Abstract
Advances in acute myeloid leukemia (AML) genomics and targeted therapies include the recently approved BCL2 inhibitor venetoclax. The association between BCL2 expression and patient outcome was analyzed in a series of 176 consecutive AML patients at diagnosis (Dx), post-induction (PI), complete remission (CR) and relapse (RL). Levels increased significantly at relapse (mean 1.07 PI/0.96 CR vs. 2.17 RL, p = 0.05/p = 0.03). In multivariate analysis, high BCL2-Dx were marginally associated with worse progression-free survival, while high PI levels or at CR had an independent negative impact on outcome (PI: HR 1.58, p = 0.014; CR: HR 1.96, p = 0.008). This behavior of high PI or CR BCL2 levels and increased risk was maintained in a homogeneous patient subgroup of age <70 and intermediate cytogenetic risk (PI: HR 2.44, p = 0.037; CR: HR 2.71, p = 0.049). Finally, for this subgroup, high BCL2 at relapse indicated worse overall survival (OS, HR 1.15, p = 0.05). In conclusion, high BCL2 levels PI or at CR had an independent negative impact on patient outcome. Therefore, BCL2 expression is a dynamic marker that may be useful during AML patient follow up, and BCL2 levels at PI and/or CR may influence response to anti-BCL2 therapy.
1. Introduction
Advances in acute myeloid leukemia (AML) genomics have revealed the broad biological heterogeneity of the disease, leading to new risk stratifications in the pathology and the incorporation of targeted therapies for a more personalized management [1,2,3]. Despite this progress in the understanding of AML pathogenesis, frontline induction therapy has not substantially changed in 40 years [4] for most of the patients, and 85% of patients will relapse within 2 to 3 years [5]. BCL2 inhibitors are promising new agents [6,7] and venetoclax was approved by the U.S. Food and Drug Administration (FDA) for the treatment of AML patients under specific indications in November 2018. However, the molecular characteristics of patients likely to respond to venetoclax remain to be determined.
To address this issue, we studied the influence of BCL2 bone marrow expression on patient outcome in a consecutive series of 176 AML patients at diagnosis (Dx), and when possible, at post-induction (PI, when the patient recovers blood count, between days 21–28 after induction [8]); morphological complete remission (CR) (i.e., <5% blasts in the bone marrow [8]) and at relapse (RL). This series included a subgroup of 52 patients aged <70 years and of intermediate cytogenetic risk.
2. Materials and Methods
This study was approved by our center’s ethics committee (Comité Ético de Investigación Clínica, approval no. CEI_HUGCDN_565/150024, 01 October 2015). Informed consent was provided by all patients and donors. All methods were conducted in accordance with the Helsinki declaration and national research regulations. The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Patients were diagnosed and treated according to the protocols PETHEMA LMA2010 and LMA2014 for patients aged ≤ 65 years or > 65 years, respectively, at the Hospital Universitario de Gran Canaria Dr. Negrín and the Complejo Hospitalario Universitario Insular Materno Infantil, Las Palmas, Spain, from January 2014 to July 2017 (see Table 1 for patient characteristics). They had a median age of 59 years (range 16–82); 18 were secondary AML (with an antecedent hematologic disorder or therapy-related) and 158 were de novo. Patients with acute promyelocytic leukemia were excluded from the analysis.

Table 1.
Summary of patients’ clinical, biological and therapeutic characteristics and its association with BCL2 level.
Standard induction chemotherapy (anthracycline + cytarabine, “7+3” [1]) was applied in 94.9% of the patients. In consolidation, 51 patients (29%) underwent hematopoietic stem cell transplantation.
Of the consecutive bone marrow samples from 176 patients, we were able to analyze the BCL2 expression of 156 samples from Dx and 86 at PI due to missing samples or poor sample quality (at PI, 56 patients were at CR and 30 had persistent disease). In total, 64 patients were studied at CR and 28 at RL. At three time points, we were able to study 23 patients at Dx, PI (18 CR and 5 with persistent disease) and RL; 20 patients at Dx, CR and RL.
Relative gene expression was determined by the 2-∆∆Ct method normalized to ABL1 and relative to a cDNA pool from 10 healthy donors as internal calibrator. BCL2 expression was analyzed in healthy donors separately and no significant variation was observed; therefore, cDNAs were pooled and introduced in each experiment. Primers annealed in exons 2 and 3 of the functional anti-apoptotic long transcript isoform [9] (ENST00000333681.5; Forward: 5′-GGATTGTGGCCTTCTTTGAG-3′; Reverse: 5′- ACAGTTCCACAAAGGCATCC -3ʹ). There was good correspondence between mRNA and protein levels (anti-Bcl-2 #610538, BD Transduction Laboratories; Supplementary Figure S1).
