Abstract
One of the key steps in the origins of life was the formation of a membrane to separate protocells from their environment. These membranes are proposed to have been formed out of single chain amphiphiles, which are less stable than the dialkyl lipids used to form modern membranes. This lack of stability, specifically for decanoate, is often used to refute ocean locations for the origins of life. This review addresses the formation of membranes in hydrothermal-vent like conditions, as well as other environmental constraints. Specifically, single chain amphiphiles can form membranes at high sea salt concentrations (150 g/L), high temperatures (65 °C), and a wide pH range (2 to 10). It additionally discusses the major challenges and advantages of membrane formation in both ocean and fresh water locations.
1. Early Earth Environments for the Origins of Life
Life is hypothesized to have originated on Earth between 4.2 bya and 3.8 bya [1,2]. Energy, water, and chemicals would have had to combine in perhaps precise amounts and sequences for this process to have occurred. As these environments are reviewed elsewhere in this issue [3], as well as in other sources, they will only briefly be reviewed here.
Hydrothermal vents are often proposed and studied as early locations for abiogenesis [4,5,6]. These deep ocean locations provide the right environments and reactants for many reactions that lead to the molecules used in modern biology [7,8,9]. This is due to the temperature, pressure, water, mineral surfaces, and simple chemicals available in these environments. However, vast oceans may dilute any products formed, and the high concentration of salt in these locations make them untenable for certain prebiotic evolutionary models.
Inland locations with access to fresh water, likely through precipitation, are also possible locations for the origin of life [10,11]. These locations could have chemical precursors available from meteoric sources, and perhaps geothermal systems, with lower concentrations of dissolved ionic solutes and dehydration as a chemical driving force for non-enzymatic polymerization reactions [12,13]. However, these regions are thought to have had lower concentrations of high energy chemicals than hydrothermal systems and would be exposed to harsh UV irradiation.
One of the main arguments used to disfavor hydrothermal vent environments is the conjecture that the primitive membranes that formed the first cell-like structures could not form due to high (divalent) salt concentrations [11,14]. We disagree: membranes composed of prebiotic amphiphiles benefit from increases in ionic strength, and prebiotic vesicles can be formed in a high ionic strength solutions, including seawater (containing divalent cations), making hydrothermal environments plausible locations for the first cell membranes to have formed. Here we present a review of these membranes and hope to steer the abiogenic environment discussion away from primitive membrane stability to more relevant topics.
2. Membranes and Life
Membranes serve many purposes in a cell and are considered a requirement for the formation of life. These amphiphilic bilayers primarily act as a hydrophobic barrier between the environment and the cytosol, preventing the diffusion of molecules into and out of the cell. They additionally can contain energy harvesting molecules like the proteins in the electron transport chain required for oxidative phosphorylation and maintain the chemical gradients necessary to use these molecules. Finally, membranes serve as a platform for evolution by defining an individual within a population for selection to act upon.
Primitive membrane models have been shown to perform many of these ‘living’ functions, as described above [15,16,17,18]. Membranes composed of just ten-carbon amphiphiles have been shown to encapsulate template directed synthesis of nucleic acids from externally added nucleotides [19]. Other biologically relevant reactions have also been demonstrated in such systems, including the polymerization of nucleic acids through dehydration, catalysis using ribozymes, and catalysis using proteins [12,20,21]. The involvement of these membranes in energy generation (i.e., photochemistry) is also well established [22,23,24,25,26]. Additionally, these membranes display growth and division cycles, allowing for the replication of primitive cells [27,28,29,30]. The research to date shows these simple membranes to be versatile components of model proto-cells.
Early membranes are proposed to have been made of chemically simpler molecules than modern cell membranes while still performing the above functions [31]. Modern membranes are composed primarily of dialkyl amphiphiles with a charged head group. However, monoalkyl amphiphiles are more likely to have formed from prebiotic chemical synthesis, including hydrothermal [7,8,32,33] and interstellar reactions [34,35].
