Abstract
Brain tumors are the most common solid neoplasms of childhood. They are frequently reported in children with Neurofibromatosis type 1 (NF1). The most frequent central nervous system malignancies described in NF1 are optic pathway gliomas and brainstem gliomas. Medulloblastoma (MB) in NF1 patients is extremely rare, and to our knowledge, only 10 cases without molecular characterization are described in the literature to date. We report the case of a 14-year-old girl with NF1 that came to our attention for an incidental finding of a lesion arising from cerebellar vermis. The mass was completely resected, revealing a localized classic medulloblastoma (MB), subgroup 4. She was treated as a standard-risk MB with a dose-adapted personalized protocol. The treatment proved to be effective, with minor toxicity. Brain and spine MRI one year after diagnosis confirmed the complete remission of the disease. To our knowledge, this is the only case of MB reported in a patient with NF1 with molecular characterization by the methylation profile. The association between NF1 and MB, although uncommon, may not be an accidental occurrence.
1. Introduction
Neurofibromatosis type 1 (NF1, OMIM #162200) is a relatively common genetic syndrome, with a prevalence ranging between 1/3000 and 1/6000 worldwide [1,2,3,4]. The disorder affects multiple systems with major cutaneous, neurologic, and orthopedic manifestations, which lead to significant morbidity and mortality [5,6,7,8]. NF1 patients have an increased risk of developing malignancies and a life expectancy of about 10–15 years shorter than the general population [9]. NF1 diagnosis is primarily based on the National Institutes of Health diagnostic criteria (Table 1): 97% of patients with NF1 meet such criteria by eight years of age and all by 20 years of age [10]. These criteria usually appear in the following predictable sequence of symptoms: café-au-lait macules, axillary freckling, Lisch nodules, and neurofibromas. The characteristic osseous lesions typically appear within the first year of life, and the mean age ranges from three to six years at diagnosis of optic gliomas [10].

Table 1.
National Institutes of Health clinical diagnostic criteria for neurofibromatosis type 1 [6].
A vast number of different pathogenic variants in the NF1 gene have been described [11,12,13,14,15]. Large deletions involving the NF1 locus (chromosomal region 17q11.2) have been associated with a more severe phenotype, including developing neurofibromas at an earlier age, having a lower mean intelligence quotient, abnormal facial features, and an elevated risk for malignant peripheral nerve sheath tumors [16,17,18].
Malignancies are the leading cause of death in NF1 [19,20]. Compared to the general population, individuals with NF1 are two-to-three times more likely to develop cancer of the esophagus, stomach, colon, or lungs and three–seven times more likely to develop cancer of the liver, thyroid, ovary, breast, malignant melanoma, non-Hodgkin’s lymphoma, or chronic myeloid leukemia in their lifetime. They are also 15 times more likely to develop small intestine tumors and 20 times more likely to develop bone cancer [21], specifically before 50 years of age [22].
Children under 10 years old are the most susceptible to the development of intracranial tumors that occur in 20% of individuals with NF1 [23]. The most common central nervous system (CNS) tumor associated with NF1 is the optic pathway glioma (OPG), reported in 15–20% of children affected with this condition [24,25]. The second-most frequent brain tumor in individuals with NF1 is brainstem glioma (BSG), which is an indolent neoplasm with a good prognosis [26,27,28] that arises in slightly older children (mean age is seven years) and are often incidentally discovered on neuroimaging studies [28,29,30]. Similar to OPGs, these tumors are usually low-grade gliomas and, in particular pilocytic astrocytomas. Gliomas can also be found in other locations, such as the temporal lobe, cerebellum, thalamus, basal ganglia, or spinal cord [31], and a larger part are asymptomatic. NF1 patients can also develop high-grade gliomas (HGG). These are uncommon in children with NF1 but increase in prevalence during early adulthood [31], with estimates of a >50-fold increased risk relative to the general population [32]. HGG usually arise in the cerebral hemispheres.
Medulloblastoma (MB) is the most common pediatric brain tumor. Advances in molecular characterization have found significant heterogeneity among MBs, which has led to the identification of four distinct molecular subgroups included in the 2016 WHO Classification of Tumors of the Central Nervous System (wingless (WNT), sonic hedgehog (SHH), group 3, and group 4) [33,34]. Moreover, new scientific data has shown that germline pathogenic variants in cancer predisposition genes account for about 5% to 6% of all MB diagnoses [35].