Student’s t-test for parametric and Wilcoxon signed-rank test for non-parametric data were used to compare continuous variables. The receiver operating characteristic (ROC) curve and area under the ROC (AUC) was used to dichotomize values of gene expression. Univariate and multivariate survival analyses were carried out simultaneously in the patient cohort and a subgroup of patients with homogeneous risk (aged < 70 years, intermediate cytogenetic risk) using the Cox proportional hazard model. All tests were two-tailed; p values < 0.05 were considered statistically significant. Analyses were performed using statistical software R (version 3.3.3; www.r-project.org/).
3. Results
Table 1 shows patients’ clinical, biological and therapeutic characteristics and their association with BCL2 level, which was considered as both a continuous and dichotomous variable (with a cutoff established from the ROC curve of the prognostic performance and AUC). BCL2 levels were lower in patients with NPM1 mutation compared to NPM1 wild-type (p = 0.052). Although patients with a higher cytogenetic and European LeukemiaNet (ELN) 2010 risk showed elevated BCL2 levels, either as a continuous or dichotomous variable, this difference was not statistically significant. Mean BCL2 mRNA expression at diagnosis was above the control bone marrow pool (n = 156, mean = 1.61 ± 1.16 SD, min = 0.02, max = 6.97). Analyzing the expression dynamics of the same patients at different time points (Supplementary Figure S2), compared to Dx, BCL2 levels showed a marked descent at PI (n = 86, mean Dx 1.64 vs. PI 0.97, p < 0.001) and when achieving CR (n = 64, Dx 1.57 vs. CR 0.95, p < 0.001; Supplementary Table S1), but raised again at RL compared to PI (n = 23, RL 2.17 vs. PI 1.07, p = 0.05) and CR (n = 20, RL 2.27 vs. CR 0.96, p = 0.03). Levels were slightly higher at RL compared to those at Dx, but this difference was not significant. No association was found between BCL2 levels and presenting CR or refractory disease at PI.
