Abstract
Background: High-resolution nerve ultrasound (HRUS) has been proven to be a valuable tool in the diagnosis of immune-mediated neuropathies, such as chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M-protein, skin changes) is an important differential diagnosis of CIDP. Until now, there have been no studies that could identify specific HRUS abnormalities in POEMS syndrome patients. Thus, the aim of this study was to assess possible changes and compare findings with CIDP patients. Methods: We retrospectively analyzed HRUS findings in three POEMS syndrome and ten CIDP patients by evaluating cross-sectional nerve area (CSA), echogenicity and additionally calculating ultrasound pattern scores (UPSA, UPSB, UPSC and UPSS) and homogeneity scores (HS). Results: CIDP patients showed greater CSA enlargement and higher UPSS (median 14 vs. 11), UPSA (median 11.5 vs. 8) and HS (median 5 vs. 3) compared with POEMS syndrome patients. However, every POEMS syndrome patient illustrated enlarged nerves exceeding reference values, which were not restricted to entrapment sites. In CIDP and POEMS syndrome, heterogeneous enlargement patterns could be identified, such as inhomogeneous, homogeneous and regional nerve enlargement. HRUS in CIDP patients visualized both increased and decreased echointensity, while POEMS syndrome patients pictured hypoechoic nerves with hyperechoic intraneural connective tissue. Discussion: This is the first study to demonstrate HRUS abnormalities in POEMS syndrome outside of common entrapment sites. Although nerve enlargement was more prominent in CIDP, POEMS syndrome patients revealed distinct echogenicity patterns, which might aid in its differentiation from CIDP. Future studies should consider HRUS and its possible role in determining diagnosis, prognosis and treatment response in POEMS syndrome.
1. Introduction
High-resolution nerve ultrasound (HRUS) has been proven to be a valuable tool in the diagnosis of peripheral nervous system diseases such as immune-mediated neuropathies or central nervous system diseases such as amyotrophic lateral sclerosis, which also affects peripheral nerves [1,2,3]. The detection rate of immune-mediated neuropathies can be even improved by 20% if HRUS is added to conventional nerve conduction studies and clinical examination [4].
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) belongs to the group of immune-mediated neuropathies and is associated with segmental or diffuse nerve enlargement (with a predominance in proximal nerve segments), i.e., increased cross sectional nerve area (CSA), greater nerve vascularization and fascicular enlargement in HRUS [5].
An important differential diagnosis of CIDP is POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M-protein, skin changes), a rare variant of multiple myeloma (MM), which requires the existence of a polyneuropathy and a monoclonal plasma-cell proliferative disorder for the diagnosis. Elevated serum levels of vascular endothelial growth factor (VEGF) are also among the diagnostic criteria and can be used as a marker for therapeutic response. In its clinical presentation, the predominantly demyelinating polyneuropathy mimics CIDP with progressive sensorimotor deficits in both proximal and distal nerve segments. Moreover, polyneuropathy presents often as the initial and isolated symptom in POEMS syndrome and is mostly symmetric, distal, painful and rapidly progressive. Since treatment is different from CIDP, an accurate diagnosis is important [6].
Until now, there have been only two studies taking account of HRUS abnormalities in POEMS syndrome. In the first study, eight POEMS syndrome patients underwent HRUS evaluation for possible changes in CSA, fascicle size and echogenicity of the median, ulnar, tibial and peroneal nerves. However, no specific HRUS abnormalities could be identified [7]. In the same year, a case report of a POEMS syndrome patient described diffusely thickened peripheral nerves of both upper limbs with increased arterial blood flow in ultrasound imaging [8]. The second study observed HRUS alterations in the median nerve of 33 POEMS syndrome patients. HRUS of these patients showed greater CSA than in healthy individuals, but still CSA did not exceed reference values [9].
Thus, the aim of our study was to assess possible HRUS pathologies in patients with POEMS syndrome and compare them with findings in CIDP patients by using some novel approaches. In contrast to the already exisiting studies, we utilized newly established CSA normal values with more measuring sites. Additionally, we analyzed the enlargement patterns of the examined nerves and calculated different quantitative scores to evaluate peripheral nerve pathologies, which might possibly aid in its differentiation from CIDP. Further, we evaluated the overall echointensity of several nerves and compared them according to known classifications.
