Ventilator Parameters in the Diagnosis and Prognosis of Acute Respiratory Distress Syndrome in Postoperative Patients: A Preliminary Study
Abstract
1. Introduction
2. Objective
3. Material and Methods
3.1. Study Design
3.2. Ventilator Parameters
3.3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ashbaugh, D.; Bigelow, D.B.; Petty, T.; Levine, B. Acute respiratory distress in adults. Lancet 1967, 2, 319–323. [Google Scholar] [CrossRef]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Morris, A.; Spragg, R. The American-European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Resp. Crit. Care Med. 1994, 149, 818–824. [Google Scholar] [CrossRef]
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L.; et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intens. Care Med. 2012, 38, 1731–1732. [Google Scholar] [CrossRef]
- D’Annoville, T.; D’Journo, X.B.; Trousse, D.; Brioude, G.; Dahan, L.; Seitz, J.F.; Doddoli, C.; Thomas, P.A. Respiratory complications after oesophagectomy for cancer do not affect disease-free survival. Eur. J. Cardiothorac. Surg. 2012, 41, e66–e73. [Google Scholar] [CrossRef] [PubMed]
- Dulu, A.; Pastores, S.M.; Park, B.; Riedel, E.; Rusch, V.; Halpern, N.A. Prevalence and mortality of acute lung injury and ARDS after lung resection. Chest 2006, 130, 73–78. [Google Scholar] [CrossRef]
- Kim, J.B.; Lee, S.W.; Park, S.I.; Kim, Y.H.; Kim, D.K. Risk factor analysis for postoperative acute respiratory distress syndrome and early mortality after pneumonectomy: The predictive value of preoperative lung perfusion distribution. J. Thorac. Cardiovasc. Surg. 2010, 140, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Kometani, T.; Okamoto, T.; Yoshida, S.; Yoshino, I. Acute respiratory distress syndrome after pulmonary resection. Gen. Thorac. Cardiovasc. Surg. 2013, 61, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Tandon, S.; Batchelor, A.; Bullock, R.; Gascoigne, A.; Griffin, M.; Hayes, N.; Hing, J.; Shaw, I.; Warnell, I.; Baudouin, S.V. Peri-operative risk factors for acute lung injury after elective oesophagectomy. Br. J. Anaesth. 2001, 86, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Ricard, J.D.; Dreyfuss, D.; Saumon, G. Ventilator-induced lung injury. Eur. Resp. J. Suppl. 2003, 42, 2s–9s. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Eng. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef]