Using the top down approach to help discern the origins of life, we can gain insight into primitive membranes by examining modern membranes. Using this approach, fatty acids are often cited as the most likely primitive amphiphile [36,37]. If modern bacterial membranes evolved from primitive membranes, the first amphiphile contained an alkyl hydrophobic group and a carboxylate head group, generating a fatty acid. These fatty acids could then have later condensed onto a glycerol backbone to form the more stable diacyl amphiphiles. However, fatty acids may also have been a later adaptation that was advantageous for other reasons (e.g., energy storage), and this approach is not the only means of unraveling life’s origins.
Using a bottom up approach, very different conclusions can be made. The prebiotic chemical library, generated from hydrothermal, atmospheric, meteoric, and perhaps other sources, was a mix of chemicals with larger abundances of simple small molecules and lower abundances of more complex, larger molecules [8,38]. These mixtures were unlikely to have any one amphiphile, and a wide range of functional groups can be found in these mixtures, including amines, alcohols, aldehydes, and carboxylates, among others. If the first cells were assembled from environmentally available amphiphiles, this would indicate that a mixture of amphiphiles would have formed the first cell membranes.
Generally, the bottom up and the top down approach meet in the middle, where a successful replicator is thought to adopt a single strategy. Therefore, while carboxylates were most likely part of the first membranes, it is unlikely they were the only amphiphile present.
3. Salt and Membranes
Using a top-down approach to determine the salinity of the first cells, neither sea water nor fresh water closely mimics contemporary cytosol (Table 1). Single celled organisms have cytosol that is highly dependent on growth media, making this task more difficult. It is also not known how contemporary cytosol differs from the cytosol of the last universal common ancestor (LUCA).

Table 1.
Concentration of ions in relevant solutions.
Fresh water was likely available from precipitation and dew formation but was by far less prevalent, as the continental mass was even smaller than it is today. All cells contain relatively concentrated ionic solutions compared to fresh water, freshwater seems an unlikely choice for the formation of life, especially considering the advantages hydrophobic self-assembly has in higher ionic strength solutions [39,40].
Seawater is much higher in total ionic strength than cytosol (>3 times), and the ratios more closely mimic blood (or extracellular environment), with high concentrations of sodium but low concentrations of potassium. Additionally, the concentration of ions in the early oceans was likely equal, if not higher than, in the modern oceans, although the ions present may have differed due to the lack of oxygen in the atmosphere and the temperature, among other factors [41]. The water present on early Earth quickly equilibrated with the atmosphere, and the mineral surfaces present to generated oceans with high ionic strength, likely 1.6 to two times saltier than today’s oceans. This makes seawater an unlikely choice for the first cytosol as well.
The first primitive membranes were reported in 1978 by Hargreaves and Deamer, demonstrating that short, saturated fatty acids and fatty acid/alcohol mixtures could self-assemble into membranes [36]. These amphiphiles were appealing, not only because they are simpler than phosphoglycerolipids, but also a chemical building block making fatty acids a clear precursor to modern lipids. Since many properties of these membranes have been explored, it is clear that they are capable of a range of ‘living’ functions [14,21,22,23,28,30].
However, carboxylates precipitate in the presence of divalent cations, specifically Mg2+ and Ca2+, which are present in seawater. To form membranes, fatty acids must be partially deprotonated (close to their pKa); therefore the membranes will precipitate in ocean-like conditions. Additionally, divalent cations are a requirement for many nucleic acid reactions, making these membranes less compatible with the RNA world hypothesis.
While many articles discuss the incompatibility of high ionic strength solutions with membranes, few discuss the need for salt in single chain amphiphile membrane formation [39,45]. The ionic strength of fresh water would likely be insufficient to form membranes from decanoic acid, as about 10 mM of salt is needed to find vesicles. Most experiments using fatty acid membranes do not report NaCl addition, as it is added inadvertently through pH vesiculation: by increasing the pH with NaOH then decreasing the pH to the pKa of the fatty acid with HCl [46]. This formation improvement is likely caused by both salting-in the hydrophobic tails and charge-shielding the carboxylate headgroups, allowing for the formation of fatty acid membranes [40,47].
