Abstract
Lung cancer is the leading cause of cancer-related death worldwide, with 1.8 million deaths annually. Early detection is vital for improving patient outcomes; however, survival rates remain low due to late-stage diagnoses. Accumulating data supports the idea that screening methods are useful for improving early diagnosis in high-risk patients. However, several barriers limit the application of lung cancer screening in real-world settings. The widespread diffusion of artificial intelligence (AI), radiomics, and machine learning has dramatically changed the current diagnostic landscape. This review explores the potential of AI and biomarker-driven methods, particularly liquid biopsy, in enhancing early lung cancer detection. We report the findings of major randomized controlled trials, cohort studies, and research on AI algorithms that use multi-modal imaging (e.g., CT and PET scans) and liquid biopsy to identify early molecular alterations. AI algorithms enhance diagnostic accuracy by automating image analysis and reducing inter-reader variability. Biomarker-driven methods identify molecular alterations in patients before imaging signs of cancer are evident. Both AI and liquid biopsy show the potential to improve sensitivity and specificity, enabling the detection of early-stage cancers that traditional methods, like low-dose CT (LDCT) scans, might miss. Integrating AI and biomarker-driven methods offers significant promise for transforming lung cancer screening. These technologies could enable earlier, more accurate detection, ultimately improving survival outcomes. AI-driven lung cancer screening can achieve over 90% sensitivity, compared to 70–80% with traditional methods, and can reduce false positives by up to 30%. AI also boosts specificity to 85–90%, with faster processing times (a few minutes vs. 30–60 min for radiologists). However, challenges remain in standardizing these approaches and integrating them into clinical practice. Ongoing research is essential to fully realize their clinical benefits and enhance timely interventions.
1. Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide, responsible for approximately 1.8 million deaths annually [1]. Despite significant advances in cancer treatment, the survival rate for lung cancer remains low, with a five-year survival rate of around 20% [1,2]. This poor prognosis is primarily attributed to late-stage diagnosis, as more than 50% of lung cancer cases are diagnosed at a stage where curative treatment options are limited [1,2]. Therefore, early detection of lung cancer is crucial for improving survival rates, yet current screening methods are still far from being applicable in a real-world setting [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. Traditionally, lung cancer screening has relied on chest X-rays and low-dose computed tomography (LDCT). Large-scale studies, such as the National Lung Screening Trial (NLST) and the NELSON trial, have demonstrated that annual LDCT screening can reduce lung cancer mortality by 20–24% in high-risk individuals (e.g., those with a significant smoking history). Beyond imaging, researchers are investigating the use of blood-based biomarkers (e.g., circulating tumor DNA) to complement LDCT screening [17,18,19,20]. However, these methods face limitations, including high false positive rates, significant inter-reader variability, and challenges in detecting early-stage malignancies. Other barriers include eligibility criteria, costs, and insurance issues. In response to these challenges, emerging technologies such as artificial intelligence (AI) and biomarker-driven strategies, including liquid biopsy, are showing great promise in enhancing the accuracy, efficiency, and accessibility of lung cancer screening. AI and machine learning are increasingly being used to analyze LDCT images, improving the accuracy of nodule detection and reducing false positives. Recent studies have shown that AI-assisted screening can enhance radiologists’ ability to identify early-stage lung cancers, potentially leading to earlier interventions. This review aims to evaluate the current state of AI and biomarker-based screening methods, examining their potential to improve early detection, reduce unnecessary interventions, and ultimately revolutionize lung cancer screening [20,21,22,23]. Liquid biopsies hold the potential to revolutionize cancer care through the non-invasive early detection of tumors. A key challenge lies in developing robust tests that can analyze high-dimensional data from numerous blood samples across diverse patient groups. Artificial intelligence (AI), with particularly deep generative models, offers promising solutions.
2. Methods
Query Strategy of Reivew of AI and Lung Cancer Screening
This review is registered in the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42025641097). A comprehensive literature search was conducted to identify studies and reviews, published between 1 January 2010 and 31 January 2025, that focused on AI in lung cancer screening and biomarker-driven approaches. The following databases were queried: PubMed, Cochrane, and Web of Science. The search terms included: “AI in lung cancer screening”, “artificial intelligence in CT imaging”, “biomarker-driven screening for lung cancer”, “liquid biopsy in lung cancer detection”, and “artificial intelligence in PET imaging”. Studies were selected based on their relevance to the topic, the quality of study design, and clinical applicability.
Inclusion criteria consisted of studies that focused on AI algorithms for CT and PET scans, as well as those discussing the role of liquid biopsy in early lung cancer detection. Studies were excluded if they focused on animal models, did not provide relevant data on AI or biomarkers in lung cancer screening, or were solely focused on pulmonary nodule detection without any connection to AI models or lung cancer screening studies. Studies that concentrated only on radiological behavior and AI without any clinical impact were also excluded.
