020. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html (accessed on 1 January 2020).
- Johnston, W.; Erdmann, F.; Newton, R.; Steliarova-Foucher, E.; Schüz, J.; Roman, E. Childhood cancer: Estimating regional and global incidence. Cancer Epidemiol. 2020, 71, 101662. [Google Scholar] [CrossRef] [PubMed]
- Force, L.M.; Abdollahpour, I.; Advani, S.M.; Agius, D.; Ahmadian, E.; Alahdab, F.; Alam, T.; Alebel, A.; Alipour, V.; Allen, C.A.; et al. The global burden of childhood and adolescent cancer in 2017: An analysis of the Global Burden of Disease Study 2017. Lancet Oncol. 2019, 20, 1211–1225. [Google Scholar] [CrossRef]
- Erdmann, F.; Frederiksen, L.E.; Bonaventure, A.; Mader, L.; Hasle, H.; Robison, L.L.; Winther, J.F. Childhood cancer: Survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2020, 71, 101733. [Google Scholar] [CrossRef] [PubMed]
- Gibson, T.M.; Mostoufi-Moab, S.; Stratton, K.L.; Leisenring, W.M.; Barnea, D.; Chow, E.J.; Donaldson, S.S.; Howell, R.M.; Hudson, M.M.; Mahajan, A.; et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018, 19, 1590–1601. [Google Scholar] [CrossRef]
- World Health Organization. Double Burden of Malnutrition. Available online: https://www.who.int/nutrition/double-burden-malnutrition/en/ (accessed on 15 September 2018).
- Rogers, P.C.; Melnick, S.J.; Ladas, E.J.; Halton, J.; Baillargeon, J.; Sacks, N. Children’s Oncology Group (COG) Nutrition Committee. Pediatr. Blood Cancer 2008, 50, 447–450. [Google Scholar] [CrossRef]
- Co-Reyes, E.; Li, R.; Huh, W.; Chandra, J. Malnutrition and obesity in pediatric oncology patients: Causes, consequences, and interventions. Pediatr. Blood Cancer 2012, 59, 1160–1167. [Google Scholar] [CrossRef]
- Brinksma, A.; Huizinga, G.; Sulkers, E.; Kamps, W.; Roodbol, P.; Tissing, W. Malnutrition in childhood cancer patients: A review on its prevalence and possible causes. Crit. Rev. Oncol. Hematol. 2012, 83, 249–275. [Google Scholar] [CrossRef]
- Iniesta, R.R.; Paciarotti, I.; Davidson, I.; McKenzie, J.M.; Brougham, M.F.; Wilson, D.C. Nutritional status of children and adolescents with cancer in Scotland: A prospective cohort study. Clin. Nutr. ESPEN 2019, 32, 96–106. [Google Scholar] [CrossRef]
- Ladas, E.J.; Sacks, N.; Meacham, L.; Henry, D.; Enriquez, L.; Lowry, G.; Hawkes, R.; Dadd, G.; Rogers, P. A Multidisciplinary Review of Nutrition Considerations in the Pediatric Oncology Population: A Perspective from Children’s Oncology Group. Nutr. Clin. Pract. 2005, 20, 377–393. [Google Scholar] [CrossRef]
- Hamilton, E.C.; Curtin, T.; Slack, R.S.; Ge, C.; Slade, A.D.; Hayes-Jordan, A.; Lally, K.P.; Austin, M.T. Surgical Feeding Tubes in Pediatric and Adolescent Cancer Patients: A Single-institution Retrospective Review. J. Pediatr. Hematol. Oncol. 2017, 39, e342–e348. [Google Scholar] [CrossRef] [PubMed]
- Brinksma, A.; Sanderman, R.; Roodbol, P.F.; Sulkers, E.; Burgerhof, J.G.M.; De Bont, E.S.J.M.; Tissing, W.J.E. Malnutrition is associated with worse health-related quality of life in children with cancer. Support. Care Cancer 2015, 23, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.B.; Hodgman, E.I.; Burkhalter, L.S.; Renkes, R.; Slone, T.; Alder, A.C. Long-term central venous access in a pediatric leukemia population. J. Surg. Res. 2016, 205, 419–425. [Google Scholar] [CrossRef]