Many simple membrane models have now been proposed from a range of single chain amphiphiles (Figure 1) [48,49,50,51,52,53,54,55]. Membranes composed of non-fatty acid amphiphiles or mixtures of fatty acids with other amphiphiles were found to be more stable than fatty acid/carboxylate membranes; they showed less divalent salt sensitivity [14,55], higher critical vesicle concentrations [22,48], more pH-range resilience [14,49,55], and less mixing between populations [48]. These amphiphiles include the often-used long chain alcohols and glycerol monoacylates but also less commonly reported charged groups like alkyl phosphates, ketocarboxylic acids, and amines.
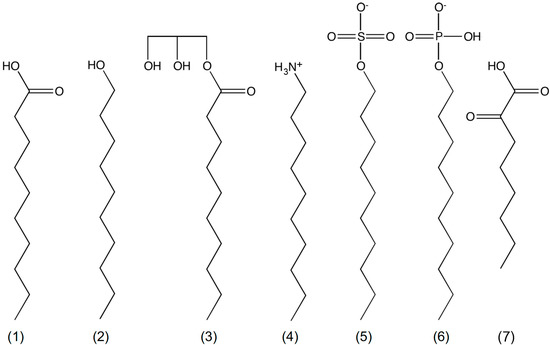
Figure 1.
Chemical structures of mono decyl amphiphiles. (1) decanoic acid; (2) decanol; (3) glycerol monodecanoate; (4) decylamine; (5) decylsulfate; (6) decylphosphate; and (7) 2-ketooctanoic acid.
It is likely that a wide variety of membranes need some amount of salt to form, and the author therefore recommends adding a fixed amount of saline to buffers to aid in aggregation (30 to 100 mM). While decanoic acid/decanoate membranes tend to ‘salt-out’ of solution around 200 mM NaCl, mixtures of single chain amphiphile membranes are often stable at much higher concentrations of salt [39].
Some mixtures of single-chain amphiphiles can form stable membranes in seawater solutions. Namani and Deamer originally showed the formation of decanoic acid/decylamine vesicles in the presence of seawater [51]. Additionally, ketocarboxylic acids and phosphate vesicles are stable in seawater solutions [49,55]. Recently, we have found that seawater can be used to form membranes out of a variety of amphiphile mixtures (Figure 2) [56]. The clear exception is carboxylates, which precipitate due to the Mg2+ content, but, if the pH is kept relatively low, even fatty acids can be included in the mixture. The concentration of the seawater can even be increased above Archean estimates, and membranes of decanoic acid/decylamine will still form (Figure 3A) [56]. Additionally, mixtures of amphiphiles would have been far more likely in the absence of a specific enzymatic synthesis, reducing the carboxylate precipitation problems commonly cited as a barrier to a saltwater/hydrothermal origin.
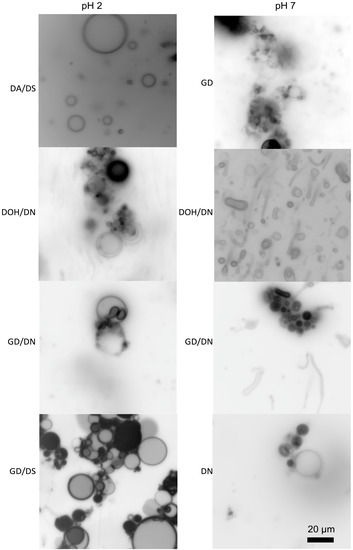
Figure 2.
Membrane formation of various amphiphiles in the presence of 35 g/L sea salt. The abbreviations used are decanoic acid (DA), decylsulfate (DS), decanol (DOH), decylamine (DN), and glycerol monodecanoate (GMD). The total concentration of lipid in each sample is 25 mM, and the mixtures of amphiphiles are in a 1:1 ratio. Original micrographs are from the author. The scale bar is the same for all micrographs.