The finding of MB in NF1 patients is extremely rare: to our knowledge, only ten cases without molecular characterization have been reported in the literature.
We hereby describe, to the best of our knowledge, the first molecularly characterized case of a subgroup 4 MB reported in a child with NF1.
2. Case Presentation
A 14-year-old mixed-race girl affected by familial NF1 (affected father) came to our attention for the incidental finding of a lesion arising from the cerebellar vermis and extending to the roof of the fourth ventricle, without detection of secondary lesions. It was revealed accidentally by a magnetic resonance imaging (MRI) that was performed for increasing headaches. The sequence of the NF1 gene (NM_000267) on DNA isolated from the blood did not reveal germline pathogenic variants. Subsequently, her genetic profile was studied by the Multiplex Ligation-dependent Probe Amplification (MLPA) analysis for microdeletions/microduplications of the NF1 gene (NM_000267), which detected the deletion of the entire NF1 gene (assay performed by Multiplex Ligation-dependent Probe Amplification (MLPA) kits P081-D1-0418 and P082-C2-0419 (Mrc Holland, Amsterdam, The Netherlands) and capillary electrophoresis with Genetic Analyzer automated sequencer (Applied Biosystems, Waltham, MA, USA).
At the physical examination, the girl presented NF1 stigmatic characteristics, which included more than six diffuse pathognomonic café-au-lait spots, neurofibromas on the left wrist and shoulders, freckling in the trunk and groin region, Lisch nodules in both eyes, and Tanner puberty stage V. The neurological examination showed there were no motor or sensory deficits and no cranial nerve abnormalities.
After preoperatory evaluation that showed a localized disease, the patient underwent a complete surgical resection of the intracranial mass. A lumbar puncture (LP) was performed 15 days after surgery, and no neoplastic cells were detected.
Microscopically, the tumor showed a vaguely nodular pattern (Figure 1A), but desmoplasia was mostly absent and limited to the areas of leptomeningeal invasion (Figure 1B). Immunohistochemical analyses revealed that the tumor tissue was diffusely positive for synaptophysin and negative for YAP1 and GAB1, whereas the β-catenin expression was limited to the cytoplasm (Figure 1C–F). Ki67 was highly expressed in the internodular areas (Figure 1G). Coherently, Sanger sequencing failed to detect pathogenic variants in the CTNNB1 gene. The tumor was interpreted as a MB, classic type, non-WNT, and non-SHH. Gain/amplification of MYC/MYCN, such as a TP53 mutation, was not found.
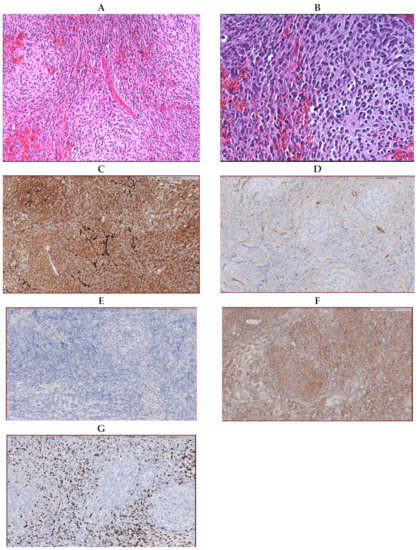
Figure 1.
Histological samples preparation of the tumor (hematoxylin-;eosin coloration) showing a vague nodular pattern (A) without an increase in reticulin (B). Immunohistochemical samples revealing the diffused expression of synaptophysin (C), YAP1 solely expressed by the endothelial cells (D), no expression of GAB1 (E), the expression of Beta-catenin limited to the cytoplasm (F), and a high expression of Ki67 in the internodular areas (G).
As previously reported, DNA methylation profiling was performed in accordance with the protocols approved by the Bambino Gesù Children’s Hospital Institutional Review Board (1556_OPBG_2018, approved on 15/01/2019) after written consent was obtained from the patient’s parents.
In the brain tumor classifier developed by Heidelberg University, Heidelberg, Germany [36], the tumor scored significantly in the “methylation class family medulloblastoma group 3 and 4, subtype VI”, reaching a raw score of 0.55 (Figure 2A) and a calibrated score of 0.98 in the “methylation class medulloblastoma, subclass group 4”. Molecular subgroups 3 and 4 that reflect non-WNT/non-SHH groups in immunohistochemistry are consistent with limited cytoplasm β-catenin expression and are YAP1- and GAB1-immunonegative, as previously described.