2. Methods
In this study we retrospectively included three patients who were diagnosed with POEMS syndrome according to the current diagnostic criteria ([10]; three men, mean age at onset 65.0 ± 6.1 years, range 58–69 years). We compared HRUS findings with ten patients who met the EFNS diagnostic criteria for definite CIDP ([11]; four men and six women, mean age at onset 56.1 ± 11.4 years, range 37–73 years). HRUS in POEMS syndrome patients was performed before treatment initiation. Since the majority of our CIDP patient sample was diagnosed years before our POEMS patients and HRUS was not always available then, HRUS in CIDP patients mainly took place after treatment onset. All patients were recruited from the Center of Neurology at the University of Tuebingen except for one POEMS syndrome patient (Department of Neurology at the University of Jena) and gave their written consent for participation in the study. Table 1 and Table 2 present the characteristics of the patients.

Table 1.
Characteristics of patients with POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M-protein, skin changes).

Table 2.
Characteristics of patients with CIDP (chronic inflammatory demyelinating polyradiculoneuropathy).
HRUS examinations were performed by different neurologists using 14–18 MHz broadband ultrasound probes (Mindray TE7, Darmstadt, Germany) and took approximately 40 min for each patient. In one POEMS syndrome patient (#3; Table 1) and in one CIDP patient (#8; Table 2), another ultrasound system was used (#3: 14 MHz broadband ultrasound probe, Aplio 400, Toshiba medical systems, Japan; #8: 24 MHz broadband ultrasound probe, Canon Aplio i800, Neuss, Germany). To avoid anisotropy, the ultrasound probe was kept perpendicular to the nerves. Moreover, to avert any artificial nerve deformity, the extremities were maintained in the neutral position and only the weight of the ultrasound probe was applied to the measurement points. HRUS measurements were repeated with intraclass coefficient correlation >90% (intra-and interobserver accuracy >90% [12]).
The evaluation for each patient was conducted according to the well-established ultrasound pattern sum score (=UPSS), which includes sensorimotor nerves (median, ulnar, tibial and peroneal nerves: ultrasound pattern score A (= UPSA)), cervical roots (C5 and C6) and vagal nerves (ultrasound pattern score B = UPSB), as well as sensory nerves (sural, superficial radial and superficial peroneal nerves: ultrasound pattern score C (= UPSC)). Measurements took place at predefined sites and comprehended CSA within the hyperechoic epineural rim for all nerves (axial plane) and diameter for C5 and C6 (longitudinal plane). The median and ulnar nerves were scanned at the wrist, at the mid-forearm, at the elbow and at the mid-upper arm. The tibial and peroneal nerves were measured at the popliteal fossa, and the tibial (besides the sural) nerves were additionally analyzed at the ankle. Furthermore, the radial superficial nerve was examined at the forearm, the peroneal superficial nerve at mid-calf level and the vagus nerve close to the carotid artery at bifurcation level. Diameters of the cervical roots 5 and 6 were assessed after exiting the intervertebral foramen [12,13]. Due to the symmetrical involvement of the polyneuropathy, most measurements were performed on the right side (in some cases on the left side as well). Figure 1 demonstrates the measurement points of the examined nerves.
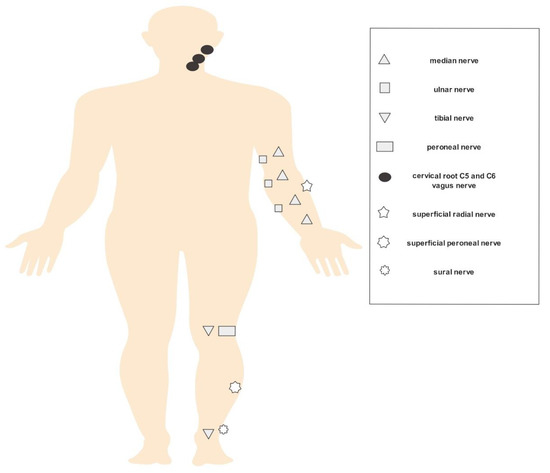
Figure 1.
HRUS (high-resolution nerve ultrasound) measurement points.
Regarding the UPSA, one point is given for every nerve enlargement >100% and <150%, and two points for each enlargement >150% of the upper normal values (UPSA maximum 16 points). For the UPSB and UPSC, each nerve exceeding normal values is rated with one point (UPSB and UPSC maximum three points, respectively). Thus, the UPSS as a total score can reach up to 22 points [12].