- Li, M.-J.; Chang, H.-H.; Yang, Y.-L.; Lu, M.-Y.; Shao, P.-L.; Fu, C.-M.; Chou, A.-K.; Liu, Y.-L.; Lin, K.-H.; Huang, L.-M.; et al. Infectious complications in children with acute lymphoblastic leukemia treated with the Taiwan Pediatric Oncology Group protocol: A 16-year tertiary single-institution experience. Pediatr. Blood Cancer 2017, 64, e26535. [Google Scholar] [CrossRef]
- Abla, O.; Ribeiro, R.C.; Testi, A.M.; Montesinos, P.; Creutzig, U.; Sung, L.; Di Giuseppe, G.; Stephens, D.; Feusner, J.H.; Powell, B.L.; et al. Predictors of thrombohemorrhagic early death in children and adolescents with t(15;17)-positive acute promyelocytic leukemia treated with ATRA and chemotherapy. Ann. Hematol. 2017, 96, 1449–1456. [Google Scholar] [CrossRef]
- Orgel, E.; Genkinger, J.M.; Aggarwal, D.; Sung, L.; Nieder, M.; Ladas, E.J. Association of body mass index and survival in pediatric leukemia: A meta-analysis. Am. J. Clin. Nutr. 2016, 103, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Amankwah, E.K.; Saenz, A.M.; Hale, G.A.; Brown, P.A. Association between body mass index at diagnosis and pediatric leukemia mortality and relapse: A systematic review and meta-analysis. Leuk. Lymphoma 2016, 57, 1140–1148. [Google Scholar] [CrossRef]
- Saenz, A.M.; Stapleton, S.; Hernandez, R.G.; Hale, G.A.; Goldenberg, N.A.; Schwartz, S.; Amankwah, E.K. Body Mass Index at Pediatric Leukemia Diagnosis and the Risks of Relapse and Mortality: Findings from a Single Institution and Meta-analysis. J. Obes. 2018, 2018, 7048078. [Google Scholar] [CrossRef]
- Hirose, E.Y.; de Molla, V.C.; Gonçalves, M.V.; Pereira, A.D.; Szor, R.S.; da Fonseca, A.R.B.M.; Fatobene, G.; Serpa, M.G.; Xavier, E.M.; Tucunduva, L.; et al. The impact of pretransplant malnutrition on allogeneic hematopoietic stem cell transplantation outcomes. Clin. Nutr. ESPEN 2019, 33, 213–219. [Google Scholar] [CrossRef]
- Ren, G.; Cai, W.; Wang, L.; Huang, J.; Yi, S.; Lu, L.; Wang, J. Impact of body mass index at different transplantation stages on postoperative outcomes in patients with hematological malignancies: A meta-analysis. Bone Marrow Transplant. 2018, 53, 708–721. [Google Scholar] [CrossRef]
- Ghafoor, T. Prognostic factors in pediatric Hodgkin lymphoma: Experience from a developing country. Leuk. Lymphoma 2020, 61, 344–350. [Google Scholar] [CrossRef]
- González, H.R.; Mejía, S.A.; Ortiz, J.O.C.; Gutiérrez, A.P.O.; López, J.E.B.; Quintana, J.E.F. Malnutrition in paediatric patients with leukaemia and lymphoma: A retrospective cohort study. Ecancermedicalscience 2021, 15, 1327. [Google Scholar] [CrossRef] [PubMed]
- Bs, J.M.S.; Cyrus, J.; Horvai, A.; Hazard, F.K.G.; Neuhaus, J.; Matthay, K.K.; Goldsby, R.; Marina, N.; DuBois, S.G. Predictors of acute chemotherapy-associated toxicity in patients with Ewing sarcoma. Pediatr. Blood Cancer 2012, 59, 611–616. [Google Scholar] [CrossRef]