For univariate and multivariate survival analyses, only patients who received first-line intensive treatment (anthracycline + cytarabine, 7 + 3 schedule) were selected.
BCL2 level was also considered as a continuous and dichotomous variable for overall survival (OS, median follow up 28.4 months; range 0.2–147.3 months) and progression-free survival, defined as the time from diagnosis to disease progression or death from any cause (PFS, median follow up 24.6 months; range 6.1–147.2 months) (Supplementary Tables S2 and S3).
At diagnosis, no association with OS was observed; however, BCL2 levels above the AUC value (Supplementary Table S4) were significantly associated with worse PFS in the univariate analysis (HR 1.63, 95% CI 1–2.66, p = 0.05) and this behavior was marginally maintained in the multivariate analysis (HR 1.589, 95% CI 0.96–2.62, p = 0.07).
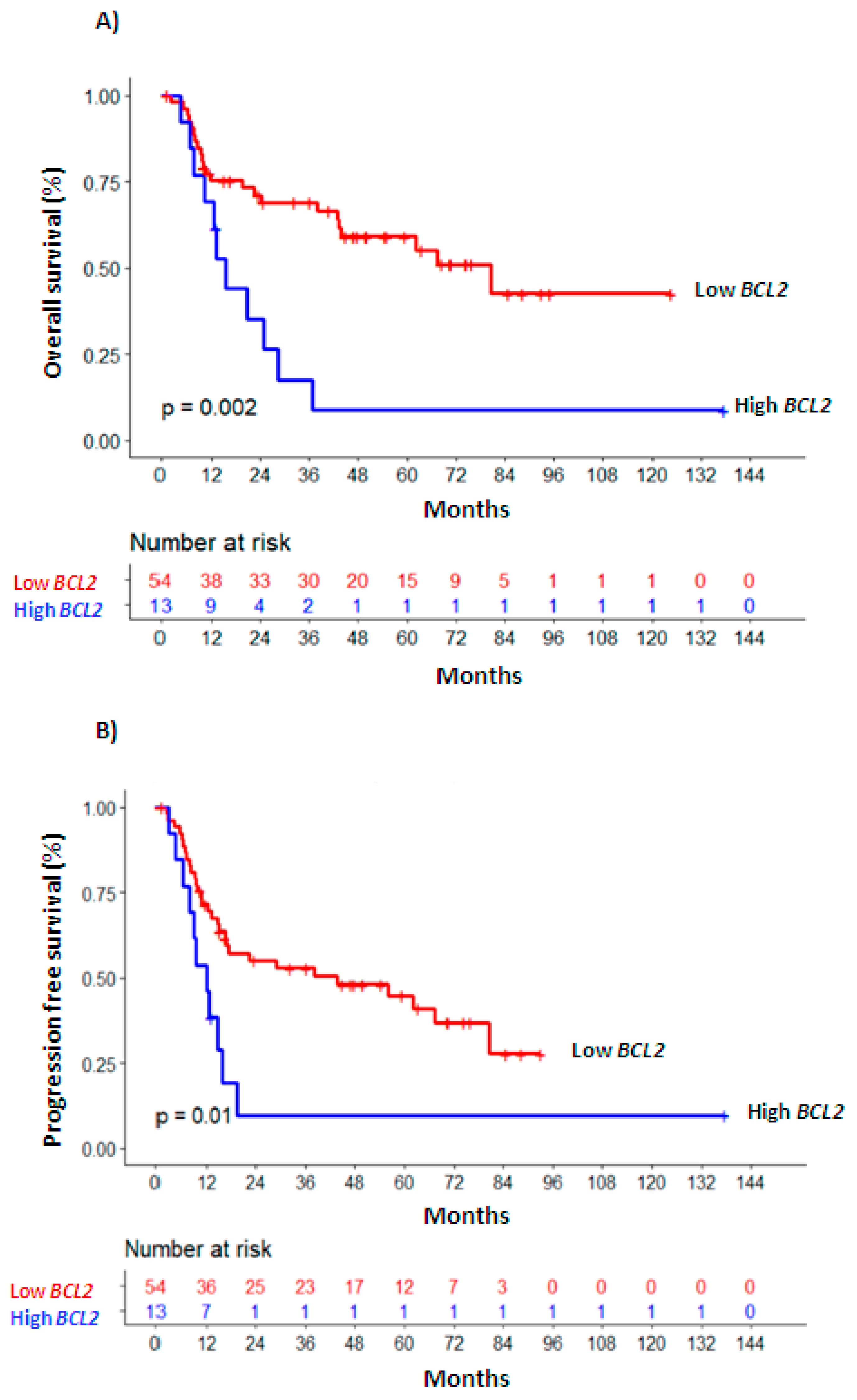
Univariate survival analyses were also performed considering BCL2 levels at the moment of PI, CR (Figure 1) and at RL (Supplementary Table S5). In multivariate analysis (Table 2), higher BCL2 levels measured at PI as a continuous variable remained as an independent marker of worse OS (HR 1.578, 95% CI 1.1–2.27, p = 0.014) and PFS (HR 1.547, 95% IC 1.09–2.19, p = 0.014); as a dichotomous variable, associated with inferior PFS (HR 1.749, 95% CI 1.04–2.95, p = 0.036) and marginally with OS (HR 1.764, 95% CI, 0.95–3.28, p = 0.073). This behavior was also maintained for OS in the subgroup of patients with homogenous risk (age < 70 years, intermediate cytogenetic risk; n = 52; BCL2-PI continuous: HR 1.63, 95% CI 1.04–2.57, p = 0.035; BCL2-PI dichotomous: HR 2.44, 95% CI 1.06–5.64; p = 0.037, Supplementary Table S5).
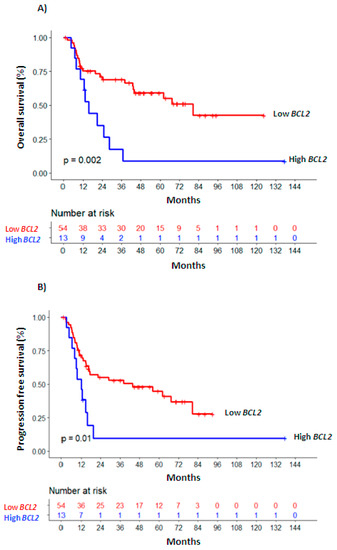
Figure 1.
Overall survival (A) and progression-free survival (B) Kaplan—Meier curves of patients with high vs. low BCL2 expression levels.

Table 2.
Multivariate survival analysis.