Additionally, homogeneity scores (=HS) for each patient were obtained. Homogeneity patterns are categorized into normal (no enlargement), regional enlargement (enlarged and normal values in the same nerve), inhomogeneous enlargement (generalized CSA enlargement in a nerve, enlargement >150% and <150% above normal limit in the same nerve), mild homogeneous enlargement (generalized nerve enlargement in a nerve <150% above normal limit) and overt homogeneous enlargement (generalized nerve enlargement in a nerve >150% above normal limit). For scoring, −1 point is given for regional enlarged nerves, 0 points for no enlargement, 1 point for inhomogeneous, 2 points for mild homogeneous and 3 points for overt homogeneous enlargement. For analysis, the median, ulnar and tibial nerves are examined and a total score of 9 points can be reached for each patient (range −3–9 points [12,14]).
Echogenicity of the nerves was rated according to Padua et al. [15] and divided into class 1 (large nerves with hypoechoic nerves/fascicles), class 2 (large nerves with heterogeneous hyper- and hypoechoic fascicles) and class 3 (normal size nerve with hyperechoic fascicles). Finally, we evaluated the intranerve CSA ratios (maximal CSA/minimal CSA without entrapment sites) as well as the entrapment site CSA ratios (CSA median nerve wrist/CSA median nerve forearm, and CSA ulnar nerve elbow/CSA ulnar nerve upper arm) for the median and ulnar nerves.
For each examined nerve (CSA and diameter) as well as for the UPSS and HS, median values of the POEMS patient group and the CIDP patient group were calculated and compared with each other. When measurements took place on both sides, the most involved nerve was used (see also [14]).
Alongside HRUS examinations, nerve conduction studies (NCS) were performed according to existing literature [11].
3. Results
HRUS showed abnormalities in both POEMS and CIDP patients for almost all examined nerves and were not restricted to entrapment sites. Table 3 gives an overview on the examined nerves.

Table 3.
HRUS median values.
The findings from the upper extremitiy nerves are summarized in Table 4 and Table 5. Both patient groups visualized nerve pathologies along the whole course of the median and ulnar nerves, but CIDP patients tended to have slightly higher CSA values compared with POEMS syndrome patients.

Table 4.
HRUS findings in upper limb nerves: median nerve CSA values in mm2.

Table 5.
HRUS findings in upper limb nerves: ulnar nerve CSA values in mm2.
POEMS patients demonstrated overt homogeneous (two patients) and regional enlargement (one patient) in the median nerve, while CIDP patients had overt homogeneous (five patients), inhomogeneous (two patients), regional (two patients) and mild homogeneous (one patient) enlargement patterns. HRUS findings of the right median nerve in POEMS syndrome patient #3 are shown in Figure 2. Enlargement patterns in the ulnar nerve were equally heterogeneous. Two POEMS syndrome patients exhibited inhomogeneous and another mild homogeneous enlargement, five CIDP patients had overt homogeneous, two inhomogeneous, one mild homogeneous and one regional enlargement (and also one patient without an enlargement pattern).
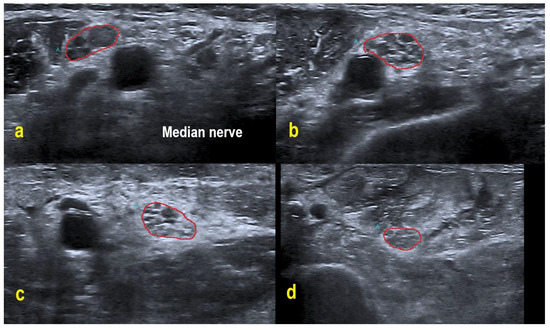
Figure 2.
HRUS in POEMS syndrome patient #3. HRUS demonstrated overt homogeneous enlargement of the CSA in the right median nerve (within red border; CSA up to 2.0-fold increased). Axilla (a): CSA 20 mm². Upper arm (b): 25 mm². Midarm (c): 23 mm². Forearm (d): 15 mm². Of note, the intraneural echointensity reflects hyperechoic interfascicular tissue next to hypoechoic fascicles.
The median value for the intranerve CSA ratio in the median nerve was 1.6 in POEMS syndrome vs. 1.55 in CIDP, and for the ulnar nerve it was 1.1 vs. 1.2. Concerning the entrapment site CSA ratio of the median nerve, the median value was 1.1 in POEMS syndrome vs. 1.2 in CIDP. However, POEMS syndrome patients depicted obviously higher entrapment site CSA ratios in the ulnar nerve in comparison to CIDP patients (median 1.5 vs. 0.8).