- Goldstein, G.; Shemesh, E.; Frenkel, T.; Jacobson, J.M.; Toren, A. Abnormal body mass index at diagnosis in patients with Ewing sarcoma is associated with inferior tumor necrosis. Pediatr. Blood Cancer 2015, 62, 1892–1896. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.R.; Vijarnsorn, C.; Potts, J.; Milner, R.; Sandor, G.G.; Fryer, C. Anthracycline induced cardiac toxicity in pediatric Ewing sarcoma: A longitudinal study. Pediatr. Blood Cancer 2013, 60, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, P.; Seidel, K.; Krailo, M.; Mascarenhas, L.; Meyers, P.; Marina, N.; Conrad, E.U.; Hawkins, D.S. Body mass index (BMI) at diagnosis is associated with surgical wound complications in patients with localized osteosarcoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2011, 57, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Altaf, S.; Enders, F.; Jeavons, E.; Krailo, M.; Barkauskas, D.A.; Meyers, P.; Arndt, C. High-BMI at diagnosis is associated with inferior survival in patients with osteosarcoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2013, 60, 2042–2046. [Google Scholar] [CrossRef]
- Rodeberg, D.A.; Stoner, J.A.; Garcia-Henriquez, N.; Randall, R.L.; Spunt, S.L.; Arndt, C.A.; Kao, S.; Paidas, C.N.; Million, L.; Hawkins, D.S. Tumor volume and patient weight as predictors of outcome in children with intermediate risk rhabdomyosarcoma: A report from Children’s Oncology Group. Cancer 2011, 117, 2541–2550. [Google Scholar] [CrossRef]
- Burke, M.E.; Lyden, E.R.; Meza, J.L.; Ladas, E.J.; Dasgupta, R.; Wiegner, E.A.; Arndt, C.A. Does body mass index at diagnosis or weight change during therapy predict toxicity or survival in intermediate risk rhabdomyosarcoma? A report from the Children’s Oncology Group soft tissue sarcoma committee. Pediatr. Blood Cancer 2013, 60, 748–753. [Google Scholar] [CrossRef]
- Fernandez, C.V.; Anderson, J.; Breslow, N.E.; Dome, J.S.; Grundy, P.; Perlman, E.; Green, D.M.; The National Wilms Tumor Study Group/Children’s Oncology Group. Anthropomorphic measurements and event-free survival in patients with favorable histology Wilms tumor: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2009, 52, 254–258. [Google Scholar] [CrossRef]
- Small, A.G.; Thwe, L.M.; Byrne, J.A.; Lau, L.; Chan, A.; Craig, E.M.; Cowell, C.T.; Garnett, S.P. Neuroblastoma, Body Mass Index, and Survival: A retrospective analysis. Medicine 2015, 94, e713. [Google Scholar] [CrossRef] [PubMed]
- Yariş, N.; Akyüz, C.; Coşkun, T.; Kutluk, T.; Büyükpamukçu, M. Nutritional status of children with cancer and its effects on survival. Turk. J. Pediatr. 2002, 44, 35–39. [Google Scholar] [PubMed]
- Tenardi, R.D.; Frühwald, M.C.; Jürgens, H.; Hertroijs, D.; Bauer, J. Nutritional status of children and young adults with Ewing sarcoma or osteosarcoma at diagnosis and during multimodality therapy. Pediatr. Blood Cancer 2012, 59, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Triarico, S.; Rinninella, E.; Natale, L.; Brizi, M.G.; Cintoni, M.; Raoul, P.; Maurizi, P.; Attinà, G.; Mastrangelo, S.; et al. Clinical Impact of Nutritional Status and Sarcopenia in Pediatric Patients with Bone and Soft Tissue Sarcomas: A Pilot Retrospective Study (SarcoPed). Nutrients 2022, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Kadenczki, O.; Nagy, A.; Kiss, C. Prevalence of Undernutrition and Effect of Body Weight Loss on Survival among Pediatric Cancer Patients in Northeastern Hungary. Int. J. Environ. Res. Public Health 2021, 18, 1478. [Google Scholar] [CrossRef] [PubMed]
- Loeffen, E.A.H.; Brinksma, A.; Miedema, K.G.E.; de Bock, G.H.; Tissing, W.J.E. Clinical implications of malnutrition in childhood cancer patients—infections and mortality. Support. Care Cancer 2015, 23, 143–150. [Google Scholar] [CrossRef]
