Abstract
Urachal cancer arises from an embryologic remnant of the urogenital sinus and allantois and accounts for approximately 1% of bladder malignancies. The most encountered histologic subtype is adenocarcinoma. We present a 76-year-old man suspected to have an advanced sigmoid cancer infiltrating nearby organs. A supplemental 18F-FDG PET/CT showed high tracer uptake in a tumorous process coherent with the dome of the bladder wall involving the sigmoid colon. Cystoscopy revealed a normal bladder wall, except for a small edematous area in the anterior bladder. Biopsies from the sigmoid colon and transurethral resection from the bladder confirmed a urothelial carcinoma originating from the urachus.
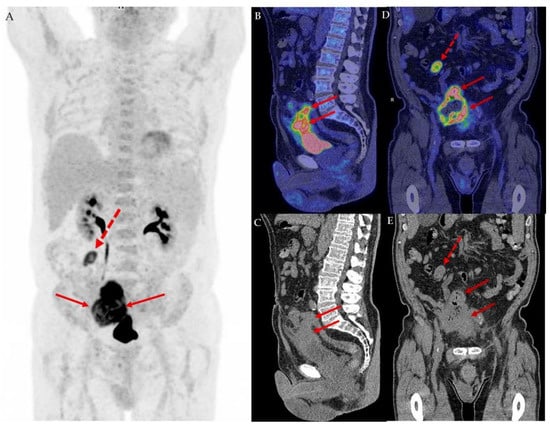
Figure 1.
We present a 76-year-old man initially suspected to have an advanced colon cancer infiltrating nearby organs including the bladder and small intestines. During colonoscopy, the operator describes an ulcerating tumor in the sigmoid colon occupying more than half of the circumference. Surprisingly, the biopsies revealed a urothelial carcinoma. A supplemental 18F-FDG PET/CT conducted as part of the Danish standard-of-care for staging of muscle invasive bladder cancer was conducted along with a cystoscopy [1,2]. The maximum intensity projection of the 18F-FDG PET/CT image in the anterior view (A) revealed a high tracer uptake in an infiltrating tumorous mass coherent with the top of the bladder (full arrows) and an enlarged metastatic lymph node with high tracer uptake (dotted red arrow) in the upper right abdomen. In the sagittal view of the fused 18F-FDG PET/CT image (B) and low-dose CT (C), the coherence of the tumor to the top of the bladder wall stretching along the urachus is elegantly illustrated, supporting the suspicion of a urachal cancer. The tumor infiltrates the peritoneum, small intestines and sigmoid colon. The intestinal involvement is apparent in the coronal fused 18F-FDG PET/CT image (D) and coronal low dose CT (E), which shows the tumor (full arrows) and lymph node metastasis (dotted arrow). The cystoscopy revealed no visible tumors and a normal bladder wall. However, a small area in the top of the anterior bladder wall looked edematous. A small transurethral resection of 3 ml tissue from this area fulfilled the criteria for the diagnosis of urothelial carcinoma, high-grade, originating from the urachus, except that no urachus remnants were found in the specimen [3]. The stage according to the Sheldon Classification [4] was IVA and according to the TNM 8th Edition [5] and TNM Supplement 5th Edition [6] pT4a.
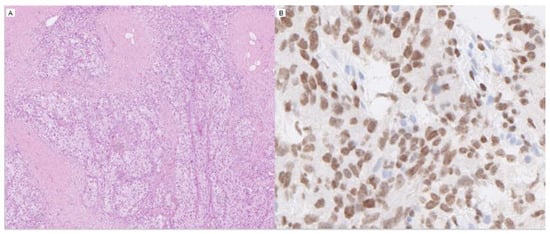
Figure 2.
Two histopathological images of urothelial carcinoma, H&E, low magnification (A), and GATA3, high magnification (B).
Urachal cancer is a rare entity which accounts for 0.5–2% of bladder cancer [7] and most urachal malignancies represents an adenocarcinoma [7,8,9,10,11,12]. According to Reis et al, 58 cases have been reported during the last [6,7] decades, representing a urothelial carcinoma [13]. We present a case of an unusual presentation of the disease which illustrates how an 18F-FDG PET/CT elegantly complements the diagnosis and staging.