In contrast to the median and ulnar nerves, POEMS syndrome patients displayed less obvious CSA enlargements in the nerves of the lower extremities (Table 6). Nerve enlargement was almost only restricted to proximal nerve segments of the tibial nerve. CIDP patients had obviously higher CSA values in the peroneal nerve and distal segments of the tibial nerve as compared to POEMS patients.

Table 6.
HRUS findings in lower limb nerves: tibial and peroneal nerve CSA values in mm2.
The enlargement patterns in the tibial nerve showed regional enlargement in two POEMS syndrome and two CIDP patients. Four CIDP patients had inhomogeneous, two mild homogeneous and one overt homogeneous nerve enlargement. One POEMS syndrome and one CIDP patient illustrated normal tibial nerves.
As demonstrated for the CSA values of the sensorimotor nerves, diameters of C5 and C6 were more increased in CIDP patients than in POEMS syndrome. Still, CIDP patients showed greater heterogeneity among the nerves (internerve variability). In every POEMS syndrome patient, diameters of C5 and C6 and CSA of the vagus nerve were enlarged, while seven out of ten CIDP patients had enlargement of C5, five out of nine enlargement of C6 and six out of ten enlargement of the vagus nerve (Table 7).

Table 7.
HRUS findings in cervical roots (diameter in mm) and vagus nerve CSA values in mm2.
Concerning the pure sensory nerves, every POEMS syndrome patient as well as seven out of nine CIDP patients displayed CSA enlargements of the sural nerve. However, again greater internerve variability could be identified in the remaining nerves. Only in one POEMS syndrome patient and only in four out of nine CIDP patients could enlargement of the superficial radial nerve be observed. The same applies to the superficial peroneal nerve with no POEMS syndrome patient and only five out of nine CIDP patients having CSA enlargement (Table 8).

Table 8.
HRUS findings in sensory nerves: sural, superf. radial and superf. peroneal nerves in mm2.
Corresponding to CSA values, quantitative scores in CIDP patients tended to be higher than in POEMS syndrome, although differences were again not prominent (median UPSA in CIDP 11.5 vs. 8 points in POEMS syndrome, median UPSB 1.5 vs. 3, median UPSC 1 vs. 1, median UPSS 14 vs. 11 and median HS 5 vs. 3; Table 9).

Table 9.
UPSA, UPSB, UPSC, UPSS, HS and echogenicity classes for POEMS and CIDP patients.
Four CIDP patients and every POEMS syndrome patient showed hypoechoic nerves (class 1), whereas the remaining CIDP patients demonstrated a more heterogeneous pattern with hypo- and hyperechoic fascicles (class 2). No patient exhibited class 3 pattern. In contrast to the patients with CIDP, the examined POEMS syndrome patients featured additional hyperechoic intraneural connective tissue. Figure 3 exemplifies the different HRUS findings.
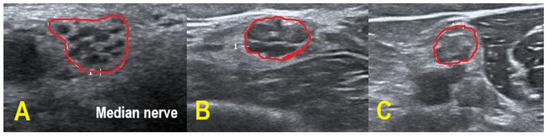
Figure 3.
HRUS of the right upper arm median nerve. POEMS syndrome patient #1 demonstrated hypoechoic fascicles and hyperechoic intraneural connective tissue ((A) within red border). In CIDP patient #6, HRUS depicted hypoechoic next to hyperechoic fascicles ((B) within red border), whereas HRUS in CIDP patient #9 showed hyperechoic fascicles ((C) within red border).
NCS in every POEMS syndrome patient revealed a demyelinating sensorimotor polyneuropathy with secondary axonal degeneration (reduced conduction velocity (CV) and compound muscle action potential (CMAP), prolonged distal motor latency in the upper extremities; severe axonal involvement in the lower extremities with loss of CMAP and sensory nerve action potential, plus not recordable CV). Similar to POEMS patients, every CIDP patient illustrated a predominantly demyelinating sensorimotor involvement and secondary axonal nerve damage.
4. Discussion
This is the first study that shows HRUS pathologies in POEMS syndrome outside of common entrapment sites [7]. CSA enlargement occurred in both CIDP and POEMS syndrome patients (as compared to normative values) and could be further quantified and categorized by using the UPSS and HS.