The independent association of increased BCL2 levels and worse OS was stronger at the time of CR (BCL2-CR continuous: HR 1.961, 95% CI 1.19–3.24, p = 0.008; BCL2-CR dichotomous: HR 3.028, 95% CI 1.34–6.86, p = 0.008, Table 2). This relationship was maintained in the homogenous subgroup, with levels of BCL2 above the AUC value at CR (HR 2.71, 95% CI 1–7.33, n = 41, p = 0.049, Supplementary Table S5). More BCL2 at CR was also significantly associated with worse PFS (BCL2-CR continuous: HR 1.73, 95% CI 1.13-2.66, p = 0.012; BCL2-CR dichotomous: HR 2.078, 95% CI 1.09–3.97, p = 0.027, Table 2).
Finally, we observed no significant association for the impact of BCL2 levels on OS at the moment of RL in multivariate analyses (BCL2-RL continuous: n = 30, HR 1.105, 95% CI 0.97–1.26, p = 0.125). Nevertheless, in the homogenous subgroup, higher BCL2 expression at RL was significantly associated with OS (n = 19, BCL2-RL continuous: HR 1.15, 95% CI 1–1.32, p = 0.05).
4. Discussion
We observed a positive association between the absence of NPM1 mutation and higher BCL2 levels. A biological explanation was not found for this association; however, recent studies show promising results for the combination of hypomethylating agents + venetoclax in older patients with a NPM1 mutation, which could be related to the lower BCL2 levels of these patients [10,11]. According to our results, BCL2 expression was higher at Dx compared to PI and CR and was high again at RL; therefore, it seems to be a dynamic marker that reflects the evolution of AML and could be informative of impending RL.
Multivariate analysis of survival in relation to BCL2 levels (as a continuous or dichotomous variable) at the different follow-up times revealed that higher BCL2 at PI negatively influenced OS and PFS. This behavior was maintained for OS in the homogeneous subgroup. The independent association with worse OS was more evident in patients with more BCL2 at CR in the whole series and homogenous subgroup. Lastly, albeit tested in a limited series of patients, high BCL2 expression at RL also related with worse OS in the homogeneous subgroup. Therefore, although BCL2 levels are generally reduced by induction chemotherapy, there is a relationship between increased BCL2 levels at PI or when achieving CR and a worse outcome. This association remained in patients aged below 70 and of intermediate cytogenetic risk, meaning that its prognostic value may contribute to the more accurate risk stratification of this wide group of patients of variable outcome.
Previous studies found that high BCL2 protein expression at diagnosis was associated with shorter survival in multivariate analysis [12] and this was also observed for mRNA levels in univariate analyses [13,14].
However, despite the BCL2 reduction observed after chemotherapy, according to our results, it is the BCL2 mRNA level after induction or at CR, rather than at diagnosis, which has the greatest influence on patient outcome. Our results reinforce the fact that the assessment of post-treatment remission by cytomorphology, defined as <5% blasts in bone marrow [8], does not mean that the patient is free of disease. It will be interesting to determine whether minimal/measurable residual disease-positive samples correlate with higher BCL2 levels at CR (and/or PI) in future studies.
Relapsed AML cases are difficult to treat and therapeutic options are needed for refractory cases. Since sensitivity to the selective BCL2 inhibitor, venetoclax, correlates with BCL2 levels [15], it is possible that BCL2 expression levels at Dx, PI, CR, and even at RL may influence the response to venetoclax treatment, in agreement with our observations and recently communicated results [10,11,16,17].
Although further analyses in a larger series with longer follow up data are needed to confirm these results, we consider that BCL2 expression may be an informative prognostic marker after induction therapy and could allow for the rapid, easy and cost-effective identification of a subgroup of patients in which BCL2 inhibition could be successful.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4418/10/12/1048/s1. Supplementary Figure S1 Correspondence between protein levels (A) via Western blot and mRNA levels (B) via QRT-PCR. Protein quantification (C) by ImageJ software. The images in (A) are taken from two different blots; the samples were derived from the same experiment and the blots were processed in parallel. Supplementary Figure S2 Evolution of BCL2 levels in 18 individual patients with follow up BCL2 values available at diagnosis (Dx), complete remission (CR) and relapse (RL). Supplementary Table S1 Comparison of BCL2 expression levels at different points during follow up. Supplementary Table S2 Dichotomized value of BCL2 expression level with the area under the ROC curve for overall survival (OS). Supplementary Table S3 Dichotomized value of BCL2 expression level with the area under the ROC curve for progression-free survival (PFS). Supplementary Table S4 Univariate and multivariate progression-free survival analysis in the whole series at diagnosis (Dx). Supplementary Table S5 Univariate survival analysis.