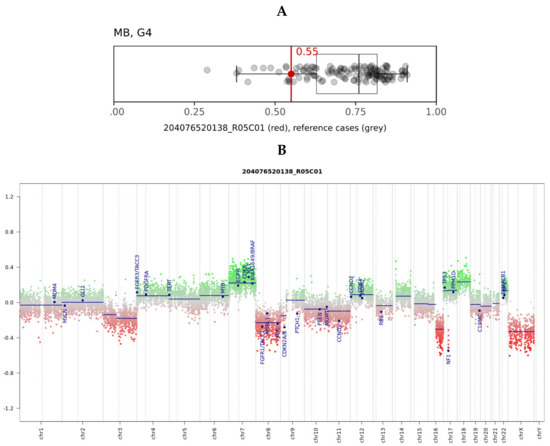
Figure 2.
(A) Box and whisker plots depict the maximum raw classification scores (0.55) of the tumor sample in the methylation class “methylation class medulloblastoma, subclass group 4 (MB, G4)”, according to brain tumor classifier v11b4. Grey dots represent the reference cases in the methylation class. (B) Copy number variation plots were calculated from the DNA to 22 and X. Gains/amplifications represent positive (green), and losses negative (red), deviations from the baseline. Twenty-nine brain tumor-relevant genomic regions are highlighted. The red arrow points out the deleted chromosome 17q region, including the NF1 gene.
The plotted copy number variation showed the partial deletion of chromosomes 3, 8, and 16q, as well as partial chromosome 7 and 18 gains. (Figure 2B). The known deletion of the entire NF1 gene locus was also evident (red arrow). Neither the methylation array nor the real-time PCR demonstrated a gain/amplification of MYC/MYCN.
Complete resected and isolated classical MB without gain/amplification of MYC/MYCN and TP53 pathogenic variants allowed for the classification as a standard-risk MB. Considering her genetic syndrome, it was not possible to enroll the patient in the MB protocol. In consideration of the increased risk of secondary malignancies, she was treated with a personalized regimen according to the European indications for standard-risk MB, based on proton therapy rather than standard radiotherapy and four courses of reduced doses of vincristine, cyclophosphamide, and cisplatin. Vincristine-related neurotoxicity was reported, and two doses of vincristine were omitted.
The brain and spine MRI one year after diagnosis confirmed the complete remission of the disease.
3. Discussion
NF1 is an autosomal-dominant tumor predisposition syndrome caused by deletions or pathogenic variants in the NF1 gene [9,11,12,13,14]. Neurofibromin, the product of the NF1 gene, is a negative regulator of the RAS/MAPK pathway [37]. Its loss of function leads to an increased RAS pathway activity, with a subsequent increased proliferation and tumorigenesis, specifically in neurocutaneous tissues [38].
Brain tumors occur in about 20% of individuals with NF1 [23]. Cerebral hemisphere and posterior fossa tumors, such as astrocytomas and medulloblastomas, are uncommon in NF1 patients and occur globally at a 1% rate [39].
MBs account for about 9.2% of pediatric brain tumors [40]. The incidence of MB peaks during the first decade of life, with a higher incidence noted in children between three and four years of age and between eight and 10 years of age [40]. A risk stratification established based on the histopathological subtype, age at diagnosis, staging, residual disease, MYC and TP53 status, and molecular subgrouping allows for a distinction of low-, standard-, and high-risk patients [41].
Low- and standard-risk patients are characterized by ages over three years old, the absence of metastatic and/or residual disease, histotypes other than anaplastic/large cells, the absence of MYC amplification, and/or TP53 pathogenic variants. On the other side, patients less than three years of age with residual postoperative tumors, metastases, anaplastic/large cell histotypes, MYC amplification, and TP53 mutations reflected high-risk MB [42]. Recently, to more accurately predict the outcome, an updated risk stratification took into account the subgroup status and genetic and cytogenetic aberrations. This new risk stratification system allocates patients to one of four risk groups: low-risk (> 90% survival), standard-risk (75–90% survival), high-risk (50–75% survival), and very high risk (< 50% survival) [42]. Four different subgroups were identified based on genetic profiling studies: Wingless (WNT), Sonic Hedgehog (SHH), Group 3, and Group 4; each of these is characterized by a specific set of demographic, clinical, and genetic features [30].