Additionally, we eliminated studies that exhibited classical biases in AI application, such as diverse algorithms, non-representative populations, and variations in the evaluation of certain variables (e.g., sex, ethnicity, and comorbidities) when these factors were not similarly accounted for by the AI software (DeepMind’s Gemini 2.0 Flash).
3. Results
The literature search identified 4280 papers. Figure 1 shows the process of evidence acquisition. Overall, 45 studies were retrieved and fully evaluated. Based on the evidence acquired we discussed: (i) the application of AI in lung cancer screening; (ii) the role of AI in lung cancer imaging and screening; and (iii) biomarker-based screening.
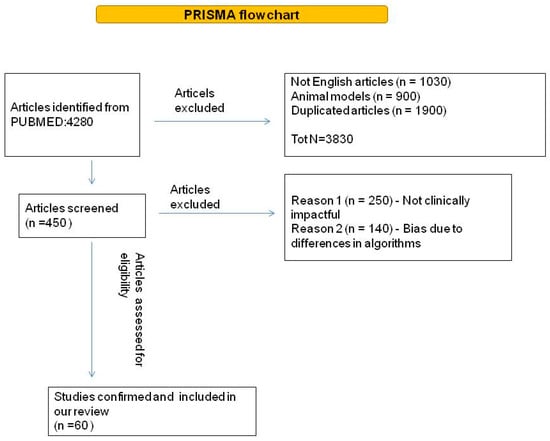
Figure 1.
Diagram of literature query flow.
3.1. Artificial Intelligence in Lung Cancer Screening
AI (including radiomics, machine learning, and deep learning algorithms) has made significant advancements in improving the sensitivity and specificity of lung cancer detection. These algorithms are especially valuable in analyzing large datasets of medical images, such as CT and PET scans, to automate image analysis and reduce inter-reader variability. A key study by Liu et al. showed the importance of adopting AI in lung cancer screening [9]. In this retrospective study, the authors evaluated 120 CT images and compared the radiologist diagnostic approach with deep learning. They demonstrated that deep learning models could detect small pulmonary nodules on low-dose CT scans with an improvement in sensitivity from 80% to 90%. This 10% increase in sensitivity can result in earlier detection of lung cancer, particularly in cases with smaller or less visible lesions [9]. Additionally, AI algorithms are capable of integrating multi-modal imaging data, combining structural information from CT with functional data from PET scans to assess metabolic activity.
Gao Liang [24] created a radiomics model using monochromatic dual-energy CT (DECT) scans to detect solitary pulmonary nodules, achieving an AUC of 0.8772 (95% CI 0.780–0.974). These results emphasize that CT characteristics can differ among solid pulmonary nodules of various sizes, and identifying size-dependent CT traits can help reduce uncertainty and differentiate benign solid nodules from malignant ones. A more refined distinction of solid pulmonary nodules holds significant clinical importance. Shi et al. [25], evaluating data from more than 2500 nodules, developed another AI-based algorithm. The authors aimed to improve the ability to differentiate between benign and malignant mixed-density ground-glass nodules with a predominant solid component (CTR ≥ 50%), and developed an AI-driven radiomics prediction model (using a Lasso regression), obtaining encouraging results [25].
3.2. The Role of Artificial Intelligence in Lung Cancer Imaging and in Lung Cancer Screening
AI’s application in medical imaging has primarily focused on the use of deep learning (DL) for the analysis of CT scans. Deep learning algorithms, such as convolutional neural networks (CNNs), are designed to learn patterns and features within complex data. Studies have shown that CNNs can improve the accuracy of nodule detection and other abnormalities, significantly impacting early diagnosis. Deep learning algorithms are capable of detecting smaller nodules compared to human radiologists, and they are particularly useful in reducing false positives and false negatives. Liu et al. [9] developed a deep learning model for detecting malignant nodules in CT data, achieving higher sensitivity compared to human radiologists [26,27].
Quanyang W et al. [28] showed that with the continuous evolution of AI technologies based on deep learning, particularly the advent of convolutional neural networks (CNNs), AI presents an expanded horizon of applications in lung cancer screening. These applications include lung segmentation, nodule detection, false positive reduction, nodule classification, and prognosis (when we focus on false positives, we always refer to interpretations such as malignancy in a nodule that is not malignant). AI demonstrates significant potential in improving nodule detection sensitivity, reducing false positive rates, and classifying nodules, while also showing value in predicting nodule growth and pathological/genetic typing [26]. AI not only analyzes images but also integrates clinical data to improve risk prediction. AI models can be combined with clinical information, such as smoking history and genetic risk factors, to optimize screening [23,24,25,26,27,28,29]. As a result, AI can identify patients at high risk of developing lung cancer with greater precision compared to traditional methods. AI models need to be trained on large, diverse datasets to ensure they can perform effectively across different clinical contexts and populations. Several clinical studies have evaluated the effectiveness of AI in lung cancer screening for the automated analysis of CT scans, such as in participants of the National Lung Screening Trial (NLST) [3], finding significant improvements in early diagnosis and a reduction in false positives. Additionally, a study by Rajpurkar et al. [30] showed that an AI model trained with millions of CT images achieved sensitivity and specificity comparable to expert radiologists in detecting malignant pulmonary nodules. The authors developed and validated a deep learning algorithm that classified clinically important abnormalities in chest radiographs at a performance level comparable to practicing radiologists [29]. Other studies corroborated these findings [4,11,12,22,30,31,32,33,34,35,36,37,38,39,40,41,42] (Table 1).