- Chen, I.C.; Kor, C.T.; Lin, C.H.; Kuo, J.; Tsai, J.Z.; Ko, W.J.; Kuo, C.D. High-frequency power of heart rate variability can predict the outcome of thoracic surgical patients with acute respiratory distress syndrome on admission to the intensive care unit: A prospective, single-centric, case-controlled study. BMC Anesthesiol. 2018, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [PubMed]
- Kuo, P.H.; Kuo, S.H.; Yang, P.C.; Wu, H.D.; Lu, B.Y.; Chen, M.T. Predictive value of rapid shallow breathing index measured at initiation and termination of a 2-h spontaneous breathing trial for weaning outcome in ICU patients. J. Formos. Med. Assoc. 2006, 105, 390–398. [Google Scholar] [CrossRef][Green Version]
- Nemer, S.N.; Barbas, C.S.; Caldeira, J.B.; Cárias, T.C.; Santos, R.G.; Almeida, L.C.; Azeredo, L.M.; Noé, R.A.; Guimarães, B.S.; Souza, P.C. A new integrative weaning index of discontinuation from mechanical ventilation. Crit. Care 2009, 13, R152. [Google Scholar] [CrossRef]
- Verceles, A.C.; Diaz-Abad, M.; Geiger-Brown, J.; Scharf, S.M. Testing the prognostic value of the rapid shallow breathing index in predicting successful weaning in patients requiring prolonged mechanical ventilation. Heart Lung 2012, 41, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Bien, U.; Souza, G.F.; Campos, E.S.; De Carvalho, E.F.; Fernandes, M.G.; Santoro, I.; Costa, D.; Arena, R.; Sampaio, L.M.M. Maximum inspiratory pressure and rapid shallow breathing index as predictors of successful ventilator weaning. J. Phys. Ther. Sci. 2015, 27, 3723–3727. [Google Scholar] [CrossRef]
- Zargar, J.A.; Naqash, I.A.; Gurcoo, S.A. Comparative evaluation of the effect of metoprolol and esmolol on rate pressure product and ECG changes during laryngoscopy and endotracheal intubation in controlled hypertensive patients. Indian J. Anaesth. 2002, 46, 363–368. [Google Scholar]
- May, G.A.; Nagle, F.J. Changes in rate-pressure product with physical training of individuals with coronary artery disease. Phys. Ther. 1984, 64, 1361–1366. [Google Scholar] [CrossRef]
- Forjaz, C.L.M.; Matsudaira, Y.; Rodrigues, F.B.; Nunes, N.; Negrão, C.E. Post-exercise changes in blood pressure, heart rate and rate pressure product at different exercise intensities in normotensive humans. Braz. J. Med. Biol. Res. 1998, 31, 1247–1255. [Google Scholar] [CrossRef]
- Máca, J.; Jor, O.; Holub, M.; Sklienka, P.; Burša, F.; Burda, M.; Janout, V.; Ševčík, P. Past and present ARDS mortality rates: A systematic review. Respir. Care 2017, 62, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Smith, G.S.; Cooper, R.S.; Murthi, S.; Netzer, G. Trauma indices for prediction of acute respiratory distress syndrome. J. Surg. Res. 2016, 201, 394–401. [Google Scholar] [CrossRef]