Overall, CSA enlargement was slightly more prominent in CIDP patients than in POEMS syndrome. However, significances have not been calculated due to the small sample size. Concerning the entrapment site CSA ratios, our POEMS patients partly portrayed even obviously higher ratios compared with CIDP. As reported before in several studies, CIDP patients demonstrated nerve enlargement with a predominance in proximal nerve segments [1,5]. Plus, CIDP patients illustrated heterogeneous morphologies, having regional, inhomogeneous and homogeneous nerve enlargement with increased or decreased echointensity, as already described in the past [14,15]. In line with this, our POEMS syndrome patients had heterogeneous nerve enlargement patterns. Proximal nerve segments were also more frequently enlarged than distal nerve segments, but, different to our CIDP patients, in POEMS syndrome, the upper extremities pictured obvious greater nerve enlargement than the lower extremities. Interestingly, clinical findings in our POEMS syndrome patients identified greater involvement of the lower limbs, which has also been presented by Nasu et al. [19]. It might be possible that nerve pathology in lower limb nerves could not be adequately seen by HRUS, as more proximal regions as the sciatic nerves have not been scanned. Electrophysiological findings in our POEMS patients are in line with past studies, and discrimination between POEMS syndrome and CIDP might be difficult when bearing in mind nerve conduction studies alone [7,19].
According to Grimm et al., a UPSA ≥ 7 points or a UPSS ≥ 10 points is suggestive of CIDP, which is consistent to our findings in CIDP patients [20]. Although the UPSS tended to be higher in CIDP, patients with POEMS syndrome also exceeded these thresholds. The same holds true for the HS in our POEMS syndrome patients, which were similar to CIDP patients in past studies [14]. Considering these results, quantitative scores such as the UPSS and HS might not be sufficient to discriminate between these diseases.
Therefore, the observed echogenicity patterns in our POEMS patients could be a promising finding. Lucchetta et al. [7] already described hypoechogenic nerves in POEMS syndrome, as was the case in our study. Our POEMS patients presented additional hyperechoic intraneural connective tissue, which is an interesting morphological observation and not known to appear in CIDP. Furthermore, hypoechoic nerves are associated with better treatment response in CIDP (possibly reflecting active inflammation) and can be used as a prognostic marker, while in clinically progressive CIDP, hyperechoic nerves are frequently found (possibly reflecting axonal degeneration [18,21,22]). Follow-up studies have to prove if these findings are applicable to POEMS syndrome patients. The histological correlate behind the increased and hyperechoic interfascicular tissue has to be clarified by histopathological studies in future projects.
We must consider that in the case of MM, several neuropathy types can occur: mainly axonal neuropathies caused by the disease itself or by chemotherapy used for the disease. However, the description of hypoechoic nerve swelling in POEMS is of note. If hypo- or hyperechoic nerve swelling is seen in MM patients, examiners must be aware of extraossar infiltration of plasmocytoma in the nerve, which is overall quite rare. Second, paraproteinemic neuropathies—next to MM—might present like CIDP, and thus, might offer the same heterogeneous echointensities as the latter. Then, therapeutic steps are the same as in classical CIDP [23,24,25].
Differential diagnosis between CIDP and POEMS syndrome remains challenging. Besides clinical examination, nerve conduction studies and HRUS, other options should be considered in the diagnostic work up in the future. For example, magnetic resonance imaging (MRI) of the brain and spine could be used to further aid in the diagnosis. Ziff et al. identified pachymeningeal thickening in POEMS syndrome patients in contrast to patients with CIDP [26]. Combining HRUS and MRI might help to achieve even greater diagnostic accuracy, but future systematic studies are needed to prove this hypothesis [27]. MRI, especially diffusion tensor imaging (DTI) based tractography, might be used in the future to verify measurements by HRUS. DTI based tractography allows us to visualize and conclude about the course of nerve tracts and is able to differentiate tissues on the basis of diffusion anisotropy differences. This imaging method has already exemplified in the past its potential value (e.g., characterize changes associated with age and neurodegenerative diseases). Therefore, by analyzing changes in parameters such as Fractional Anisotropy, it might be possible to additionally infer on the state of the examined nerves [28,29,30]. To obtain sufficient precision of the research, it will be important to use suitable methods to eliminate spatial systematic errors [31,32].