Table 1.
Published evidence about AI and lung cancer screening.
3.3. Biomarker-Driven Screening: Liquid Biopsy
Biomarker-driven screening, particularly through liquid biopsy, offers a promising complementary approach to imaging-based screening. Biomarker-driven screening has the aim of reducing the false positive rates of conventional imaging-based screening. Liquid biopsy involves the detection of circulating tumor DNA (ctDNA), microRNAs, and other genetic markers in blood samples to identify cancer at early stages, even before tumors are detectable through imaging. This ability to detect molecular alterations before the emergence of visible lesions could significantly enhance early detection and lead to better patient outcomes.
Liang W et al. [28] demonstrated that ctDNA analysis could detect EGFR mutations in 85% of patients with early-stage lung cancer, even before tumors were visible on CT scans. Similarly, Bordi et al. [30] found that liquid biopsy could identify KRAS mutations in 70% of patients with Stage I lung cancer. These findings suggest that liquid biopsy has the potential to detect cancers at stages when curative interventions are still possible.
Recent studies have also suggested that combining liquid biopsy with LDCT screening could enhance diagnostic accuracy. By integrating genetic testing with imaging, the combined approach could help identify high-risk individuals more effectively, reduce unnecessary follow-up procedures, and allow for earlier, more targeted interventions. However, liquid biopsy remains in the validation phase, and more studies are needed to standardize the assays and assess their clinical utility [4,22,29,30,31,32].
4. Discussion
AI-driven screening is a generalized term encompassing the application of artificial intelligence techniques in screening processes. This broad category could involve a variety of AI methodologies, ranging from expert systems that rely on predefined rules to more sophisticated machine learning (ML) models. However, it lacks the precision necessary to differentiate between various AI technologies and specify the particular underlying algorithms employed, making it less useful for technical clarity in a scientific context. “Machine learning-based screening” specifically refers to the utilization of machine learning algorithms in the screening process. ML methods involve the development of models that learn patterns and make predictions by analyzing large datasets. This approach distinguishes itself from rule-based AI systems by relying on data-driven techniques, where the model iteratively improves its performance through exposure to training data. Supervised, unsupervised, and reinforcement learning are the primary paradigms within machine learning, and these methods can vary significantly in their approach to learning and pattern recognition. Consequently, machine learning-based screening provides a more structured and algorithmically defined framework than general AI-driven approaches, offering higher accuracy and adaptability in predictive tasks.
“Deep learning models”, a subset of machine learning, utilize multi-layered neural networks, often referred to as deep neural networks (DNNs), to model complex relationships within data. These models are particularly effective in handling large-scale, high-dimensional data, such as images, speech, or textual information. Deep learning relies on hierarchical architectures, where each successive layer extracts increasingly abstract features from the input data. This capability makes deep learning models exceptionally powerful for tasks like image classification, object detection, and natural language understanding. However, deep learning models require significantly more computational resources and vast amounts of labeled training data to achieve optimal performance compared to traditional machine learning models. While deep learning represents a more specialized approach within machine learning, its increased computational demands and complexity make it suitable for highly specific and data-intensive applications. Lung cancer screening, particularly using low-dose computed tomography (LDCT), has proven effective in reducing mortality in high-risk populations. The National Lung Screening Trial (NLST) [26] demonstrated that LDCT could reduce lung cancer mortality by 20% in high-risk individuals. Further studies, such as the NELSON trial, have reinforced these findings, with a 26% reduction in mortality in men and a 61% reduction in women [6,9]. Similarly, the ITALUNG trial showed a 39% reduction in mortality for men and 50% for women [32]. These results underscore the effectiveness of LDCT in reducing lung cancer mortality when applied to high-risk populations [6] (see the details in Table 2). However, despite these advancements, several challenges remain. One of the primary issues is the high false positive rate associated with LDCT, which leads to unnecessary biopsies and follow-up imaging. These procedures are not only costly but also contribute to patient anxiety. Furthermore, overdiagnosis remains a concern, particularly in the case of indolent cancers that would not have caused symptoms during the patient’s lifetime [3]. The published evidence supports the efficacy of LDCT as a screening tool for reducing lung cancer mortality in high-risk populations. In his thorough analysis, Field JK et al. [6] showed that the results align with those of landmark trials such as the NLST and the European Randomized Study of Screening for Lung Cancer (NELSON) [9], both of which demonstrated significant reductions in lung cancer mortality with LDCT. However, the effect size varies, with some trials showing larger benefits than others. The observed heterogeneity may be attributed to differences in study design, participant characteristics, and screening protocols.