- Becher, R.D.; Colonna, A.L.; Enniss, T.M.; Weaver, A.A.; Crane, D.K.; Martin, R.S.; Mowery, N.T.; Miller, P.R.; Stitzel, J.D.; Hoth, J.J. An innovative approach to predict the development of adult respiratory distress syndrome in patients with blunt trauma. J. Trauma Acute Care Surg. 2012, 73, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.G.; Arozullah, A.M.; Neumayer, L.; Henderson, W.G.; Hosokawa, P.; Khuri, S.F. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: Results from the patient safety in surgery study. J. Am. Coll. Surg. 2007, 204, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J.E.; Calfee, C.S.; Goldstein, B.A.; Vojnik, R.; Matthay, M.A. Early acute lung injury: Criteria for identifying lung injury prior to the need for positive pressure ventilation. Crit. Care Med. 2013, 41, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
| Variables | Non-ARDS (n = 11) | ARDS (n = 21) | p |
|---|---|---|---|
| General characteristics | |||
| Age (years) | 61 ± 8 | 60 ± 17 | 0.687 |
| Gender (M/F) | 5/6 | 17/4 | 0.056 |
| BH (cm) | 158.5 ± 10.3 | 164.7 ± 6.9 | 0.050 |
| BW (kg) | 65.6 ± 9.4 | 65.1 ± 8.5 | 0.882 |
| BMI (kg/m2) | 26.3 ± 4.4 | 24.0 ± 2.8 | 0.138 |
| Clinical characteristics | |||
| Lung cancer/esophageal cancer | 6/5 | 9/5 | 0.697 |
| SICU stay (days) | 4 (2, 4) | 25 (14, 36) | <0.001 * |
| Pneumonia (yes/no) | 0/11 | 4/17 | 0.272 |
| Hypertension (yes/no) | 3/8 | 4/17 | 0.667 |
| ASA classification | 1 (1, 2) | 2 (2, 3) | 0.005 * |
| APACHE II | 9 (6, 12) | 16 (11, 19) | 0.002 * |
| RASS | 0 (0, 0) | −1 (−1, 0) | 0.001 * |
| Ventilator parameters | |||
| Endo size | 7 (7, 7.5) | 7.5 (7, 7.5) | 0.134 |
| PEEP (cmH2O) | 5.8 ± 1.8 | 10.4 ± 3.0 | <0.001 * |
| PIP (cmH2O) | 20 (20, 20) | 20 (18, 22) | 0.983 |
| RI (s) | 5.0 (4.6, 5.0) | 3.5 (2.3, 3.8) | <0.001 * |
| Ti (s) | 0.9 (0.9, 1.0) | 0.9 (0.8, 1.0) | 0.532 |
| Te (s) | 3.8 ± 0.6 | 2.3 ± 0.9 | <0.001 * |
| (mL/s) | 574.8 ± 92.5 | 464.7 ± 119.1 | 0.012 * |
| VT (mL) | 538.5 ± 93.8 | 427 ± 117.4 | 0.011 * |
| RR (bpm) | 12 (12, 13) | 17 (16, 26) | <0.001 * |
| MV (L/min) | 7.1 ± 2.0 | 8.6 ± 3.2 | 0.160 |
| Cdyn (mL/cmH2O) | 28.6 ± 6.2 | 22.9 ± 7.6 | 0.041 * |
| Raw (cmH2O·s/mL) | 0.034 ± 0.006 | 0.044 ± 0.012 | 0.020 * |
| RSBIv (bpm/L) | 25.0 (20.3, 26.1) | 56.3 (37.7, 69.6) | <0.001 * |