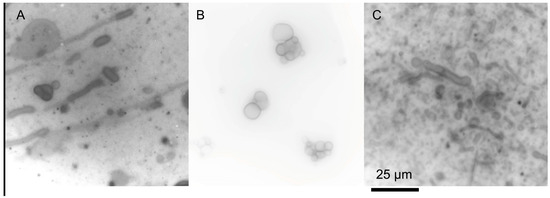
Figure 3.
Decanoic acid/decylamine mixtures (equimolar, 25 mM total concentration) at higher concentrations of sea salt. (A) 60 g/L of sea salt at room temperature. (B) 150 g/L sea salt at room temperature. (C) 150 g/L sea salt at 65 °C. Original micrographs are from the author. The scale bar is the same for all micrographs.
4. Other Environmental Factors
Simple membranes are impacted by other environmental conditions, although each factor’s impact is specific to the composition of the amphiphile in the membrane. For example, some compositions are only stable as membranes at low temperatures, while others only form membranes at high temperatures [48,57]. This is means that there are compositions of single chain amphiphiles that can form membranes under most conditions, but a composition of single chain amphiphiles that forms membranes under one extreme condition is unlikely to form membranes under different conditions. Hydrothermal environments have many ‘extreme’ conditions, but it is this author’s conjecture that perhaps it is not truly impossible to find an amphiphile composition that will form membranes.
The pH of contemporary oceanic vents can vary from 2 to 3 in black smoker chimneys [58] to a much higher pH of almost 10 in white smoker chimneys [59], compared to an ocean pH of about 8. Some single chain amphiphiles are known to form membranes under these conditions. Decylphosphate was capable of forming membranes at low pH [49,60], and mixtures of decylamine with a wide range of co-surfactants produce membranes that are pH stable at both low and high pH, even in the presence of sea salt ([51,56]). However, if we assume that fatty acids alone were the precursor to modern membranes, the pH of the solution in which membranes form would be dependent on the hydrocarbon tail length of the fatty acid and likely be between 6 and 9 [22]. River pH is generally between these values [61]; cytosolic pH in human cells is generally 7.2 [42].
All membranes, even biological membranes, have temperature dependent formation. When the temperature is reduced below the phase transition temperature, the membranes lose integrity, becoming leaky, and preventing the function of membrane bound protein components (although the phase transition temperature of palmitoyl oleoyl phosphatidylcholine (POPC) is −2.5 °C [62]). Single chain amphiphile membranes are often more instable, precipitating and sometimes crystallizing if the temperature is decreased. Upon heating, these membranes can return to solution. Additionally, as the temperature is increased, the solubility of the amphiphiles increases, increasing the critical vesicle concentration, which dissolves the membranes. It has also been shown that increasing temperatures increases membrane fusion between individual vesicles [35]. White smoker hydrothermal vents are generally between 40 °C to 70 °C, while black smokers are much hotter, measuring between 60 °C to 400 °C. The ocean floor in these regions is generally around 2 °C, giving a gradient of temperatures around a vent system. Single chain amphiphile membranes can be observed at temperatures above room temperature. For example, dodecanoic acid/glycerol dodecanoate mixtures have been used to encapsulate fluorescent molecules at 42 °C [57]. Additionally, mixtures of decanoic acid/decylamine have been observed to form membranes at 65 °C (Figure 3C), and it is likely that other membranes are stable at these higher temperatures as well. The temperature may have fluctuated more rapidly in surface environments, where weather and day/night cycles would have a much greater impact.
Perhaps the greatest obstacle to the formation of membranes under prebiotic conditions is the accumulation of amphiphiles in sufficient concentrations to form membranes. For amphiphile molecules to accumulate, they either must be continuously synthesized in sufficient numbers to compensate for dilution or aggregate in a specific location. Synthesis in tectonic fault zones, for example, could account for the continuous release of amphiphiles into a prebiotic ocean [63]. In contrast, the build-up of amphiphiles in continental locations could occur by washing materials into natural pools that concentrate amphiphiles through dehydration [10]. However, this would also concentrate any other materials that are with the amphiphiles, like salts or oily contaminants, creating new challenges. Issues with low concentrations of amphiphiles are also mitigated by more complex mixtures of surfactants, which have lower critical vesicle concentrations [48,51,64]. With regards to generating sufficient concentrations of amphiphiles, the plausibility of either ocean or land origins are suspect; both locations are problematic in their own way.