Table 2.
Summary of key historical studies on lung cancer screening.
Despite its benefits, lung cancer screening with LDCT presents several challenges, including overdiagnosis and false positives. The rate of overdiagnosis remains a significant concern, as some detected cancers may never progress to causing harm. The high rate of false positives leads to unnecessary follow-up tests, biopsies, and patient anxiety [5]. Additionally, the high cost of LDCT and follow-up procedures poses a challenge for large-scale implementation, especially in low- and middle-income countries. Cost-effectiveness analyses suggest that lung cancer screening is only cost-effective in high-risk populations, particularly those aged 55–74 years with a smoking history [7,8,9,10,11,12].
Several studies [4,12,22,32,33,34,35,36,37,38,39] have explored the role of AI in detecting lung cancer, particularly in analyzing CT scans and aiding radiologists in screening and diagnosis. Mehta et al. [40] demonstrated that semi-supervised learning (where AI is trained on partially labeled data) for convolutional neural networks (CNNs) provides a performance comparable to supervised learning, despite using less labeled data. This method enhances AI’s ability to work with more diverse datasets, which is a key advantage [34]. Chamberlin et al. [4] showed that AI’s agreement with expert radiologists in detecting lung cancer on low-dose CT scans was high, with excellent sensitivity (0.929–1) and specificity (0.708–0.960). Chauvin et al. emphasized the role of AI in enhancing the positive predictive value (PPV) during lung cancer screening, thereby reducing false positives and negatives [22]. Cheng et al. [33] compared AI’s performance in detecting lung nodules to radiologists with varying levels of experience. While AI performed similarly to junior radiologists, it was outperformed by more experienced physicians [33]. This suggests that AI can serve as a useful screening tool but still requires expert supervision. Zhang et al. [12] assessed two AI models (CNN and radiomics) and found that while the CNN model had better specificity, radiologists showed higher sensitivity, indicating that AI could match junior radiologists in nodule detection [12]. Studies like those by Armato et al. and Shah et al. have demonstrated that AI can not only detect but also accurately measure tumor size, a crucial step in lung cancer diagnosis [35,36,37]. Yoo et al. found that AI-assisted reading of chest radiographs improved sensitivity for junior radiologists, while senior radiologists saw an improvement in specificity, underscoring AI’s value for both novice and expert clinicians [41]. The AI system tested by Park et al. [36] showed potential in automating Lung-RADS categorization, reducing observer variability. Additionally, Adams et al. demonstrated that AI could enhance Lung-RADS by adding a malignancy risk score (mSI), improving sensitivity and specificity, and reducing diagnostic delays [11,38,39,40,41].
Most AI algorithms, particularly deep learning (DL) models, require large, well-labeled datasets, which are difficult and time-consuming to curate. Strategies like using publicly available databases, generative adversarial networks (GANs), and transfer learning are being explored to overcome this. Challenges remain in dataset quality and consistency, as pathological data can vary in interpretation, and inconsistent terminology is often used to describe nodules. Standardization of labeling practices is needed. Ethical and legal concerns regarding data sharing, privacy, and regulatory compliance are also obstacles, compounded by international data protection laws. Additionally, deep learning models often face overfitting, where performance is good on specific datasets but poor on new data. This can be addressed with larger, more diverse datasets and model validation techniques like cross-validation. The lack of interpretability of AI models remains a concern, but incorporating non-imaging data may help with acceptance. Lastly, rigorous validation is essential to ensure AI algorithms perform well in clinical practice, with careful management of overfitting and underfitting during model training [45].
Liquid biopsies hold the potential to revolutionize cancer care through the non-invasive early detection of tumors. A key challenge lies in developing robust tests that can analyze high-dimensional data from numerous blood samples across diverse patient groups. Artificial intelligence (AI), particularly deep generative models, offers promising solutions. For example, the Orion model can learn generalizable signatures of blood-based biomarkers, such as orphan non-coding RNAs (oncRNAs), by using variational autoencoders. The Orion model uses variational inference to learn a Gaussian distribution from oncRNA data. It surpasses traditional methods in performance and generalizability, achieving high sensitivity and specificity in cancer detection. Orion leverages a two-arm semi-supervised multi-input variational autoencoder to model the expression of oncRNAs and annotated small RNAs, while also incorporating classification and contrastive learning objectives to improve label prediction and remove confounders. This approach enables the model to minimize technical variations and improve the overall accuracy in cancer detection [44,46,47,48,49].
Artificial intelligence (AI) has gained attention for its potential in diagnosing Ground-Glass Opacities (GGOs), with most research focusing on CT images [42,50,51]. However, there is limited research on AI methods for predicting the malignant risk of GGOs using 18F-FDG PET/CT images, particularly in light of the new WHO classification for GGOs [52,53,54]. The 3D nnU-net, an automatic segmentation method based on convolutional neural networks, demonstrates excellent generalization, accuracy, reliability, and efficiency in medical image processing. This study applied the 3D nnU-net with a majority voting method to predict the malignant risk of GGOs using dual-time-point 18F-FDG PET/CT images according to the new WHO classification [55,56,57,58].