There are some limitations of the study. While there were similar HRUS findings in POEMS syndrome in the mentioned previous study (hypoechogenic nerves [7]), we also report new findings (general CSA enlargement exceeding reference values, hyperechoic intraneural connective tissue) in a much smaller patient sample. Thus, our findings have to be treated with caution and need confirmation in future studies. Secondly, we did not examine nerve vascularity or abnormalities of single fascicles, which might have helped in the differentiation between CIDP and POEMS syndrome. Additionally, we have to critically point out that HRUS in our CIDP patients was partly performed before and partly after treatment onset, while HRUS in POEMS syndrome took place before treatment initiation, which further restricts comparison of the two patient groups. Nerve enlargement may vary with disease duration and treatment. Some studies found that longer disease duration or longer intervals between symptom onset and treatment in CIDP patients is associated with larger nerve size [33,34]. Consequently, we cannot rule out that our findings are biased to a certain degree. However, they depict daily routine in outpatient hospitals as diagnosis is often delayed in CIDP as well as in POEMS syndrome and treatment had already begun. Despite the fact that these results cannot be necessarily applied to POEMS syndrome, future studies should consider this and include more treatment naïve patients to evaluate the role of HRUS in determining prognosis and treatment efficacy in POEMS syndrome. At last, it should be mentioned that the position of the ultrasound probe, particularly the arm angulation, is an important factor on the assessment of the CSA. Since examinations were performed by different neurologists, we have to take into consideration the subjectivity concerning the positioning of the probe.
5. Conclusions
Demonstrating for the first time specific HRUS nerve abnormalities in POEMS syndrome is an important finding of this study, since HRUS has already proven in the past to be a helpful tool in the diagnosis and monitoring of disease course of peripheral nervous system diseases [35]. Particularly, the discovery of distinct echogenicity patterns in POEMS syndrome might aid in its differentiation from CIDP. Future follow-up studies have to analyze if HRUS could not only serve as a door opener in immune-mediated neuropathies such as CIDP, but also in POEMS syndrome, and should focus on the addressed issues.
Author Contributions
Conceptualization and design, M.D., M.C., A.G., N.W.; Literature research, M.D., M.C., A.G., N.W.; Data analysis and interpretation, M.D., M.C., F.S., J.-H.S., C.K., J.W., M.K., S.W., S.S., A.G., N.W.; Critical revisions, M.C., F.S., J.-H.S., C.K., J.W., M.K., S.W., S.S., A.G., N.W.; Writing—creating Tables, M.D.; Writing—Figures, M.D., M.C.; Writing—Original Draft Preparation, M.D; Writing—Review & Editing, M.D., M.C., S.S., A.G., N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the University of Tuebingen (702/2015BO2, 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
M.K. received a reimbursement for congress participation by Biogene GmbH. A.G. received speakers honorary from CSL Behring, Grifols, Pfizer, Alnylam. N.W. received travel support to attend scientific meetings by Canon Medical Systems and CSL Behring Pharmaceuticals. N.W. further received research funding by the German Ultrasound Society (DEGUM) and is currently receiving a scholarship by the Medical Faculty Tuebingen (clinician scientist). The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, the writing of the manuscript, or in the decision to publish the results.
References
- Zaidman, C.M.; Harms, M.B.; Pestronk, A. Ultrasound of inherited vs. acquired demyelinating polyneuropathies. J. Neurol. 2013, 260, 2580–2587. [Google Scholar] [CrossRef]