The final environmental factor often considered in amphiphile chemistry is the presence of a mineral surface. The diversity of surfaces considered, both submarine and terrestrial, results in some surfaces being detrimental to membrane formation [65], while others are beneficial [66]. In ocean environments, the surface could play a lesser role, but most hydrothermal locations are ideal because of their mineral chemistry, so it is unlikely that the first cells would be removed too far from the often-catalytic locales. Therefore, both ocean and land locations have similar mineral challenges for vesicle formation.
5. Conclusions
Membranes are an essential component of cells, even protocells; therefore the environment for the origins of life must have permitted membrane formation to occur. Because decanoate, which precipitates in the presence of divalent cations, is often thought of as the precursor to modern membranes, ocean environments are sometimes referred to as unfavorable for membrane formation. The missing logic in this argument is that decanoate is only one among many possible early membrane-forming molecules, and mixtures of amphiphiles, even containing decanoate, are stabilized against divalent cation precipitation. In fact, membrane formation is often enhanced in the presence of salts due to changes in hydrophobicity and interactions between salts and charged amphiphile headgroups.
By varying the composition of single chain amphiphile mixtures, it is possible to find stable membranes in most environmental conditions suggested for the origins of life. Therefore, the ability of a specific amphiphile (e.g., decanoate) to form membranes in an environment should not be used to determine the suitability of that environment for abiogenesis. Both ocean and land environments pose challenges to membrane formation but none that are truly insurmountable. Indeed, membrane formation through the self-assembly of single chain amphiphiles may be the easiest step in the formation of a functional biological cell.
Acknowledgments
I would like to thank Bruce Damer and David Deamer for their work in the implementation of the Astrobiology Science Conference sessions devoted to this topic in April 2017. This work was supported by the 2015–2016 Connecticut State University American Association of University Professors (CSU-AAUP) Research Grant (Grant ID: ARMAUJ).
Conflicts of Interest
The author declares no conflicts of interest.
References
- Knoll, A.H.; Barghoorn, E.S. Archean microfossils showing cell division from the swaziland system of South Africa. Science 1977, 198, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Wacey, D.; Kilburn, M.R.; Saunders, M.; Cliff, J.; Brasier, M.D. Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of Western Australia. Nat. Geosci. 2011, 4, 698–702. [Google Scholar] [CrossRef]
- Special Issue: Hydrothermal Vents or Hydrothermal Fields: Challenging Paradigms. Life 2017. Available online: http://www.mdpi.com/journal/life/special_issues/hydrothermal (accessed on 15 September 2017).
- Holm, N.G. Why are hydrothermal systems proposed as plausible environments for the origin of life? Orig. Life Evol. Biosph. 1992, 22, 5–14. [Google Scholar] [CrossRef]
- Sojo, V.; Herschy, B.; Whicher, A.; Camprubi, E.; Lane, N. The origin of life in alkaline hydrothermal vents. Astrobiology 2016, 16, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Chyba, C.F. The violent environment of the origin of life–progress and uncertainties. Geochim. Cosmochim. Acta 1993, 57, 3351–3358. [Google Scholar] [CrossRef]
- Simoneit, B.R.T. Prebiotic organic synthesis under hydrothermal conditions: An overview. Adv. Sp. Res. 2004, 33, 88–94. [Google Scholar] [CrossRef]
- McCollom, T.M.; Simoneit, B.R.T. Abiotic formation of hydrocarbons and oxygenated compounds during thermal decomposition of iron oxalate. Orig. Life Evol. Biosph. 1999, 29, 167–186. [Google Scholar] [CrossRef] [PubMed]