In our case, the patient was affected by the classic group 4 MB. Group 4 tumors form the largest molecular subgroup of MBs, comprising about 35% of the overall cases. Similar to SHH subgroup tumors, Group 4 tumors have a prognosis that is intermediate between WNT and Group 3 MBs. Within Group 4 MBs, significantly inferior outcomes have been observed in patients with metastatic disease or MYCN amplification [40].
The treatment of MB includes a combination of surgery, radiation therapy (in patients >three years old), and chemotherapy and is associated with significant morbidity, especially in the youngest patients.
MB has been reported in association with certain cancer predisposition syndromes [43], such as the MB SHH subtype with Gorlin-Goltz syndrome (PTCH1 and SUFU genes) [43,44]. The elevated frequency of MBs has also been observed in patients with Turcot syndrome (APC gene and MMR genes), where colon polyps and brain tumors are associated in a context of alterations of the WNT pathway [45]. Similarly, numerous MBs have been reported in patients with Nijmegen syndrome as a result of defects in the DNA repair signaling pathways [46]. Few cases of MBs have been associated with Down syndrome [47]. Other rare genetic syndromes associated with MB involve pathogenic variants in the BRCA2, PALB2, GPR161, and ELP genes that were recently identified, even if cases of NF1 patients are not reported [43].
MB is very rarely associated with NF1. To the best of our knowledge, a total of ten cases are described in the scientific literature to date. In Table 2, we briefly described the principal characteristics of our case and the other 10 cases described in the literature. Information regarding the MB histology of the reported patients is very poor, and no patient, other than ours, has a known molecular subgroup.
The first case was described in 1969 by Corkill and Ross [48]. They described an eight-year-old boy with NF1 and multiple tumors (medulloblastoma, multiple neurofibromas/sarcomas, and radiation-induced thyroid carcinoma). The patient died nine years after due to the metastatic involvement of sarcomatous nerve tumors. Perilongo et al. [49] described in 1993 an eight-year-old girl with NF1 who developed four consecutively primary malignant tumors: a Wilms tumor, T-cell acute lymphoblastic leukemia, MB, and acute myeloid leukemia. Magimairajan et al. [50] described a case of NF1 with MB and immunohistochemistry for mismatch repair positivity.
In this study, we reported the eleventh case of a patient with NF1 and MB subgroup 4. This is the first case investigated by DNA methylation profiling. The association between MB and NF1 is not well-established, and further studies are needed to elucidate the possible molecular mechanisms underlying this correlation. As declared by Martinez-Lage et al. [51], this association, although uncommon, may not be an accidental occurrence, and neurofibromatosis should be included in the cancer predisposition syndrome for medulloblastoma. One of the possible second hits in individuals with NF1 is a somatic rearrangement leading to isochromosome 17q, causing the loss of the remaining wild-type allele of the NF1 gene and triggering tumorous degeneration in the affected tissue. Such a isochromosome, 17q, has been reported in several cases of MBs belonging to the GP4 group. This possible connection may provide further validation to the mechanistic link between NF1 and MBs.
Moreover, no significant clinical-pathological correlation or molecular profile were described until now in medulloblastoma related to NF1. To the best of our knowledge, molecular methylation profile characterization was described for the first time in our case, and more cases are needed to establish if NF1 gene pathogenic variants or deletions can drive medulloblastoma development.
In the future, it will be crucial to molecularly classify these tumors in order to better understand their biology and to allow prognostic evaluations of this peculiar patient population.

Table 2.
Medulloblastoma in patients with Neurofibromatosis type 1 (NF1) described in the literature.
Table 2.
Medulloblastoma in patients with Neurofibromatosis type 1 (NF1) described in the literature.