The integration of AI into lung cancer screening offers a promising solution to some of these challenges. AI has been shown to reduce inter-reader variability, automate image analysis, and improve the sensitivity and specificity of nodule detection. AI algorithms can also assist in risk stratification, enabling clinicians to identify high-risk patients who may require more intensive monitoring or immediate intervention [11,12,22,33,34,35,36,37,38,39,40,41,42,45]. Biomarker-driven approaches, particularly liquid biopsy, could further enhance screening accuracy by detecting genetic mutations associated with lung cancer at an early stage, before lesions become visible on imaging. Liquid biopsy can complement traditional imaging methods, offering a non-invasive and highly sensitive means of early cancer detection. However, the clinical implementation of liquid biopsy is still in the validation phase, and there are ongoing efforts to standardize testing protocols and establish its utility in routine practice.
5. Conclusions
AI and biomarker-driven approaches represent transformative tools for the future of lung cancer screening. AI can enhance diagnostic accuracy by automating image analysis and integrating multi-modal data, while liquid biopsy provides a promising method for detecting genetic alterations at an early stage, even before visible lesions appear, and for reducing the false positive rate. These advancements hold significant potential to improve early detection, reduce unnecessary interventions, and ultimately enhance patient outcomes. AI has the potential to significantly improve healthcare efficiency, especially in regions with limited access to physicians. AI’s introduction in lung cancer screening could lower costs and improve early detection, leading to better patient outcomes. It is anticipated that AI will complement, rather than replace, radiologists in the near future, enhancing clinical decision-making and potentially expanding screening programs worldwide. AI shows great promise in supporting lung cancer screening, particularly in the categorization of findings and reducing the workload of radiologists. While it is unlikely to replace radiologists in the next two decades, AI will become a vital adjunct in clinical decision-making, potentially transforming lung cancer diagnosis and treatment. However, the widespread adoption of these technologies faces several challenges, including the need for rigorous validation, standardization, and addressing disparities in access to care. Further research and collaboration among clinicians, data scientists, and regulatory bodies are essential to overcoming these challenges. If these obstacles can be addressed, AI and biomarker-driven methods could revolutionize lung cancer screening, reducing mortality and improving survival rates globally.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-prof sectors.
Conflicts of Interest
The authors certify that have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90, Erratum in CA Cancer J. Clin. 2011, 61, 134. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424, Erratum in CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chamberlin, J.; Kocher, M.R.; Waltz, J.; Snoddy, M.; Stringer, N.F.C.; Stephenson, J.; Sahbaee, P.; Sharma, P.; Rapaka, S.; Schoepf, U.J.; et al. Automated detection of lung nodules and coronary artery calcium using artificial intelligence on low-dose CT scans for lung cancer screening: Accuracy and prognostic value. BMC Med. 2021, 19, 55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Lung Screening Trial Research Team. Lung Cancer Incidence and Mortality with Extended Follow-up in the National Lung Screening Trial. J. Thorac. Oncol. 2019, 14, 1732–1742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Field, J.K.; Vulkan, D.; Davies, M.P.A.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Gosney, J.; Green, B.A.; Holemans, J.A.; et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg. Health Eur. 2021, 10, 100179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Croswell, J.M.; Baker, S.G.; Marcus, P.M.; Clapp, J.D.; Kramer, B.S. Cumulative incidence of false-positive test results in lung cancer screening: A randomized trial. Ann. Intern. Med. 2010, 152, 505–512, Erratum in Ann. Intern. Med. 2010, 152, 759. [Google Scholar] [CrossRef] [PubMed]