- Grimm, A.; Décard, B.F.; Athanasopoulou, I.; Schweikert, K.; Sinnreich, M.; Axer, H. Nerve ultrasound for differentiation between amyotrophic lateral sclerosis and multifocal motor neuropathy. J. Neurol. 2015, 262, 870–880. [Google Scholar] [CrossRef]
- Schreiber, S.; Vielhaber, S.; Schreiber, F.; Cartwright, M.S. Peripheral nerve imaging in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2020, 131, 2315–2326. [Google Scholar] [CrossRef]
- Herraets, I.J.; Goedee, H.S.; Telleman, J.A.; Van Eijk, R.P.; Van Asseldonk, J.T.; Visser, L.H.; Berg, L.H.V.D.; Van Der Pol, W.L. Nerve ultrasound improves detection of treatment-responsive chronic inflammatory neuropathies. Neurology 2020, 94, e1470–e1479. [Google Scholar] [CrossRef]
- Telleman, J.A.; Grimm, A.; Goedee, S.; Visser, L.H.; Zaidman, C.M. Nerve Ultrasound In Polyneuopathies. Muscle Nerve 2018, 57, 716–728. [Google Scholar] [CrossRef]
- Brown, R.; Ginsberg, L. POEMS syndrome: Clinical update. J. Neurol. 2019, 266, 268–277. [Google Scholar] [CrossRef]
- Lucchetta, M.; Pazzaglia, C.; Granata, G.; Briani, C.; Padua, L. Ultrasound evaluation of peripheral neuropathy in POEMS syndrome. Muscle Nerve 2011, 44, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Yanik, B.; Conkbayır, I.; Keyik, B.; Yoldas, T.K. Sonographic findings in a case of polyneuropathy associated with POEMS syndrome. J. Clin. Ultrasound 2011, 39, 473–476. [Google Scholar] [CrossRef]
- Mitsuma, S.; Misawa, S.; Shibuya, K.; Isose, S.; Sekiguchi, Y.; Iwai, Y.; Beppu, M.; Watanabe, K.; Amino, H.; Kuwabara, S. Altered axonal excitability properties and nerve edema in POEMS syndrome. Clin. Neurophysiol. 2015, 126, 2014–2018. [Google Scholar] [CrossRef][Green Version]
- Dispenzieri, A. POEMS syndrome: 2017 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2017, 92, 814–829. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First Revision. Eur. J. Neurol. 2010, 17, 356–363. [Google Scholar]
- Grimm, A.; Axer, H.; Heiling, B.; Winter, N. Nerve ultrasound normal values—Readjustment of the ultrasound pattern sum score UPSS. Clin. Neurophysiol. 2018, 129, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Winter, N.; Rattay, T.W.; Härtig, F.; Dammeier, N.M.; Auffenberg, E.; Koch, M.; Axer, H. A look inside the nerve—Morphology of nerve fascicles in healthy controls and patients with polyneuropathy. Clin. Neurophysiol. 2017, 128, 2521–2526. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Vittore, D.; Schubert, V.; Lipski, C.; Heiling, B.; Décard, B.F.; Axer, H. Ultrasound pattern sum score, homogeneity score and regional nerve enlargement index for differentiation of demyelinating inflammatory and hereditary neuropathies. Clin. Neurophysiol. 2016, 127, 2618–2624. [Google Scholar] [CrossRef]
- Padua, L.; Granata, G.; Sabatelli, M.; Inghilleri, M.; Lucchetta, M.; Luigetti, M.; Coraci, D.; Martinoli, C.; Briani, C. Heterogeneity of root and nerve ultrasound pattern in CIDP patients. Clin. Neurophysiol. 2014, 125, 160–165. [Google Scholar] [CrossRef]
- Aseem, F.; Williams, J.W.; Walker, F.O.; Cartwright, M.S. Neuromuscular Ultrasound in Patients with Carpal Tunnel Syndrome And Normal Nerve Conduction Studies. Muscle Nerve 2017, 55, 913–916. [Google Scholar] [CrossRef]
- Cartwright, M.S.; Walker, F.O. Neuromuscular Ultrasound in Common Entrapment Neuropathies. Muscle Nerve 2013, 48, 696–704. [Google Scholar] [CrossRef]
- Härtig, F.; Ross, M.; Dammeier, N.M.; Fedtke, N.; Heiling, B.; Axer, H.; Décard, B.F.; Auffenberg, E.; Koch, M.; Rattay, T.W.; et al. Nerve ultrasound predicts treatment response in chronic-inflammatory demyelinating polyradiculoneuropathy—A prospective follow-up. Neurotherapeutics 2018, 15, 439–451. [Google Scholar] [CrossRef]
- Nasu, S.; Misawa, S.; Sekiguchi, Y.; Shibuya, K.; Kanai, K.; Fujimaki, Y.; Ohmori, S.; Mitsuma, S.; Koga, S.; Kuwabara, S. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J. Neurol. Neurosurg. Psychiatry 2012, 83, 476–479. [Google Scholar] [CrossRef]