| Article | Cases/NF1 Patients | Age at MB Diagnosis (y) | Histology | Outcome | FU | Other Findings |
|---|---|---|---|---|---|---|
| Corkill et al. (1969) [48] | 1 | 8 | unk | Died at 17 y because of metastatic sarcomatous nerve tumors in MB CR | 9 y | Multiple neurofibromas, thyroid carcinoma, and sarcomatous nerve tumor at age of 17 y |
| Robles Cascallar et al. (1992) [52] | 1 | 1 | unk | unk | unk | no |
| Perilongo et al. (1993) [49] | 1 | 8 | unk | unk | unk | Wilms tumor, T-cell acute lymphoblastic, and myeloid leukemia |
| Martinez-Lage et al. (2002) [51] | 1 | 6 | Classic | Alive in CR | 5 y | no |
| Rosenfeld et al. (2010) [32] | 1/740 | 16 | Anaplastic | Alive in CR | 11 m | no |
| Pascual-Castrovejo et al. (2010) [39] | 1/600 | 4 | unk | Died | 3 y | no |
| Varan et al. (2015) [53] | 2/473 | #1: 4 #2: 9 | unk | #1: Died #2: Alive in CR | #1: 1 m #2: 11 y | #1: no #2: no |
| Magimairajan et al. (2016) [50] | 1 | unk | unk | Alive in CR | 6 m | MMRD |
| Marinău et al. (2017) [54] | 1 | 4 | Desmoplastic | Died for metastatic progression | 6 m | no |
| Ranalli et al. (our case) | 1 | 14 | Classic—subgroup 4 | Alive in CR | 6 m | no |
Unk, unknown; CR, complete remission; FU, follow-up; y, years; m, months; MMRD, mismatch repair defect genes; and MB, medulloblastoma.
Author Contributions
A.M. coordinated the study; M.R. (Marco Ranalli), A.B., A.M.C., G.D.B. reviewed the literature and wrote the manuscript; A.M., A.C. (Andrea Carai) and L.B. reviewed the manuscript; S.R. and E.M. performed the pathologic findings; R.C., A.C. (Antonella Cacchione), G.D.B., I.A., A.M.C., M.A.D.I. contributed to patient management and revision; A.C. (Andrea Carai) contributed to neurosurgery and revision; S.V. contributed to protontherapy treatment and revision, G.S.C. acquired and elaborated the images; E.A. and M.R. (Martina Rinelli) performed the genetic analysis; A.C., L.B., and A.M. critically revised the manuscript for intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors thank Megan Eckley for helping with the English final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lammert, M.; Friedman, J.M.; Kluwe, L.; Mautner, V.F. Prevalence of Neurofibromatosis 1 in German Children at Elementary School Enrollment. Arch. Dermatol. 2005, 141, 71–74. [Google Scholar] [CrossRef]
- Evans, D.G.; Howard, E.; Giblin, C.; Clancy, T.; Spencer, H.; Huson, S.M.; Lalloo, F. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. Am. J. Med. Genet. Part A 2010, 152A, 327–332. [Google Scholar] [CrossRef]
- Huson, S.M.; Compston, D.A.; Clark, P.; Harper, P.S. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J. Med. Genet. 1989, 26, 704–711. [Google Scholar] [CrossRef]
- Kallionpää, R.A.; Uusitalo, E.; Leppävirta, J.; Pöyhönen, M.; Peltonen, S.; Peltonen, J. Prevalence of neurofibromatosis type 1 in the Finnish population. Genet. Med. 2017, 20, 1082–1086. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Yang, Q.; Friedman, J. Mortality in Neurofibromatosis 1: An Analysis Using U.S. Death Certificates. Am. J. Hum. Genet. 2001, 68, 1110–1118. [Google Scholar] [CrossRef]
- Zöller, M.; Rembeck, B.; Akesson, H.O.; Angervall, L. Life expectancy, mortality and prognostic factors in neurofibromatosis type 1. A twelve-year follow-up of an epidemiological study in Göteborg, Sweden. Acta Derm Venereol. 1995, 75, 136–140. [Google Scholar]
- Madanikia, S.A.; Bergner, A.; Ye, X.; Blakeley, J.O. Increased risk of breast cancer in women with NF1. Am. J. Med. Genet. Part A 2012, 158A, 3056–3060. [Google Scholar] [CrossRef]