| RPPv (cmH2O * bpm) | 240 (240, 260) | 340 (300, 540) | 0.010 * |
| RPVI (cmH2O * bpm/mL) | 0.48 (0.39, 0.52) | 0.98 (0.71, 1.35) | 0.001 * |
| IER | 0.24 (0.22, 0.25) | 0.36 (0.32, 0.66) | <0.001 * |
| MW (cmH2O * L) | 10.3 ± 2.1 | 8.2 ± 2.6 | 0.030 * |
| MW/IER (cmH2O * L) | 41.1 (37.0, 43.7) | 18.7 (12.8, 27.3) | <0.001 * |
| Outcome | |||
| Survivors | 11 (100%) | 16 (76.2%) | 0.138 |
| Non-survivors | 0 (0%) | 5 (23.8%) |
| Variables | Survivors (n = 16) | Non-survivors (n = 5) | p |
|---|---|---|---|
| General characteristics | |||
| Age (years) | 57 ± 17 | 69 ± 12 | 0.148 |
| Gender (M/F) | 14/2 | 3/2 | 0.228 |
| BH (cm) | 165.3 ± 6.6 | 162.7 ± 8.3 | 0.493 |
| BW (kg) | 64.5 ± 7.6 | 67.2 ± 11.6 | 0.546 |
| BMI (kg/m2) | 23.62 ± 2.49 | 25.34 ± 3.49 | 0.235 |
| Clinical characteristics | |||
| Lung cancer/esophageal cancer | 5/4 | 4/1 | 0.123 |
| ICU stay (days) | 20.5 (11, 40.5) | 25 (25, 36) | 0.509 |
| Pneumonia (yes/no) | 4/12 | 0/5 | 0.532 |
| ASA classification | 2 (2, 3) | 2 (2, 3) | 0.768 |
| APACHE II | 14 (10, 19) | 18 (14, 29) | 0.185 |
| LIS | 13 (10, 14) | 13 (12, 15) | 0.557 |
| SOFA score | 8 (7, 10) | 7 (7, 8) | 0.313 |
| RASS | −1 (−1, 0) | −2 (−2, −1) | 0.040 * |
| WBC (×103/μL) | 13.3 (7.7, 13.4) | 12.9 (11.1, 14.0) | 0.905 |
| CRP (mg/dL) | 21.5 (10.9, 22.6) | 12.4 (11.0, 19.7) | 0.495 |
| Ventilator parameters | |||
| Endo size | 7.5 (7.0, 7.5) | 7.0 (7.0, 7.5) | 0.314 |
| PEEP (cmH2O) | 10.0 (9.0, 12.0) | 10.2 (10.0, 11.9) | 0.523 |
| PIP (cmH2O) | 18 (15.5, 20) | 22 (20, 22) | 0.035 * |
| RI (s) | 3.41 ± 0.94 | 2.58 ± 0.66 | 0.087 |
| Ti (s) | 0.9 (0.8,1.0) | 0.9 (0.8,0.9) | 0.520 |
| Te (s) | 2.48 ± 0.88 | 1.70 ± 0.70 | 0.088 |
| VT (mL) | 436.3 ± 119.5 | 397.2 ± 117.9 | 0.530 |
| RR (breaths/min) | 17 (16, 22) | 26 (24, 27) | 0.145 |
| MV (L/min) | 8.19 ± 2.84 | 9.96 ± 4.08 | 0.285 |
| RSBIv (bpm/L) | 47.8 ± 20.0 | 62.9 ± 10.4 | 0.045 * |
| RPPv (cmH2O * bpm) | 359.5 ± 148.9 | 514.8 ± 92.7 | 0.042 * |
| RPVI (cmH2O * bpm/mL) | 0.88 ± 0.39 | 1.35 ± 0.24 | 0.023 * |
| Cdyn (mL/cmH2O) | 21.6 (18.9, 30.7) | 19.0 (17.7, 21.3) | 0.215 |
| (mL/s) | 470.3 ± 122.1 | 446.9 ± 120.3 | 0.712 |
| Raw (cmH2O·s/mL) | 0.041 ± 0.010 | 0.051 ± 0.017 | 0.105 |
| MW (cmH2O * L) | 8.11 ± 2.72 | 8.46 ± 2.54 | 0.801 |
| IER | 0.35 (0.32, 0.45) | 0.66 (0.60, 0.67) | 0.230 |
| MW/IER (cmH2O * L) | 21.7 (12.2, 30.3) | 12.9 (12.8, 18.1) | 0.240 |
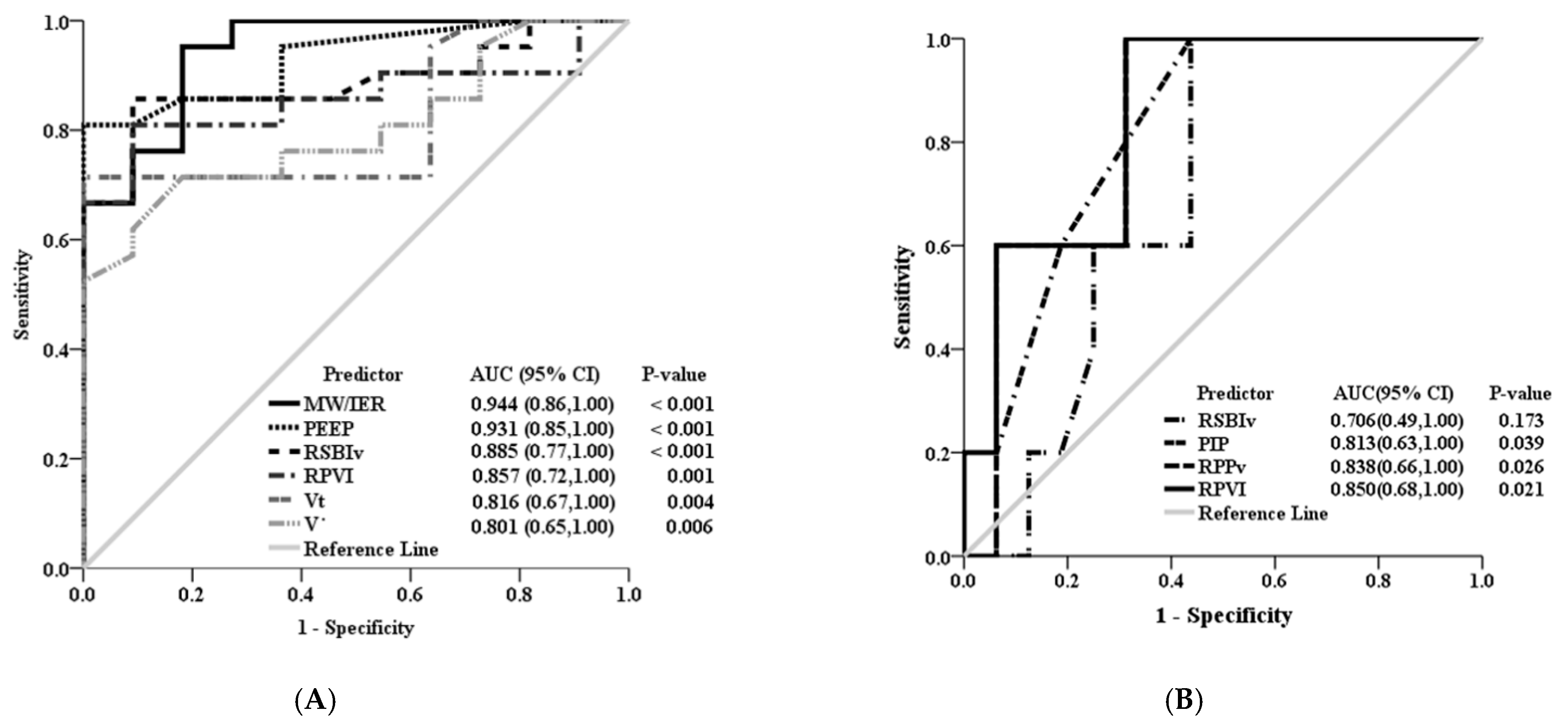
| Variables | AUC Analysis | Principal Component Analysis | |