- McCollom, T.M.; Ritter, G.; Simoneit, B.R.T. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig. Life Evol. Biosph. 1999, 29, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Damer, B.; Deamer, D. Coupled phases and combinatorial selection in fluctuating hydrothermal pools: A scenario to guide experimental approaches to the origin of cellular life. Life 2015, 5, 872–887. [Google Scholar] [CrossRef] [PubMed]
- Damer, B. A field trip to the archaean in search of darwin’s warm little pond. Life 2016, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, S.; Vlassov, A.; Benner, S.; Coombs, A.; Olasagasti, F.; Deamer, D. Lipid-assisted synthesis of RNA-like polymers from mononucleotides. Orig. Life Evol. Biosph. 2008, 38, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, M.; Surman, A.J.; Cooper, G.J.T.; Suárez-Marina, I.; Hosni, Z.; Lee, M.P.; Cronin, L. Formation of oligopeptides in high yield under simple programmable conditions. Nat. Commun. 2015, 6, 8385. [Google Scholar] [CrossRef] [PubMed]
- Monnard, P.A.; Apel, C.L.; Kanavarioti, A.; Deamer, D.W. Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: Implications for a prebiotic aqueous medium. Astrobiology 2004, 2, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Bedau, M.A.; McCaskill, J.S.; Packard, N.H.; Parke, E.C.; Rasmussen, S.R. Introduction to recent developments in living technology. Artif. Life 2013, 19, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Constantinescu, A.; Svaneborg, C. Generating minimal living systems from non-living materials and increasing their evolutionary abilities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150440. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Chen, L.H.; Deamer, D.; Krakauer, D.C.; Packard, N.H.; Stadler, P.F.; Bedau, M.A. Transitions from nonliving to living matter. Science 2004, 303, 963–965. [Google Scholar] [CrossRef] [PubMed]
- Bedau, M.A.; McCaskill, J.S.; Packard, N.H.; Rasmussen, S. Living technology: Exploiting life’s principles in technology. Artif. Life 2010, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Mansy, S.S.; Schrum, J.P.; Krishnamurthy, M.; Tobe, S.; Treco, D.A.; Szostak, J.W. Template-directed synthesis of a genetic polymer in a model protocell. Nature 2008, 454, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Adamala, K.P.; Engelhart, A.E.; Szostak, J.W. Collaboration between primitive cell membranes and soluble catalysts. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.A.; Salehi-Ashtiani, K.; Szostak, J.W. RNA catalysis in model protocell vesicles. J. Am. Chem. Soc. 2005, 127, 13213–13219. [Google Scholar] [CrossRef] [PubMed]
- Cape, J.L.; Monnard, P.A.; Boncella, J.M. Prebiotically relevant mixed fatty acid vesicles support anionic solute encapsulation and photochemically catalyzed trans-membrane charge transport. Chem. Sci. 2011, 2, 661–671. [Google Scholar] [CrossRef]
- Maurer, S.E.; DeClue, M.S.; Albertsen, A.N.; Dorr, M.; Kuiper, D.S.; Ziock, H.; Rasmussen, S.; Boncella, J.M.; Monnard, P.A. Interactions between catalysts and amphiphilic structures and their implications for a protocell model. ChemPhysChem 2011, 12, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Volkov, G.; Gugeshashvili, M.I.; Deamer, D.W. Energy conversion at liquid/liquid interfaces: Artificial photosynthetic systems. Electrochim. Acta 1995, 40, 2849–2868. [Google Scholar] [CrossRef]
- Deamer, D.W. Polycyclic aromatic hydrocarbons: Primitive pigment systems in the prebiotic environment. Adv. Sp. Res. 1992, 12, 183–189. [Google Scholar] [CrossRef]
- Zhu, T.F.; Adamala, K.; Zhang, N.; Szostak, J.W. Photochemically driven redox chemistry induces protocell membrane pearling and division. Proc. Natl. Acad. Sci. USA 2012, 109, 9828–9832. [Google Scholar] [CrossRef] [PubMed]