Author Contributions
Study design: M.T.G.-C., C.R.-M., C.B.-S. Study conduct: Y.F., S.S.-S., C.R.-M., G.S., M.T.G., C.B.-S. Data collection: Y.F., S.S.-S., C.R.-M., G.S., E.G.-P., N.C.-C., R.F., T.M.L. Data analysis: J.M.G.M., M.N.S. Data interpretation: C.R.-M., R.S., M.T.G, C.B.-S. Drafting the manuscript: R.S., C.B.-S. Revising the manuscript content: R.S., M.T.G., C.B.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness ISCIII-FIS (grant no. PI14/02220).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. New Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.M. Progress and predictions: AML in 2018. Best Pract. Res. Clin. Haematol. 2018, 31, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, H.F.; Sun, Z.; Yao, X.; Litzow, M.R.; Luger, S.M.; Paietta, E.M.; Racevskis, J.; Dewald, G.W.; Ketterling, R.P.; Bennett, J.M.; et al. Anthracycline dose intensification in acute myeloid leukemia. New Engl. J. Med. 2009, 361, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.; Wetzler, M.; Lowenberg, B. Therapeutic advances in acute myeloid leukemia. J. Clin. Oncol. 2011, 29, 487–494. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Stricklands, S.A.; Roboz, G.J.; Hou, J.Z.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Chyla, B.; Popovic, R.; et al. Phase 1/2 study of venetoclax with low-dose cytarabine in treatment-naive, elderly patients with acute myeloid leukemia unfit for intensive chemotherapy: 1-year outcomes. Blood 2017, 130, 890. [Google Scholar]
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Büchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [PubMed]
- Akgul, C.; Moulding, D.A.; Edwards, S.W. Alternative splicing of Bcl-2-related genes: functional consequences and potential therapeutic applications. Cell. Mol. Life Sci. 2004, 61, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; Loghavi, S.; Kadia, T.M.; Daver, N.; Borthakur, G.; Pemmaraju, N.; Naqvi, K.; Alvarado, Y.; Yilmaz, M.; Short, N.; et al. Outcomes of older patients with NPM1-mutated AML: current treatments and the promise of venetoclax-based regimens. Blood Adv. 2020, 14, 1311–1320. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.; Rouault, J.P.; Sabido, O.; Oriol, P.; Roubi, N.; Vasselon, C.; Archimbaud, E.; Magaud, J.P.; Guyotat, D. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood 1993, 81, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Karakas, T.; Maurer, U.; Weidmann, E.; Miething, C.C.; Hoelzer, D.; Bergmann, L. High expression of bcl-2 mRNA as a determinant of poor prognosis in acute myeloid leukemia. Ann. Oncol. 1998, 9, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Karakas, T.; Miething, C.C.; Maurer, U.; Weidmann, E.; Ackermann, H.; Hoelzer, D.; Bergmann, L. The coexpression of the apoptosis-related genes bcl-2 and wt1 in predicting survival in adult acute myeloid leukemia. Leukemia 2002, 16, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Hogdal, L.J.; Benito, J.M.; Bucci, D.; Han, L.; Borthakur, G.; Cortes, J.; DeAngelo, D.J.; Debose, L.; Mu, H.; et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014, 4, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Chyla, B.J.; Harb, J.; Mantis, C.; Riehm, J.J.; Ross, J.A.; Sun, Y.; Huang, X.; Jiang, Q.; Dail, M. Response to venetoclax in combination with low intensity therapy (ldac or hma) in untreated patients with acute myeloid leukemia patients with IDH, FLT3 and other mutations and correlations with BCL2 family expression. Abstract 546. In Proceedings of the ASH Annual Meeting, Orlando, FL, USA, 7–10 December 2019; Available online: https://ash.confex.com/ash/2019/webprogram/Paper128373.html (accessed on 2 February 2020).
- Maiti, A.; DiNardo, C.D.; Rausch, C.R.; Cortes, J.E.; Pemmaraju, N.; Daver, N.G.; Ravandi, F.; Garcia-Manero, G.; Borthakur, G.M.; Naqvi, K.; et al. Ten-day decitabine with venetoclax (DEC10-VEN) in acute myeloid leukemia: Updated results of a phase II trial. Abstract 2637. In Proceedings of the ASH Annual Meeting, Orlando, FL, USA, 7–10 December 2019; Available online: https://ash.confex.com/ash/2019/webprogram/Paper127803.html (accessed on 2 February 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).