|---|---|---|---|
| Component 1 | Component 2 | ||
| PEEP (cmH2O) | 0.931 | 0.760 | 0.327 |
| RSBIv (bpm/L) | 0.885 | 0.992 | 0.041 |
| RPVI (cmH2O * bpm/mL) | 0.857 | 0.949 | −0.091 |
| MW/IER (cmH2O * L) | 0.944 | 0.877 | −0.025 |
| RR (breaths/min) | 0.881 | 0.828 | −0.554 |
| RI (s) | 0.881 | 0.828 | −0.554 |
| Te (s) | 0.907 | 0.808 | −0.577 |
| IER (per 0.1 increment) | 0.905 | 0.759 | −0.578 |
| RPPv (cmH2O * bpm) | 0.779 | 0.743 | −0.587 |
| Raw (cmH2O·s/mL)(per 0.01 increment) | 0.788 | 0.695 | 0.576 |
| Cdyn (mL/cmH2O) | 0.784 | 0.686 | 0.511 |
| (mL/s) | 0.801 | 0.603 | 0.755 |
| MW (cmH2O * L) | 0.639 | 0.504 | 0.753 |
| VT (mL) | 0.816 | 0.677 | 0.729 |
| Significant Predictors of the Development of ARDS | ||||||
| Variables | Model 1a | Model 2a | Model 3a | |||
| Adj. OR | p | Adj. OR | p | Adj. OR | p | |
| MW/IER | 0.82 (0.71, 0.94) | 0.004 * | 0.81 (0.70, 0.95) | 0.008 * | ||
| VT | 0.99 (0.98, 1.00) | 0.033 * | 1.00 (0.99, 1.01) | 0.651 | ||
| Predictors of Mortality in Patients with ARDS | ||||||
| Variables | Model 1b | Model 2b | Model 3b | |||
| Adj. OR | p | Adj. OR | p | Adj. OR | p | |
| PIP | 1.35 (0.90, 2.02) | 0.150 | ||||
| RSBIv | 1.05 (0.98, 1.13) | 0.190 | ||||
| RPPv | 1.01 (1.00, 1.02) | 0.090 | ||||
| RPVI | 28.4 (0.8, 1034.8) | 0.068 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kor, C.-T.; Lin, K.-H.; Wang, C.-H.; Lin, J.-F.; Kuo, C.-D. Ventilator Parameters in the Diagnosis and Prognosis of Acute Respiratory Distress Syndrome in Postoperative Patients: A Preliminary Study. Diagnostics 2021, 11, 648. https://doi.org/10.3390/diagnostics11040648
Kor C-T, Lin K-H, Wang C-H, Lin J-F, Kuo C-D. Ventilator Parameters in the Diagnosis and Prognosis of Acute Respiratory Distress Syndrome in Postoperative Patients: A Preliminary Study. Diagnostics. 2021; 11(4):648. https://doi.org/10.3390/diagnostics11040648
Chicago/Turabian StyleKor, Chew-Teng, Kai-Huang Lin, Chen-Hsu Wang, Jui-Feng Lin, and Cheng-Deng Kuo. 2021. "Ventilator Parameters in the Diagnosis and Prognosis of Acute Respiratory Distress Syndrome in Postoperative Patients: A Preliminary Study" Diagnostics 11, no. 4: 648. https://doi.org/10.3390/diagnostics11040648
APA StyleKor, C.-T., Lin, K.-H., Wang, C.-H., Lin, J.-F., & Kuo, C.-D. (2021). Ventilator Parameters in the Diagnosis and Prognosis of Acute Respiratory Distress Syndrome in Postoperative Patients: A Preliminary Study. Diagnostics, 11(4), 648. https://doi.org/10.3390/diagnostics11040648