- Uusitalo, E.; Rantanen, M.; Kallionpää, R.A.; Pöyhönen, M.; Leppävirta, J.; Ylä-Outinen, H.; Riccardi, V.M.; Pukkala, E.; Pitkäniemi, J.; Peltonen, S.; et al. Distinctive Cancer Associations in Patients With Neurofibromatosis Type 1. J. Clin. Oncol. 2016, 34, 1978–1986. [Google Scholar] [CrossRef]
- Bergqvist, C.; Network, N.F.; Servy, A.; Valeyrie-Allanore, L.; Ferkal, S.; Combemale, P.; Wolkenstein, P. Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet J. Rare Dis. 2020, 15, 1–23. [Google Scholar] [CrossRef]
- DeBella, K.; Szudek, J.; Friedman, J.M. Use of the national institutes of health criteria for diagnosis of neurofibromatosis 1 in chil-dren. Pediatrics 2000, 105 (3 Pt 1), 608–614. [Google Scholar] [CrossRef]
- Messiaen, L.M.; Callens, T.; Mortier, G.; Beysen, D.; Vandenbroucke, I.; Van Roy, N.; Speleman, F.; Paepe, A.D. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum. Mutat. 2000, 15, 541–555. [Google Scholar] [CrossRef]
- Ars, E.; Kruyer, H.; Morell, M.; Pros, E.; Serra, E.; Ravella, A.; Estivill, X.; Lázaro, C. Recurrent mutations in the NF1 gene are common among neuro-fibromatosis type 1 patients. J. Med. Genet. 2003, 40, e82. [Google Scholar] [CrossRef]
- Wimmer, K.; Yao, S.; Claes, K.; Kehrer-Sawatzki, H.; Tinschert, S.; De Raedt, T.; Legius, E.; Callens, T.; Beiglböck, H.; Maertens, O.; et al. Spectrum of single- and multiexon NF1 copy number changes in a cohort of 1,100 unselected NF1 patients. Genes Chromosom. Cancer 2005, 45, 265–276. [Google Scholar] [CrossRef]
- Pros, E.; Gómez, C.; Martín, T.; Fábregas, P.; Serra, E.; Lázaro, C. Nature and mRNA effect of 282 differentNF1point mutations: Focus on splicing alterations. Hum. Mutat. 2008, 29, E173–E193. [Google Scholar] [CrossRef]
- Sabbagh, A.; Pasmant, E.; Imbard, A.; Luscan, A.; Soares, M.; Blanche, H.; Laurendeau, I.; Ferkal, S.; Vidaud, M.; Pinson, S.; et al. NF1 molecular characterization and neurofibroma-tosis type I genotype-phenotype correlation: The French experience. Hum. Mutat. 2013, 34, 1510–1518. [Google Scholar] [CrossRef]
- De Raedt, T.; Brems, H.; Wolkenstein, P.; Vidaud, D.; Pilotti, S.; Perrone, F.; Mautner, V.; Frahm, S.; Sciot, R.; Legius, E. Elevated Risk for MPNST in NF1 Microdeletion Patients. Am. J. Hum. Genet. 2003, 72, 1288–1292. [Google Scholar] [CrossRef]
- Leppig, K.A.; Kaplan, P.; Viskochil, D.; Weaver, M.; Ortenberg, J.; Stephens, K. Familial neurofibromatosis 1 microdeletions: Cosegregation with distinct facial phenotype and early onset of cutaneous neurofibromata. Am. J. Med. Genet. 1997, 73, 197–204. [Google Scholar] [CrossRef]
- Pasmant, E.; Sabbagh, A.; Spurlock, G.; Laurendeau, I.; Grillo, E.; Hamel, M.-J.; Martin, L.; Barbarot, S.; Leheup, B.; Rodriguez, D.; et al. NF1 microdeletions in neurofibromatosis type 1: From genotype to phenotype. Hum. Mutat. 2010, 31, E1506–E1518. [Google Scholar] [CrossRef]
- Duong, T.A.; Sbidian, E.; Valeyrie-Allanore, L.; Vialette, C.; Ferkal, S.; Hadj-Rabia, S.; Glorion, C.; Lyonnet, S.; Zerah, M.; Kemlin, I.; et al. Mortality Associated with Neurofibro-matosis 1: A Cohort Study of 1895 Patients in 1980–2006 in France. Orphanet J. Rare Dis. 2011, 6, 18. [Google Scholar] [CrossRef]
- Patil, S.; Chamberlain, R.S. Neoplasms Associated with Germline and Somatic NF1 Gene Mutations. Oncology 2011, 17, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Seminog, O.O.; Goldacre, M.J. Risk of benign tumours of nervous system, and of malignant neoplasms, in people with neurofi-bromatosis: Populationbased record-linkage study. Br. J. Cancer 2013, 108, 193–198. [Google Scholar] [CrossRef]
- Walker, L.M.; Thompson, D.; Easton, D.F.; Ponder, B.A.J.; Ponder, M.; Frayling, I.M.; Baralle, D. A prospective study of neurofibromatosis type 1 cancer incidence in the UK. Br. J. Cancer 2006, 95, 233–238. [Google Scholar] [CrossRef]
