Abstract
A 16-year-old male patient underwent 18F-FDG PET/CT staging after multiple surgical resections and radiotherapy of an uncommon metastatic pediatric sebaceous carcinoma of the parotid gland. Initial PET/CT imaging exhibited a recurrent paravertebral metastasis (C4) as well as a metabolically active tumor tissue at the primary site. Follow-up PET/CT after radiotherapy of the cervical spine (C4) and four cycles of chemotherapy with cisplatin and palbociclib revealed complete functional remission in the cervical spine and partial remission at the primary site. This case illustrates the 18F-FDG-uptake behavior and the disease course of a very rare malignant epithelial tumor of the salivary glands.
We present a 16-year-old male patient with recurrent metastatic sebaceous carcinoma in the left parotid gland. The tumor was diagnosed at the age of 9 years. Previous multimodal treatments between the age of 9 and 16 years included radical parotidectomy with partial resection of the left mandible, lower jaw and facial nerve reconstruction, repeated local re-resections and local adjuvant radiotherapy for local tumor recurrences, as well as spinal surgery for vertebral metastasis. Prior to further therapies, 18F-FDG PET/CT imaging with 232 MBq 18F-FDG and contrast-enhanced CT exhibited local re-recurrence in the left lower jaw with highly elevated glucose consumption (SUVmax 8.3; see Figure 1A) as well as an intra- and extraspinal recurrence of metastasis in the cervical spine (C4) with similarly elevated metabolism (SUVmax 8.8; see Figure 2A). Subsequently, a combined radiotherapy of C4 and chemotherapy with cisplatin and palbociclib, a cyclin-dependent kinase inhibitor, was initiated. The patient returned for follow-up after three months. Whereas no morphological changes were seen at the primary site (see red arrow in Figure 1), 18F-FDG PET imaging revealed a slightly decreased metabolic activity (SUVmax 7.1; see Figure 1B). The spinal metastasis showed a complete functional remission (see Figure 2B) on 18F-FDG PET.
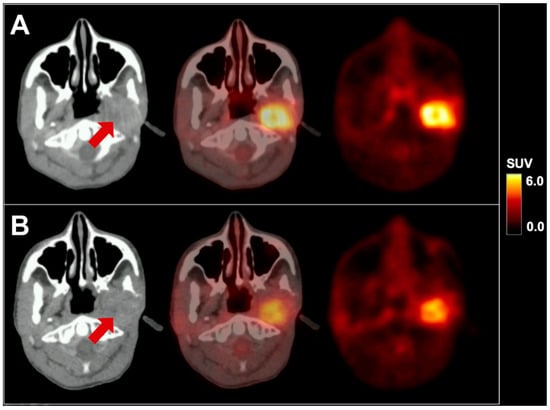
Figure 1.
18F-FDG PET/CT in axial planes of a pediatric sebaceous carcinoma before (A) and after therapy (B). The red arrow indicates the primary site in the left parotid gland. SUV = standardized uptake value.
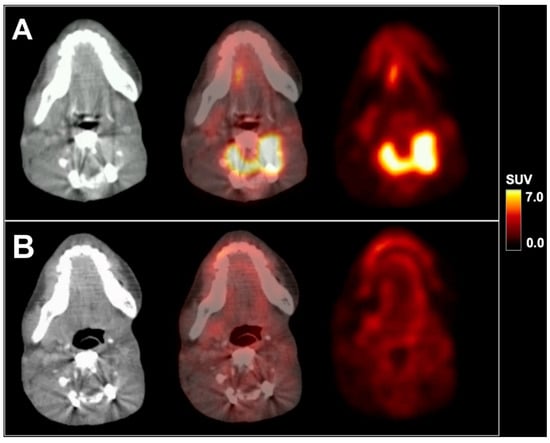
Figure 2.
18F-FDG PET/CT in axial planes of a metastasis in the cervical spine before (A) and after radiochemotherapy (B). SUV = standardized uptake value.
Sebaceous carcinoma is an aggressive malignant tumor originating from the pilosebaceous unit, usually occurring in the periorbital area and less frequently in extraocular regions with an overall incidence of 1–2 per 1 million individuals per year [1,2,3]. Ectopic occurrence in the parotid gland, however, is a very rare condition that has only been reported in few cases [4,5]. The current patient is relatively young with an age of 9 years at initial diagnosis, whereas the median age at diagnosis of sebaceous carcinoma is 73 years [1,2]. Only very few cases of pediatric sebaceous carcinoma have been reported so far, but none of them with parotid location as in the current case [6,7,8]. An association of sebaceous carcinoma with Muir–Torre syndrome, a variant of Lynch syndrome (HNPCC), has been described [2,9]. First-line therapy generally consists of surgical removal [9]. Only a single case of 18F-FDG PET/CT imaging in a sebaceous carcinoma of the parotid gland in an adult patient has been reported so far, demonstrating the general feasibility of 18F-FDG PET/CT imaging in this tumor entity [10].