- Grimm, A.; Décard, B.F.; Axer, H.; Fuhr, P. The Ultrasound pattern sum score—UPSS. A new method to differentiate acute and subacute neuropathies using ultrasound of the peripheral nerves. Clin. Neurophysiol. 2015, 126, 2216–2225. [Google Scholar] [CrossRef]
- Fisse, A.L.; Pitarokoili, K.; Motte, J.; Gamber, D.; Kerasnoudis, A.; Gold, R.; Yoon, M.-S. Nerve echogenicity and intranerve CSA variability in high-resolution nerve ultrasound (HRUS) in chronic inflammatory demyelinating polyneuropathy (CIDP). J. Neurol. 2019, 266, 468–475. [Google Scholar] [CrossRef]
- Gamber, D.; Motte, J.; Kerasnoudis, A.; Yoon, M.; Gold, R.; Pitarokoili, K.; Fisse, A.L. High-Resolution nerve ultrasound to assess nerve echogenicity, fascicular count, and cross-sectional area using semiautomated analysis. J. Neuroimaging 2020, 30, 493–502. [Google Scholar] [CrossRef]
- Ropper, A.H.; Gorson, K.C. Neuropathies associated with paraproteinemia. N. Engl. J. Med. 1998, 338, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulou, I.M.; Rasenack, M.; Grimm, C.; Axer, H.; Sinnreich, M.; Décard, B.F.; Grimm, A. Ultrasound of the nerves—An appropriate addition to nerve conduction studies to differentiate paraproteinemic neuropathies. J. Neurol. Sci. 2016, 362, 188–195. [Google Scholar] [CrossRef]
- Nobile-Orazio, E.; Bianco, M.; Nozza, A. Advances in the Treatment of Paraproteinemic Neuropathy. Curr. Treat. Options. Neurol. 2017, 19, 43. [Google Scholar] [CrossRef]
- Ziff, O.J.; Hoskote, C.; Keddie, S.; D′Sa, S.; Davangnanam, I.; Lunn, M.P. Frequent central nervous system, pachymeningeal and plexus MRI changes in POEMS syndrome. J. Neurol. 2019, 266, 1067–1072. [Google Scholar] [CrossRef]
- Schreiber, S.; Schreiber, F.; Peter, A.; Isler, E.; Dörner, M.; Heinze, H.-J.; Petri, S.; Tempelmann, C.; Nestor, P.J.; Grimm, A.; et al. 7T MR neurography-ultrasound fusion for peripheral nerve imaging. Muscle Nerve 2020, 4, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, K.; Krzyzak, A.T. Analysis and correction of errors in DTI-based tractography due to diffusion gradient inhomogeneity. J. Magentic Reson. 2018, 296, 5–11. [Google Scholar] [CrossRef]
- Kierońska, S.; Sokal, P.; Dura, M.; Jabłońska, M.; Rudaś, M.; Jabłońska, R. Tractography-Based Analysis of Morphological and Anatomical Characteristics of the Uncinate Fasciculus in Human Brains. Brain Sci. 2020, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.; Eierud, C.; Banks, D.; Harshbarger, T.; Michael, A.; Rammell, C. Effects of Lifelong Musicianship on White Matter Integrity and Cognitive Brain Reserve. Brain Sci. 2021, 11, 67. [Google Scholar] [CrossRef]
- Morez, J.; Sijbers, J.; Vanhevel, F.; Jeurissen, B. Constrained spherical deconvolution of nonspherically sampled diffusion MRI data. Hum. Brain Mapp. 2021, 42, 521–538. [Google Scholar] [CrossRef]
- Mazur, W.; Urbańczyk-Zawadzka, M.; Banyś, R.; Obuchowicz, R.; Trystuła, M.; Krzyżak, A.T. Diffusion as a Natural Contrast in MR Imaging of Peripheral Artery Disease (PAD) Tissue Changes. A Case Study of the Clinical Application of DTI for a Patient with Chronic Calf Muscles Ischemia. Diagnostics 2021, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, A.; Morino, S.; Loreti, S.; Bucci, E.; Vanacore, N.; Antonini, G. Peripheral nerve ultrasound changes in CIDP and correlations with nerve conduction velocitiy. Neurology 2015, 84, 803–809. [Google Scholar] [CrossRef]
- Grimm, A.; Vittore, D.; Schubert, V.; Rasenack, M.; Décard, B.F.; Heiling, B.; Hammer, N.; Axer, H. Ultrasound aspects in therapy-naïve CIDP compared to longterm treated CIDP. J. Neurol. 2016, 263, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Winter, N.; Dammeier, N.; Schäffer, E.; Bornemann, A.; Stahl, J.-H.; Herlan, S.; Schuhmann, M.U.; Grimm, A. Nerve Ultrasonography as an Additive Tool to Clinical Examination and Electrodiagnostics in Sporadic Mononeuritis—Imaging is the Key. Ultraschall Med. 2019, 40, 465–472. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).