- Rosser, P.R.T. Intracranial neoplasms in children with neurofibromatosis 1. J. Child Neurol. 2002, 17, 630–637. [Google Scholar] [CrossRef]
- Lewis, R.A.; Gerson, L.P.; Axelson, K.A.; Riccardi, V.M.; Whitford, R.P. Von Recklinghausen neurofibromatosis. II. Incidence of optic gliomata. Ophthalmology 1984, 91, 929–935. [Google Scholar] [CrossRef]
- Listernick, R.; Charrow, J.; Greenwald, M.; Mets, M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: A longitudinal study. J. Pediatr. 1994, 125, 63–66. [Google Scholar] [CrossRef]
- Guillamo, J.; Créange, A.; Kalifa, C.; Grill, J.; Rodriguez, D.; Doz, F.; Barbarot, S.; Zerah, M.; Sanson, M.; Bastuji-Garin, S.; et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): A retrospective study of 104 patients. Brain 2002, 126, 152–160. [Google Scholar] [CrossRef]
- Sellmer, L.; Farschtschi, S.; Marangoni, M.; Heran, M.K.S.; Birch, P.; Wenzel, R.; Friedman, J.M.; Mautner, V.-F. Non-optic glioma in adults and children with neurofibromatosis 1. Orphanet J. Rare Dis. 2017, 12, 34. [Google Scholar] [CrossRef]
- Mahdi, J.; Shah, A.C.; Sato, A.; Morris, S.M.; McKinstry, R.C.; Listernick, R.; Packer, R.J.; Fisher, M.J.; Gutmann, D.H. A multi-institutional study of brainstem gliomas in children with neurofibromatosis type 1. Neurology 2017, 88, 1584–1589. [Google Scholar] [CrossRef]
- Ullrich, N.J.; Raja, A.I.; Irons, M.B.; Kieran, M.W.; Goumnerova, L. Brainstem Lesions in Neurofibromatosis Type 1. Neurosurgery 2007, 61, 762–767. [Google Scholar] [CrossRef]
- Bilaniuk, L.T.; Molloy, P.T.; Zimmerman, R.A.; Phillips, P.C.; Vaughan, S.N.; Liu, G.T.; Sutton, L.N.; Needle, M. Neurofibromatosis type 1: Brain stem tumours. Neuroradiology 1997, 39, 642–653. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Rasmussen, S.A.; Wolkenstein, P.; MacCollin, M.M.; Guha, A.; Inskip, P.D.; North, K.N.; Poyhonen, M.; Birch, P.H.; Friedman, J.M. Gliomas presenting after age 10 in individuals with neurofibromatosis type 1 (NF1). Neurology 2002, 59, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, A.; Listernick, R.; Charrow, J.; Goldman, S. Neurofibromatosis type 1 and high-grade tumors of the central nervous system. Child’s Nerv. Syst. 2009, 26, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.-J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2011, 123, 465–472. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Waszak, S.M.; Northcott, P.A.; Buchhalter, I.; Robinson, G.W.; Sutter, C.; Groebner, S.; Grund, K.B.; Brugières, L.; Jones, D.T.W.; Pajtler, K.W.; et al. Spectrum and prevalence of genetic pre-disposition in medulloblastoma: A retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol. 2018, 19, 785–798. [Google Scholar] [CrossRef]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Xu, G.F.; O’Connell, P.; Viskochil, D.; Cawthon, R.; Robertson, M.; Culver, M.; Dunn, D.; Stevens, J.; Gesteland, R.; White, R.; et al. The neurofbromatosis type 1 gene encodes a protein related to GAP. Cell 1990, 62, 599–608. [Google Scholar] [CrossRef]
- Basu, T.N.; Gutmann, D.H.; Fletcher, J.A.; Glover, T.W.; Collins, F.S.; Downward, J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nat. Cell Biol. 1992, 356, 713–715. [Google Scholar] [CrossRef]
- Pascual-Castroviejo, I.; Pascual-Pascual, S.I.; Viaño, J.; Carceller, F.; Gutiérrez-Molina, M.; Morales, C.; Frutos-Martinez, R. Posterior fossa tumors in children with neurofibromatosis type 1 (NF1). Child’s Nerv. Syst. 2010, 26, 1599–1603. [Google Scholar] [CrossRef]