In clinical routine, sebaceous carcinoma has to be considered as a very rare differential diagnosis of a tumor in the parotid gland, even in young patients (ORPHA 276145). As illustrated by the current case, hybrid imaging using 18F-FDG PET/CT depicts changes of glucose consumption during therapy in sebaceous carcinoma, and may provide additional information for evaluation of therapy response beyond the morphological extent on CT. However, it still has to be clarified, whether assessment of glucose consumption in pediatric sebaceous carcinoma of the parotid gland improves clinical management and results in better patient outcomes. Due to the extreme rarity of the entity, prospective studies including PET/CT imaging are unlikely to be available; however the assumption of an added value seems plausible—e.g., when functional remission as in the current case indicates a therapy response—as a benefit of PET imaging in therapy management has likewise been shown for other conditions in pediatric oncology [11,12,13]. Hence, a mere unspecific, treatment-related uptake seems highly unlikely. Overall, hybrid imaging using PET/CT, but also PET/MRI should be considered for therapy monitoring in this entity, particularly in young patients.
Author Contributions
A.H.—clinical management, draft manuscript. T.P.—consultation pediatric imaging, increased intellectual content. I.S., O.G.-L.—clinical management, revision of the manuscript, increased intellectual content. N.L.A., W.G.K., J.R., P.B.—revision manuscript, increased intellectual content. M.U.—manuscript draft, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Consent for Publication
The pediatric patient’s parent gave written consent prior to PET/CT exam. The ethics committee waives additional approval for case reports.
References
- Dasgupta, T.; Wilson, L.D.; Yu, J.B. A retrospective review of 1349 cases of sebaceous carcinoma. Cancer 2009, 115, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Dores, G.M.; Curtis, R.E.; Toro, J.R.; Devesa, S.S.; Fraumeni, J.F., Jr. Incidence of cutaneous sebaceous carcinoma and risk of associated neoplasms: insight into Muir-Torre syndrome. Cancer 2008, 113, 3372–3381. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, T.; Samie, F.H. Sebaceous Carcinoma: A Review of the Scientific Literature. Curr. Treat. Options Oncol. 2017, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Khmou, M.; Laadam, K.; Cherradi, N. Parotid gland, an exceptional localization of sebaceous carcinoma: case report. BMC Clin. Pathol. 2016, 16, 10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marnouche el, A.; Maghous, A.; Kadiri, S.; Berhili, S.; Touil, A.; Kettani, F.; Majjaoui, S.; Elkacemi, H.; Kebdani, T.; Benjaafar, N. Sebaceous carcinoma of the parotid gland: a case report and review of the literature. J. Med Case Rep. 2016, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Boussofara, L.; Trabelsi, A.; Denguezli, M.; Sriha, B.; Belajouza, C.; Nouira, R. Undifferentiated sebaceous carcinoma: an unusual childhood cancer. Pediatric Dermatol. 2007, 24, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Mirzamani, N.; Sundram, U.N. A case of sebaceous carcinoma diagnosed in an adolescent male. J. Cutan. Pathol. 2011, 38, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Stacey, S.K.; Moss, T.A.; Kobayashi, T.T. Sebaceous carcinoma on the arm of a 10-year-old girl. Dermatol. Online J. 2017, 23, 6. [Google Scholar]
- Owen, J.L.; Kibbi, N.; Worley, B.; Kelm, R.C.; Wang, J.V.; Barker, C.A.; Behshad, R.; Bichakjian, C.K.; Bolotin, D.; Bordeaux, J.S.; et al. Sebaceous carcinoma: evidence-based clinical practice guidelines. Lancet. Oncol. 2019, 20, e699–e714. [Google Scholar] [CrossRef]
- Kumabe, A.; Kawase, T.; Miura, K.; Tada, Y.; Masubuchi, T.; Kamata, S.E.; Tanada, S.; Kubo, A.; Tajima, Y.; Shigematsu, N. Sebaceous carcinoma of the parotid gland: F-18 FDG PET/CT findings. Clin. Nucl. Med. 2010, 35, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Masselli, G.; De Angelis, C.; Sollaku, S.; Casciani, E.; Gualdi, G. PET/CT in pediatric oncology. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 83–94. [Google Scholar] [PubMed]
- Schmidkonz, C.; Krumbholz, M.; Atzinger, A.; Cordes, M.; Goetz, T.I.; Prante, O.; Ritt, P.; Schaefer, C.; Agaimy, A.; Hartmann, W.; et al. Assessment of treatment responses in children and adolescents with Ewing sarcoma with metabolic tumor parameters derived from (18)F-FDG-PET/CT and circulating tumor DNA. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Shapira-Zaltsberg, G.; Wilson, N.; Trejo Perez, E.; Abbott, L.; Dinning, S.; Kapoor, C.; Davila, J.; Smith, B.; Miller, E. Whole-Body Diffusion-Weighted MRI Compared to (18 F)FDG PET/CT in Initial Staging and Therapy Response Assessment of Hodgkin Lymphoma in Pediatric Patients. Can. Assoc. Radiol. J. 2020, 71, 217–225. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).