- Croswell, J.M.; Kramer, B.S.; Kreimer, A.R.; Prorok, P.C.; Xu, J.L.; Baker, S.G.; Fagerstrom, R.; Riley, T.L.; Clapp, J.D.; Berg, C.D.; et al. Cumulative incidence of false-positive results in repeated, multimodal cancer screening. Ann. Fam. Med. 2009, 7, 212–222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, Y.; Liu, B. The effectiveness of deep learning model in differentiating benign and malignant pulmonary nodules on spiral CT. Technol. Health Care 2024, 32, 5129–5140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Jiang, B.; Zhang, L.; Greuter, M.J.W.; de Bock, G.H.; Zhang, H.; Xie, X. Lung Nodule Detectability of Artificial Intelligence-assisted CT Image Reading in Lung Cancer Screening. Curr. Med. Imaging 2022, 18, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wei, Y.; Shi, F.; Ren, J.; Zhou, Q.; Li, W.; Chen, B. The diagnostic and prognostic value of radiomics and deep learning technologies for patients with solid pulmonary nodules in chest CT images. BMC Cancer 2022, 22, 1118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maki, K.G.; Talluri, R.; Toumazis, I.; Shete, S.; Volk, R.J. Impact of U.S. Preventive Services Task Force lung cancer screening update on drivers of disparities in screening eligibility. Cancer Med. 2023, 12, 4647–4654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomonaga, Y.; Ten Haaf, K.; Frauenfelder, T.; Kohler, M.; Kouyos, R.D.; Shilaih, M.; Lorez, M.; de Koning, H.J.; Schwenkglenks, M.; Puhan, M.A. Cost-effectiveness of low-dose CT screening for lung cancer in a European country with high prevalence of smoking-A modelling study. Lung Cancer 2018, 121, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Behr, C.M.; Oude Wolcherink, M.J.; IJzerman, M.J.; Vliegenthart, R.; Koffijberg, H. Population-Based Screening Using Low-Dose Chest Computed Tomography: A Systematic Review of Health Economic Evaluations. Pharmacoeconomics 2023, 41, 395–411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grover, H.; King, W.; Bhattarai, N.; Moloney, E.; Sharp, L.; Fuller, L. Systematic review of the cost-effectiveness of screening for lung cancer with low dose computed tomography. Lung Cancer 2022, 170, 20–33. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Cancer statistics for African Americans, 2019. CA Cancer J. Clin. 2019, 69, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Tammemägi, M.C.; Katki, H.A.; Hocking, W.G.; Church, T.R.; Caporaso, N.; Kvale, P.A.; Chaturvedi, A.K.; Silvestri, G.A.; Riley, T.L.; Commins, J.; et al. Selection criteria for lung-cancer screening. N. Engl. J. Med. 2013, 368, 728–736, Erratum in N. Engl. J. Med. 2013, 369, 394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoo, S.J.; Park, Y.S.; Choi, H.; Kim, D.S.; Goo, J.M.; Yoon, S.H. Prospective evaluation of deep learning image reconstruction for Lung-RADS and automatic nodule volumetry on ultralow-dose chest CT. PLoS ONE 2024, 19, e0297390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buys, D.R.; Rennekamp, R. Cooperative Extension as a Force for Healthy, Rural Communities: Historical Perspectives and Future Directions. Am. J. Public Health 2020, 110, 1300–1303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zgodic, A.; Zahnd, W.E.; Advani, S.; Eberth, J.M. Low-dose CT lung cancer screening uptake: A rural-urban comparison. J. Rural Health 2022, 38, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Chauvie, S.; De Maggi, A.; Baralis, I.; Dalmasso, F.; Berchialla, P.; Priotto, R.; Violino, P.; Mazza, F.; Melloni, G.; Grosso, M.; et al. Artificial intelligence and radiomics enhance the positive predictive value of digital chest tomosynthesis for lung cancer detection within SOS clinical trial. Eur. Radiol. 2020, 30, 4134–4140. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.E.; Reuland, D.S.; Reddy, S.M.; Nagle, M.; Clark, S.D.; Weber, R.P.; Enyioha, C.; Malo, T.L.; Brenner, A.T.; Armstrong, C.; et al. Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 325, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Castello, A.; Morenghi, E.; Tanzi, D.; Cavuto, S.; Lutman, F.; Chiesa, G.; Vanni, E.; Alloisio, M.; Infante, M. Cost-effectiveness of second-line diagnostic investigations in patients included in the DANTE trial: A randomized controlled trial of lung cancer screening with low-dose computed tomography. Nucl. Med. Commun. 2019, 40, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Balbi, M.; Sabia, F.; Ledda, R.E.; Rolli, L.; Milanese, G.; Ruggirello, M.; Valsecchi, C.; Marchianò, A.; Sverzellati, N.; Pastorino, U. Surveillance of subsolid nodules avoids unnecessary resections in lung cancer screening: Long-term results of the prospective BioMILD trial. ERJ Open Res. 2024, 10, 00167–02024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, G.; Yu, W.; Liu, S.Q.; Xie, M.G.; Liu, M. The value of radiomics based on dual-energy CT for differentiating benign from malignant solitary pulmonary nodules. BMC Med. Imaging 2022, 22, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, W.; Hu, Y.; Chang, G.; Qian, H.; Yang, Y.; Song, Y.; Wei, Z.; Gao, L.; Yi, H.; Wu, S.; et al. Development of a clinical prediction model for benign and