- Millard, N.E.; De Braganca, K.C. Medulloblastoma. J. Child Neurol. 2016, 31, 1341–1353. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, S.C.; Doz, F.; Kool, M.; Dufour, C.; Vassal, G.; Milde, T.; et al. Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathol. 2016, 131, 821–831. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Faria, C.C.; Perreault, S.; Cho, Y.-J.; Shih, D.J.; Luu, B.; Dubuc, A.M.; Northcott, P.A.; et al. Recurrence patterns across medulloblastoma subgroups: An integrated clinical and molecular analysis. Lancet Oncol. 2013, 14, 1200–1207. [Google Scholar] [CrossRef]
- Carta, R.; Del Baldo, G.; Miele, E.; Po, A.; Besharat, Z.M.; Nazio, F.; Colafati, G.S.; Piccirilli, E.; Agolini, E.; Rinelli, M.; et al. Cancer Predisposition Syndromes and Medullo-blastoma in the Molecular Era. Front. Oncol. 2020, 10, 566822. [Google Scholar] [CrossRef]
- Garrè, M.L.; Cama, A.; Bagnasco, F.; Morana, G.; Giangaspero, F.; Brisigotti, M.; Gambini, C.; Forni, M.; Rossi, A.; Haupt, R.; et al. Medulloblastoma Variants: Age-Dependent Occurrence and Relation to Gorlin Syndrome—A New Clinical Perspective. Clin. Cancer Res. 2009, 15, 2463–2471. [Google Scholar] [CrossRef]
- Hamilton, S.R.; Liu, B.; Parsons, R.E.; Papadopoulos, N.; Jen, J.; Powell, S.M.; Krush, A.J.; Berk, T.; Cohen, Z.; Tetu, B.; et al. The molecular basis of Turcot’s syndrome. N. Eng. J. Med. 1995, 332, 839–847. [Google Scholar] [CrossRef]
- Saran, A. Medulloblastoma: Role of developmental pathways, DNA repair signaling, and other players. Curr. Mol. Med. 2009, 9, 1046–1057. [Google Scholar] [CrossRef]
- Boni, A.; Ranalli, M.; Del Baldo, G.; Carta, R.; Lodi, M.; Agolini, E.; Rinelli, M.; Valentini, D.; Rossi, S.; Alesi, V.; et al. Medulloblastoma Associated with Down Syndrome: From a Rare Event Leading to a Pathogenic Hypothesis. Diagnostics 2021, 11, 254. [Google Scholar] [CrossRef]
- Corkill, A.G.; Ross, C.F. A case of neurofibromatosis complicated by medulloblastoma, neurogenic sarcoma, and radiation-induced carcinoma of thyroid. J. Neurol. Neurosurg. Psychiatry 1969, 32, 43–47. [Google Scholar] [CrossRef][Green Version]
- Perilongo, G.; Felix, C.A.; Meadows, A.T.; Nowell, P.; Biegel, J.; Lange, B.J. Sequential development of Wilms tumor, T-cell acute lymphoblastic leukemia, medulloblastoma and myeloid leukemia in a child with type 1 neurofibromatosis: A clinical and cy-togenetic case report. Leukemia 1993, 7, 912–915. [Google Scholar]
- Vanan, M.I.; McDonald, P.; Marles, S.; Frosk, P.; Krawitz, S.; Moffat, H. Rare-22. Medulloblastoma in a child with neurofibromatosis-1: Case report and review. Neuro-Oncol. 2016, 18, vi164. [Google Scholar] [CrossRef][Green Version]
- Martínez-Lage, J.; Salcedo, C.; Corral, M.; Poza, M. Medulloblastomas in neurofibromatosis type 1. Case report and literature review. Neurocirugía 2002, 13, 128–131. [Google Scholar] [CrossRef]
- Robles Cascallar, P.; Contra Gómez, T.; Martín Ramos, N.; Scaglione Ríos, C.; Madero López, L. Asociación de neurofibromatosis tipo I y méduloblastoma [Association of neurofibromatosis type 1 and medulloblastoma]. An. Esp. Pediatr. 1992, 37, 57–58. [Google Scholar] [PubMed]
- Varan, A.; Şen, H.; Aydın, B.; Yalçın, B.; Kutluk, T.; Akyüz, C. Neurofibromatosis type 1 and malignancy in childhood. Clin. Genet. 2015, 89, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Marinău, L.D.; Singer, C.E.; Meşină, C.; Niculescu, E.C.; Puiu, I.; Petrescu, I.O.; Geormăneanu, C.; Enculescu, A.C.; Tache, D.E.; Purcaru, Ş.O.; et al. Two girl patients with medulloblastoma. Case reports. Rom. J. Morphol. Embryol. 2017, 58, 1103–1108. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).