- Hanczyc, M.M.; Szostak, J.W. Replicating vesicles as models of primitive cell growth and division. Curr. Opin. Chem. Biol. 2004, 8, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.A.; Szostak, J.W. Membrane growth can generate a transmembrane pH gradient in fatty acid vesicles. Proc. Natl. Acad. Sci. USA 2004, 101, 7965–7970. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.A.; Szostak, J.W. A kinetic study of the growth of fatty acid vesicles. Biophys. J. 2004, 87, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.F.; Szostak, J.W. Coupled growth and division of model protocell membranes. J. Am. Chem. Soc. 2009, 131, 5705–5713. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J.M.; Hicks, M. Ufasomes are stable particles surrounded by unsaturated fatty-acid membranes. Nature 1973, 243, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Rabenau, A. The role of hydrothermal synthesis in preparative chemistry. Angew. Chem. Int. Ed. Engl. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Rushdi, A.I.; Deamer, D.W. Abiotic formation of acylglycerols under simulated hydrothermal conditions and self-assembly properties of such lipid products. Adv. Sp. Res. 2007, 40, 1649–1656. [Google Scholar] [CrossRef]
- Dworkin, L.P.; Deamer, D.W.; Sandford, S.A.; Allamandola, L.J. Self-Assembling amphiphilic molecules: Synthesis in simulated interstellar/precometary ices. Proc. Natl. Acad. Sci. USA 2001, 98, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W. Boundary structures are formed by organic-components of the murchison carbonaceous chondrite. Nature 1985, 317, 792–794. [Google Scholar] [CrossRef]
- Hargreaves, W.R.; Deamer, D.W. Liposomes from ionic, single-chain amphiphiles. Biochemistry 1978, 17, 3759–3768. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, W.R.; Mulvihill, S.J.; Deamer, D.W. Synthesis of phospholipids and membranes in prebiotic conditions. Nature 1977, 266, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Dalai, P.; Kaddour, H.; Sahai, N. Incubating life: Prebiotic sources of organics for the origin of life. Elements 2016, 12, 401–406. [Google Scholar] [CrossRef]
- Maurer, S.E.; Nguyen, G. Prebiotic vesicle formation and the necessity of salts. Orig. Life Evol. Biosph. 2016, 46, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Tanford, C. Hydrophobicity of long chain n-Alkyl carboxylic acids, as measured by their distribution between heptane and aqueous solutions. Proc. Natl. Acad. Sci. USA 1973, 70, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Knauth, L.P. Temperature and salinity history of Precambrian ocean: Implications for the course of microbial evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 53–69. [Google Scholar] [CrossRef]
- Theillet, F.X.; Binolfi, A.; Frembgen-Kesner, T.; Hingorani, K.; Sarkar, M.; Kyne, C.; Li, C.; Crowley, P.B.; Gierasch, L.; Pielak, G.J.; et al. Physicochemical properties of cells and their effects on intrinsically disordered proteins (IDPs). Chem. Rev. 2014, 114, 6661–6714. [Google Scholar] [CrossRef] [PubMed]
- Lodish, H.F. Molecular Cell Biology, 6th ed.; W.H. Freeman: New York, NY, USA, 2008. [Google Scholar]
- Huang, X.; Sillanpää, M.; Gjessing, E.T.; Vogt, R.D. Water quality in the Tibetan Plateau: Major ions and trace elements in the headwaters of four major Asian rivers. Sci. Total Environ. 2009, 407, 6242–6254. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Tan, X.; Li, T.; Chen, Y.; Huang, X. Influence of salt and polymer on the critical vesicle concentration in aqueous mixture of zwitterionic/anionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2006, 276, 28–33. [Google Scholar] [CrossRef]
- Monnard, P.A.; Deamer, D.W. Preparation of vesicles from nonphospholipid amphiphiles. Liposomes 2003, 372 Pt B, 133–151. [Google Scholar]
- Tanford, C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes, 2nd ed.; J. Wiley: New York, NY, USA, 1980. [Google Scholar]