malignant pulmonary nodules with a CTR ≥ 50% utilizing artificial intelligence-driven radiomics analysis. BMC Med. Imaging 2025, 25, 21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quanyang, W.; Yao, H.; Sicong, W.; Linlin, Q.; Zewei, Z.; Donghui, H.; Hongjia, L.; Shijun, Z. Artificial intelligence in lung cancer screening: Detection, classification, prediction, and prognosis. Cancer Med. 2024, 13, e7140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, W.; Zhao, Y.; Huang, W.; Gao, Y.; Xu, W.; Tao, J.; Yang, M.; Li, L.; Ping, W.; Shen, H.; et al. Non-invasive diagnosis of early-stage lung cancer using high-throughput targeted DNA methylation sequencing of circulating tumor DNA (ctDNA). Theranostics 2019, 9, 2056–2070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajpurkar, P.; Irvin, J.; Ball, R.L.; Zhu, K.; Yang, B.; Mehta, H.; Duan, T.; Ding, D.; Bagul, A.; Langlotz, C.P.; et al. Deep learning for chest radiograph diagnosis: A retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med. 2018, 15, e1002686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bordi, P.; Tiseo, M.; Rofi, E.; Petrini, I.; Restante, G.; Danesi, R.; Del Re, M. Detection of ALK and KRAS Mutations in Circulating Tumor DNA of Patients With Advanced ALK-Positive NSCLC With Disease Progression During Crizotinib Treatment. Clin. Lung Cancer 2017, 18, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Paci, E.; Puliti, D.; Lopes Pegna, A.; Carrozzi, L.; Picozzi, G.; Falaschi, F.; Pistelli, F.; Aquilini, F.; Ocello, C.; Zappa, M.; et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017, 72, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wen, H.; You, H.; Hua, L.; Xiaohua, W.; Qiuting, C.; Jiabao, L. Recognition of Peripheral Lung Cancer and Focal Pneumonia on Chest Computed Tomography Images Based on Convolutional Neural Network. Technol. Cancer Res. Treat. 2022, 21, 15330338221085375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venkadesh, K.V.; Aleef, T.A.; Scholten, E.T.; Saghir, Z.; Silva, M.; Sverzellati, N.; Pastorino, U.; van Ginneken, B.; Prokop, M.; Jacobs, C. Prior CT Improves Deep Learning for Malignancy Risk Estimation of Screening-detected Pulmonary Nodules. Radiology 2023, 308, e223308. [Google Scholar] [CrossRef] [PubMed]
- Armato, S.G., 3rd; Oxnard, G.R.; Kocherginsky, M.; Vogelzang, N.J.; Kindler, H.L.; MacMahon, H. Evaluation of semiautomated measurements of mesothelioma tumor thickness on CT scans. Acad. Radiol. 2005, 12, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, H.; Lee, S.M.; Ahn, Y.; Kim, W.; Jung, K.; Seo, J.B. Application of computer-aided diagnosis for Lung-RADS categorization in CT screening for lung cancer: Effect on inter-reader agreement. Eur. Radiol. 2022, 32, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.K.; McNitt-Gray, M.F.; Rogers, S.R.; Goldin, J.G.; Suh, R.D.; Sayre, J.W.; Petkovska, I.; Kim, H.J.; Aberle, D.R. Computer aided characterization of the solitary pulmonary nodule using volumetric and contrast enhancement features. Acad. Radiol. 2005, 12, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.J.; Madtes, D.K.; Burbridge, B.; Johnston, J.; Goldberg, I.G.; Siegel, E.L.; Babyn, P.; Nair, V.S.; Calhoun, M.E. Clinical Impact and Generalizability of a Computer-Assisted Diagnostic Tool to Risk-Stratify Lung Nodules With CT. J. Am. Coll. Radiol. 2023, 20, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.G.; Kim, H.J.; Lee, E.H.; Hong, W.; Park, J.; Hwang, E.J.; Park, C.M.; Goo, J.M. Value of a deep learning-based algorithm for detecting Lung-RADS category 4 nodules on chest radiographs in a health checkup population: Estimation of the sample size for a randomized controlled trial. Eur. Radiol. 2022, 32, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Jain, A.; Mangalagiri, J.; Menon, S.; Nguyen, P.; Chapman, D.R. Lung Nodule Classification Using Biomarkers, Volumetric Radiomics, and 3D CNNs. J. Digit. Imaging 2021, 34, 647–666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoo, H.; Lee, S.H.; Arru, C.D.; Doda Khera, R.; Singh, R.; Siebert, S.; Kim, D.; Lee, Y.; Park, J.H.; Eom, H.J.; et al. AI-based improvement in lung cancer detection on chest radiographs: Results of a multi-reader study in NLST dataset. Eur. Radiol. 2021, 31, 9664–9674. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Choi, D.; Choi, J.Y.; Hyun, S.H. Performance evaluation of a Deep Learning System for Differential diagnosis of Lung Cancer with Conventional CT and FDG PET/CT using transfer learning and Metadata. Clin. Nucl. Med. 2021, 46, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qi, S.; Wu, Y.; Pan, X.; Yao, Y.; Qian, W.; Guan, Y. Multi-scale segmentation squeeze-and-excitation UNet with conditional random field for segmenting lung tumor from CT images. Comput. Methods Programs Biomed. 2022, 222, 106946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Grant, B.M.M.; Hope, A.J.; Hung, R.J.; Warkentin, M.T.; Lam, A.C.L.; Aggawal, R.; Xu, M.; Shepherd, F.A.; Tsao, M.S.; et al. Using Recurrent Neural Networks to Extract High-Quality Information From Lung Cancer Screening Computerized Tomography Reports for Inter-Radiologist Audit and Feedback Quality Improvement. JCO Clin. Cancer Inform. 2023, 7, e2200153. [Google Scholar] [CrossRef] [PubMed]