Due to the infiltrating tumorous growth involving the sigmoid colon, small intestines and lymph node metastases no primary tumor resection was performed. The patient was offered palliating oncologic treatment.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Einerhand, S.M.H.; van Gennep, E.J.; Mertens, L.S.; Hendricksen, K.; Donswijk, M.L.; van der Poel, H.G.; van Rhijn, B.W.G. 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in muscle-invasive bladder cancer. Curr. Opin. Urol. 2020, 30, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Vind-Kezunovic, S.; Bouchelouche, K.; Ipsen, P.; Høyer, S.; Bell, C.; Bjerggaard Jensen, J. Detection of Lymph Node Metastasis in Patients with Bladder Cancer using Maximum Standardised Uptake Value and (18)F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography: Results from a High-volume Centre Including Long-term Follow-up. Eur. Urol. Focus 2019, 5, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Paner, G.P.; Lopez-Beltran, A.; Sirohi, D.; Amin, M.B. Updates in the Pathologic Diagnosis and Classification of Epithelial Neoplasms of Urachal Origin. Adv. Anat. Pathol. 2016, 23, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, C.A.; Clayman, R.V.; Gonzalez, R.; Williams, R.D.; Fraley, E.E. Malignant urachal lesions. J. Urol. 1984, 131, 1–8. [Google Scholar] [CrossRef]
- Brierley, J.D.; Wittekind, M.K.G.E.C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; p. 272. [Google Scholar]
- Wittekind, C.; Anne Lee, J.D.B.E.; van Eycken, E. (Eds.) TNM Supplement: A Commentary on Uniform Use, 5th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; p. 328. [Google Scholar]
- Chen, D.; Li, Y.; Yu, Z.; Su, Z.; Ni, L.; Gui, Y.; Yang, S.; Shi, B.; Lai, Y. Investigating urachal carcinoma for more than 15 years. Oncol. Lett. 2014, 8, 2279–2283. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Hodge, G.B.; Abdul-Karim, F.W.; Ayala, A.G. Urachal carcinoma. Urology 1985, 26, 218–221. [Google Scholar] [CrossRef]
- Nakanishi, K.; Kawai, T.; Suzuki, M.; Torikata, C. Prognostic factors in urachal adenocarcinoma. A study in 41 specimens of DNA status, proliferating cell-nuclear antigen immunostaining, and argyrophilic nucleolar-organizer region counts. Hum. Pathol. 1996, 27, 240–247. [Google Scholar] [CrossRef]
- Quan, J.; Pan, X.; Jin, L.; He, T.; Hu, J.; Shi, B.; Peng, J.; Chen, Z.; Yang, S.; Mao, X.; et al. Urachal carcinoma: Report of two cases and review of the literature. Mol. Clin. Oncol. 2017, 6, 101–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Riva, G.; Mian, C.; Luchini, C.; Girolami, I.; Ghimenton, C.; Cima, L.; Novelli, L.; Hanspeter, E.; Mazzoleni, G.; Schwienbacher, C.; et al. Urachal carcinoma: From gross specimen to morphologic, immunohistochemical, and molecular analysis. Virchows Arch. 2019, 474, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T.; Módos, O.; Niedworok, C.; Reis, H.; Szendröi, A.; Szász, M.A.; Nyirády, P. Clinical, prognostic, and therapeutic aspects of urachal carcinoma-A comprehensive review with meta-analysis of 1010 cases. Urol. Oncol. 2016, 34, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Reis, H.; Krafft, U.; Niedworok, C.; Módos, O.; Herold, T.; Behrendt, M.; Al-Ahmadie, H.; Hadaschik, B.; Nyirady, P.; Szarvas, T. Biomarkers in Urachal Cancer and Adenocarcinomas in the Bladder: A Comprehensive Review Supplemented by Own Data. Dis. Markers 2018, 2018, 7308168. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).