- Triarico, S.; Rinninella, E.; Cintoni, M.; Capozza, A.M.; Mastrangelo, S.; Mele, M.C.; Ruggiero, A. Impact of malnutrition on survival and infections among pediatric patients with cancer: A retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1165–1175. [Google Scholar]
- De Araujo, O.L.; Da Trindade, K.M.; Trompieri, N.M.; Fontenele, J.B.; Felix, F.H.C. Analysis of survival and prognostic factors of pediatric patients with brain tumor. J. Pediatr. 2011, 87, 425–432. [Google Scholar] [CrossRef]
- Lee, J.W.; Cho, B. Prognostic factors and treatment of pediatric acute lymphoblastic leukemia. Korean J. Pediatr. 2017, 60, 129–137. [Google Scholar] [CrossRef]
- Okui, T. Socioeconomic Predictors of Trends in Cancer Mortality among Municipalities in Japan, 2010–2019. Asian Pac. J. Cancer Prev. 2021, 22, 499–508. [Google Scholar] [CrossRef]
- Porojnicu, A.C.; Dahlback, A.; Moan, J. Sun Exposure and Cancer Survival in Norway: Changes in the Risk of Death with Season of Diagnosis and Latitude. In Sunlight, Vitamin D and Skin Cancer; Springer: New York, NY, USA, 2008; pp. 43–54. [Google Scholar] [CrossRef]
- Barr, R.D.; Stevens, M. The influence of nutrition on clinical outcomes in children with cancer. Pediatr. Blood Cancer 2020, 67, e28117. [Google Scholar] [CrossRef] [PubMed]
- Viani, K.; Trehan, A.; Manzoli, B.; Schoeman, J. Assessment of nutritional status in children with cancer: A narrative review. Pediatr. Blood Cancer 2020, 67, e28211. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Jürgens, H.; Frühwald, M.C. Important Aspects of Nutrition in Children with Cancer. Adv. Nutr. Int. Rev. J. 2011, 2, 67–77. [Google Scholar] [CrossRef]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachex-Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef]
- Prado, C.M.; Antoun, S.; Sawyer, M.B.; Baracos, E.V. Two faces of drug therapy in cancer: Drug-related lean tissue loss and its adverse consequences to survival and toxicity. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 250–254. [Google Scholar] [CrossRef]
- Tah, P.C.; Shanita, S.N.; Poh, B.K. Nutritional status among pediatric cancer patients: A comparison between hematological malignancies and solid tumors. J. Spéc. Pediatr. Nurs. 2012, 17, 301–311. [Google Scholar] [CrossRef]
- Hopkins, J.; Sawyer, M.B. A review of body composition and pharmacokinetics in oncology. Expert Rev. Clin. Pharmacol. 2017, 10, 947–956. [Google Scholar] [CrossRef]
- Hoed, M.A.H.D.; Pluijm, S.M.F.; De Groot-Kruseman, H.A.; Winkel, M.L.T.; Fiocco, M.; Akker, E.V.D.; Hoogerbrugge, P.; Berg, H.V.D.; Leeuw, J.; Bruin, M.; et al. The negative impact of being underweight and weight loss on survival of children with acute lymphoblastic leukemia. Haematologica 2015, 100, 62–69. [Google Scholar] [CrossRef]
- Horowitz, N.S.; Wright, A.A. Impact of obesity on chemotherapy management and outcomes in women with gynecologic malignancies. Gynecol. Oncol. 2015, 138, 201–206. [Google Scholar] [CrossRef]