- Tandon, Y.K.; Bartholmai, B.J.; Koo, C.W. Putting artificial intelligence (AI) on the spot: Machine learning evaluation of pulmonary nodules. J. Thorac. Dis. 2020, 12, 6954–6965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karimzadeh, M.; Momen-Roknabadi, A.; Cavazos, T.B.; Fang, Y.; Chen, N.C.; Multhaup, M.; Yen, J.; Ku, J.; Wang, J.; Zhao, X.; et al. Deep generative AI models analyzing circulating orphan non-coding RNAs enable detection of early-stage lung cancer. Nat. Commun. 2024, 15, 10090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, Y.; Mu, W.; Shi, Y.; Qi, Q.; Wang, W.; He, Y.; Sun, X.; Yang, B.; Cui, P.; Li, C.; et al. Development and validation of an integrated system for lung cancer screening and post-screening pulmonary nodules management: A proof-of-concept study (ASCEND-LUNG). EClinicalMedicine 2024, 75, 102769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, J.; Wang, B.; Tao, J.; Liu, Q.; Peng, M.; Xiong, S.; Li, J.; Cheng, B.; Li, C.; Jiang, S.; et al. Accurate classification of pulmonary nodules by a combined model of clinical, imaging, and cell-free DNA methylation biomarkers: A model development and external validation study. Lancet Digit. Health 2023, 5, e647–e656. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yu, H.; Feng, H.; Duan, J.; Wang, K.; Tong, B.; Zhang, Y.; Li, W.; Wang, Y.; Liang, C.; et al. Enhancing the differential diagnosis of small pulmonary nodules: A comprehensive model integrating plasma methylation, protein biomarkers, and LDCT imaging features. J. Transl. Med. 2024, 22, 984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwyzer, M.; Martini, K.; Benz, D.C.; Burger, I.; Ferraro, D.; Kudura, K.; Treyer, V.; von Schulthess, G.K.; Kauhmann, P.A.; Huellner, M.W.; et al. Artificial intelligence for detecting small FDG-positive lung nodules in digital PET/CT: Impact of image reconstructions on diagnostic performance. Eur. Radiol. 2020, 30, 2031–2040. [Google Scholar] [CrossRef]
- Apostolopoulos, I.D.; Pintelas, E.G.; Livieris, I.E.; Apostolopoulos, D.J.; Papathanasiou, N.D.; Pintelas, P.E.; Panayiotakis, G.S. Automatic classification of solitary pulmonary nodules in PET/CT imaging employing transfer learning techniques. Med. Biol. Eng. Comput. 2021, 59, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zheng, D.; Chen, H.; Wang, Y.; Chen, C.; Xu, L.; Li, G.; Wang, Y.; He, X.; Li, W. Fusion of CT images and clinical variables based on deep learning for predicting invasiveness risk of stage I lung adenocarcinoma. Med. Phys. 2022, 49, 6384–6394. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yang, Z.; Wang, M.; Zhao, W.; Zhu, Q.; Shi, W.; Yu, H.; Liang, Z.; Chen, L. A computerized tomography-based radiomic model for assessing the invasiveness of lung adenocarcinoma manifesting as ground-glass opacity nodules. Respir. Res. 2022, 23, 96. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, N.; Huang, N.; Liu, X.; Zheng, Y.; Fu, Y.; Li, X.; Wu, H.; Xu, J.; Cheng, J. Determining the invasiveness of ground-glass nodules using a 3D multi-task network. Eur. Radiol. 2021, 31, 7162–7171. [Google Scholar] [CrossRef]
- Yin, P.; Wang, W.; Wang, S.; Liu, T.; Sun, C.; Liu, X.; Chen, L.; Hong, N. The potential for different computed tomography-based machine learning networks to automatically segment and differentiate pelvic and sacral osteosarcoma from Ewing’s sarcoma. Quant. Imaging Med. Surg. 2023, 13, 3174–3184. [Google Scholar] [CrossRef]
- Cheng, D.; Zhuo, Z.; Du, J.; Weng, J.; Zhang, C.; Duan, Y.; Sun, T.; Wu, M.; Guo, M.; Hua, T.; et al. A fully automated Deep-Learning Model for Predicting the Molecular subtypes of posterior Fossa Ependymomas using T2-Weighted images. Clin. Cancer Res. 2024, 30, 150–158. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Du, B.; Li, Y.; Li, X. Predicting malignant risk of ground-glass nodules using convolutional neural networks based on dual-time-point 18F-FDG PET/CT. Cancer Imaging 2025, 25, 17. [Google Scholar] [CrossRef] [PubMed Central]
- Chen, G.; Zhang, J.; Zhuo, D.; Pan, Y.; Pang, C. Identification of pulmonary nodules via CT images with hierarchical fully convolutional networks. Med. Biol. Eng. Comput. 2019, 57, 1567–1580. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).