- Maurer, S.E.; Deamer, D.W.; Boncella, J.M.; Monnard, P.A. Chemical evolution of amphiphiles: Glycerol monoacyl derivatives stabilize plausible prebiotic membranes. Astrobiology 2009, 9, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, A.N.; Duffy, C.D.; Sutherland, J.D.; Monnard, P.A. Self-Assembly of phosphate amphiphiles in mixtures of prebiotically plausible surfactants. Astrobiology 2014, 14, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Caschera, F.; de la Serna, J.B.; Loffler, P.M.G.; Rasmussen, T.E.; Hanczyc, M.M.; Bagatolli, L.A.; Monnard, P.A. Stable vesicles composed of monocarboxylic or dicarboxylic fatty acids and trimethylammonium amphiphiles. Langmuir 2011, 27, 14078–14090. [Google Scholar] [CrossRef] [PubMed]
- Namani, T.; Deamer, D.W. Stability of model membranes in extreme environments. Orig. Life Evol. Biosph. 2008, 38, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Apel, C.L.; Deamer, D.W.; Mautner, M.N. Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for the encapsulation of biopolymers. Biochim. Biophys. Acta Biomembr. 2002, 1559, 1–9. [Google Scholar] [CrossRef]
- Gotoh, M.; Miki, A.; Nagano, H.; Ribeiro, N.; Elhabiri, M.; Gumienna-Kontecka, E.; Albrecht-Gary, A.; Schmutz, M.; Ourisson, G.; Nakatani, Y. Membrane properties of branched polyprenyl phosphates, postulated as primitive membrane constituents. Chem. Biodivers. 2006, 3, 434–455. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Madanamoothoo, W.; Berlioz-Barbier, A.; Maniti, O.; Girard-Egrot, A.; Buchet, R.; Strazewski, P. Giant vesicles from rehydrated crude mixtures containing unexpected mixtures of amphiphiles formed under plausibly prebiotic conditions. Org. Biomol. Chem. 2017, 15, 4231–4240. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Du, N.; Song, Y.; Song, S.; Hou, W. Vesicles of 2-ketooctanoic acid in water. Soft Matter 2017, 13, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.E.; Monnard, P.A.; Hanczyc, M.M. Vesicle self-assembly of monoalkyl amphiphiles under hydrothermal vent-like conditions: Effects of high ionic strength, extreme pH and high temperature environments. Langmuir 2017. in preparation. [Google Scholar]
- Piedrafita, G.; Montero, F.; Morán, F.; Cárdenas, M.L.; Cornish-Bowden, A. A Simple self-maintaining metabolic system: Robustness, autocatalysis, bistability. PLoS Comput. Biol. 2010, 6, e1000872. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Karson, J.A.; Blackman, D.K.; Früh-Green, G.L.; Butterfield, D.A.; Lilley, M.D.; Olson, E.J.; Schrenk, M.O.; Roe, K.K.; Lebon, G.T.; et al. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30 degrees N. Nature 2001, 412, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Wessicken, M.; Rädler, U.; Berclaz, N.; Conde-Frieboes, K.; Luisi, P.L. Preparation and characterization of vesicles from mono-n-alkyl phosphates and phosphonates. J. Phys. Chem. B 1997, 101, 8. [Google Scholar] [CrossRef]
- Lauerwald, R.; Hartmann, J.; Moosdorf, N.; Kempe, S.; Raymond, P.A. What controls the spatial patterns of the riverine carbonate system? A case study for North America. Chem. Geol. 2013, 337–338, 114–127. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta Rev. Biomembr. 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Mayer, C.; Schreiber, U.; Dávila, M.J. Periodic vesicle formation in tectonic fault zones: An ideal scenario for molecular evolution. Orig. Life Evol. Biosph. 2015, 45, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.; Deamer, D.W.; Kros, A.; Ehrenfreund, P. Polycyclic aromatic hydrocarbons as plausible prebiotic membrane components. Orig. Life Evol. Biosph. 2012, 42, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Oleson, T.A.; Sahai, N. Oxide-Dependent adsorption of a model membrane phospholipid dipalmitoylphosphatidylcholine: Bulk adsorption isotherms. Langmuir 2008, 24, 4865–4873. [Google Scholar] [CrossRef] [PubMed]
- Hanczyc, M.M.; Mansy, S.S.; Szostak, J.W. Mineral surface directed membrane assembly. Orig. Life Evol. Biosph. 2007, 37, 67–82. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).