- Silvestris, N.; Argentiero, A.; Natalicchio, A.; D’Oronzo, S.; Beretta, G.; Acquati, S.; Adinolfi, V.; Di Bartolo, P.; Danesi, R.; Faggiano, A.; et al. Antineoplastic dosing in overweight and obese cancer patients: An Associazione Italiana Oncologia Medica (AIOM)/Associazione Medici Diabetologi (AMD)/Società Italiana Endocrinologia (SIE)/Società Italiana Farmacologia (SIF) multidisciplinary consensus position paper. ESMO Open 2021, 6, 100153. [Google Scholar] [CrossRef]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, M.S.; Dent, M.S.; Hall, K. The Insulin-Like Growth Factor System in Obesity, Insulin Resistance and Type 2 Diabetes Mellitus. J. Clin. Med. 2014, 3, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. EBioMedicine 2018, 30, 14–28. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Casabonne, D.; Benavente, Y.; Costas, L.; Robles, C.; Gonzalez-Barca, E.; Banda, E.; Alonso, E.; Aymerich, M.; Campo, E.; Marcos-Gragera, R.; et al. Insulin-like growth factor levels and chronic lymphocytic leukaemia: Results from the MCC -Spain and EpiLymph-Spain studies. Br. J. Haematol. 2019, 185, 608–612. [Google Scholar] [CrossRef]
- Orgel, E.; Sea, J.L.; Mittelman, S.D. Mechanisms by Which Obesity Impacts Survival from Acute Lymphoblastic Leukemia. J. Natl. Cancer Inst. Monogr. 2019, 2019, 152–156. [Google Scholar] [CrossRef]
- Fabozzi, F.; Trovato, C.M.; Diamanti, A.; Mastronuzzi, A.; Zecca, M.; Tripodi, S.I.; Masetti, R.; Leardini, D.; Muratore, E.; Barat, V.; et al. Management of Nutritional Needs in Pediatric Oncology: A Consensus Statement. Cancers 2022, 14, 3378. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Totadri, S.; Trehan, A.; Mahajan, D.; Viani, K.; Barr, R.; Ladas, E.J. Validation of an algorithmic nutritional approach in children undergoing chemotherapy for cancer. Pediatr. Blood Cancer 2019, 66, e27980. [Google Scholar] [CrossRef]
- Elbarbary, N.S.; Ismail, E.A.R.; Farahat, R.K.; El-Hamamsy, M. ω-3 fatty acids as an adjuvant therapy ameliorates methotrexate-induced hepatotoxicity in children and adolescents with acute lymphoblastic leukemia: A randomized placebo-controlled study. Nutrition 2016, 32, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hagag, A.; AbdElaal, A.; Elfaragy, M.; Hassan, S.; Elzamarany, E. Therapeutic Value of Black Seed Oil in Methotrexate Hepatotoxicity in Egyptian Children with Acute Lymphoblastic Leukemia. Infect. Disord. Drug Targets 2015, 15, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; Smith, M.; Henderson, M.; Reid, U.; Lewis, I.; Kinsey, S.; Allgar, V.; Bowers, D.; Picton, S.V. The effect of high-dose enteral glutamine on the incidence and severity of mucositis in paediatric oncology patients. Eur. J. Clin. Nutr. 2009, 63, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Mukherjee, P.; Kiebish, A.M.; Markis, W.T.; Mantis, J.G.; Seyfried, T.N. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr. Metab. 2007, 4, 5. [Google Scholar] [CrossRef]
- Touyz, L.M.; Cohen, J.; Neville, K.A.; Wakefield, C.E.; Garnett, S.P.; Mallitt, K.-A.; Grech, A.M.; Cohn, R.J. Changes in body mass index in long-term survivors of childhood acute lymphoblastic leukemia treated without cranial radiation and with reduced glucocorticoid therapy. Pediatr. Blood Cancer 2017, 64, e26344. [Google Scholar] [CrossRef]
- Fleming, C.A.; Cohen, J.; Murphy, A.; Wakefield, C.E.; Cohn, R.J.; Naumann, F.L. Parent feeding interactions and practices during childhood cancer treatment. A qualitative investigation. Appetite 2015, 89, 219–225. [Google Scholar] [CrossRef]
- Zhang, F.F.; Liu, S.; Chung, M.; Kelly, M.J. Growth patterns during and after treatment in patients with pediatric ALL: A meta-analysis. Pediatr. Blood Cancer 2015, 62, 1452–1460. [Google Scholar] [CrossRef]
- Chaput, C.; Beaulieu-Gagnon, S.; Bélanger, V.; Drouin, S.; Bertout, L.; Lafrance, L.; Olivier, C.; Robitaille, M.; Laverdière, C.; Sinnett, D.; et al. Research- and Practice-Based Nutrition Education and Cooking Workshops in Pediatric Oncology: Protocol for Implementation and Development of Curriculum. JMIR Res. Protoc. 2018, 7, e2. [Google Scholar] [CrossRef]
- Beaulieu-Gagnon, S.; Bélanger, V.; Meloche, C.; Curnier, D.; Sultan, S.; Laverdière, C.; Sinnett, D.; Marcil, V. Nutrition education and cooking workshops for families of children with cancer: A feasibility study. BMC Nutr. 2019, 5, 52. [Google Scholar] [CrossRef]
- Bélanger, V.; Delorme, J.; Napartuk, M.; Bouchard, I.; Meloche, C.; Curnier, D.; Sultan, S.; Laverdière, C.; Sinnett, D.; Marcil, V. Early Nutritional Intervention to Promote Healthy Eating Habits in Pediatric Oncology: A Feasibility Study. Nutrients 2022, 14, 1024. [Google Scholar] [CrossRef]
- Li, R.; Raber, M.; Chandra, J.; Tee, S.-H.; Balter, K. Developing a Healthy Web-Based Cookbook for Pediatric Cancer Patients and Survivors: Rationale and Methods. JMIR Res. Protoc. 2015, 4, e37. [Google Scholar] [CrossRef] [PubMed]
- Wartenberg, L.; Raber, M.; Chandra, J. Unique features of a web-based nutrition website for childhood cancer populations: Availability, features, and content. (Preprint). J. Med. Internet Res. 2021, 23, e24515. [Google Scholar] [CrossRef]
- Touyz, L.M.; Cohen, J.; Garnett, S.P.; Grech, A.M.; Gohil, P.; Cohn, R.J.; Wakefield, C.E. Acceptability and feasibility of a parent-targeted dietary intervention in young survivors of childhood cancer: “Reboot”. Pediatr. Blood Cancer 2020, 67, e28533. [Google Scholar] [CrossRef] [PubMed]
- Chargi, N.; Bashiri, F.; Wendrich, A.W.; Smid, E.J.; de Jong, P.A.; Huitema, A.D.R.; Devriese, L.A.; de Bree, R. Image-based analysis of skeletal muscle mass predicts cisplatin dose-limiting toxicity in patients with locally advanced head and neck cancer. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 3685–3694. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, M.; Jouinot, A.; Burns, R.; Pigneur, F.; Mounier, R.; Gondin, J.; Neuzillet, C.; Goldwasser, F. Body composition and sarcopenia: The next-generation of personalized oncology and pharmacology? Pharmacol. Ther. 2019, 196, 135–159. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.; Suzan, V.; Samanci, N.S.; Suzan, A.A.; Karadag, M.; Sahin, S.; Aslan, M.S.; Yavuzer, H.; Demirci, N.S.; Doventas, A.; et al. Sarcopenia assessment by new EWGSOP2 criteria for predicting chemotherapy dose-limiting toxicity in patients with gastrointestinal tract tumors. Eur. Geriatr. Med. 2022, 13, 267–274. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Ai, L.; Zhang, H.; Li, G.; Wang, Z.; Jiang, X.; Yan, G.; Liu, Y.; Wang, C.; et al. Linear Skeletal Muscle Index and Muscle Attenuation May Be New Prognostic Factors in Colorectal Carcinoma Treated by Radical Resection. Front. Oncol. 2022, 12, 839899. [Google Scholar] [CrossRef]
- Division of Nutrition PA and ONC for CDP and HP. Obesity Is a Common, Serious, and Costly Disease. 2022. Available online: https://www.cdc.gov/obesity/data/adult.html (accessed on 1 January 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).