Abstract
The suppressive effect of bisphosphonates (BPs) on bone metabolism is considered to be a major cause of medication-related osteonecrosis of the jaw (MRONJ). Enamel matrix derivative (EMD) stimulates and activates growth factors, leading to the regeneration of periodontal tissues. In this study, we aimed to explore the potential of EMD in reversing the detrimental effects of BPs on human fetal osteoblasts (hFOBs) and osteosarcoma-derived immature osteoblasts (MG63s) by assessing cell viability, apoptosis, migration, gene expression, and protein synthesis. While the suppressive effect of zoledronate (Zol) on cell viability and migration was observed, the addition of EMD significantly mitigated this effect and enhanced cell viability and migration. Furthermore, an increased apoptosis rate induced by Zol was decreased with the addition of EMD. The decreased gene expression of alkaline phosphatase (ALP), osteocalcin (OC), and the receptor activator of nuclear factors kappa-B ligand (RANKL) caused by BP treatment was reversed by the co-addition of EMD to hFOB cells. This trend was also observed for ALP and bone sialoprotein (BSP) levels in MG63 cells. Furthermore, suppressed protein levels of OC, macrophage colony-stimulating factor (M-CSF), BSP, and type 1 collagen (COL1) were recovered following the addition of EMD. This finding suggests that EMD could mitigate the effects of BPs, resulting in the recovery of cell survival, migration, and gene and protein expression. However, the behavior of the osteoblasts was not fully restored, and further studies are necessary to confirm their effects at the cellular level and to assess their clinical usefulness in vivo for the prevention and treatment of MRONJ.
1. Introduction
Bisphosphonates (BPs) are pyrophosphate-derived synthetic drugs that are commonly used as anti-resorptive agents for patients with osteoporosis, Paget’s disease, osteogenesis imperfecta, and multiple myeloma, as well as for the prevention of skeletal-related events and the treatment of bone metastases associated with malignancies such as breast and prostate cancers [1,2,3,4,5,6]. BPs influence bone metabolism by inhibiting bone resorption mediated by osteoclasts, thereby decreasing bone remodeling [7]. Additionally, these drugs can affect impact various cell types, including osteoblasts, fibroblasts, and endothelial cells [8], leading to reduced cell proliferation and alterations in the gene and protein expression of various cytokines [9].
Medication-related osteonecrosis of the jaw (MRONJ), first recognized in the early 2000s, is now a well-known complication in patients taking anti-resorptive medications. Although the exact accurate pathogenesis of MRONJ is unclear [10,11], impaired bone remodeling caused by the suppression of osteoclasts has been suggested as a primary mechanism [12]. In addition to the anti-osteoclastic effects of BPs [7], their role in suppressing osteoblasts’ proliferation, differentiation, and activity is thought to contribute to the development of MRONJ by inhibiting bone formation [8,13,14,15,16]. The inhibition of alkaline phosphatase (ALP) expression, along with the suppression of bone-related proteins, disrupts osteoblast differentiation, especially in the early stages of osteoblast lineage, such as in osteoprogenitor cells [15]. Several studies have shown that BPs not only inhibit ALP and osteocalcin (OC) expression, but also suppress early osteoblast differentiation by hindering the expression of other extracellular matrix proteins, specifically type 1 collagen (COL1), a marker of early osteoblast differentiation [9,17,18]. Moreover, given that the macrophage colony-stimulating factor (M-CSF) and the receptor activator of nuclear factors κB ligand (RANKL) that are secreted by osteoblasts regulate osteoclastogenesis, the role of osteoblasts cannot be overlooked [19,20].
Several pharmacological strategies are being considered to prevent the development of MRONJ and improve treatment outcomes, including the use of antibiotics, antiseptic mouth rinses, hyperbaric oxygen therapy, teriparatide as a parathyroid hormone analog, and various experimental pharmacological substances [10,21,22,23]. However, these strategies have yet to be established as effective measures for the prevention and treatment of MRONJ.
Enamel matrix derivative (EMD) is composed of enamel proteins such as amelogenin, enamelin, ameloblastin, amelotin, and apin, along with various proteinases extracted from porcine fetal teeth [24], and it can promote the periodontal regeneration of alveolar bone, the periodontal ligament, and cementum [25]. Several studies have demonstrated that EMD stimulates bone formation by enhancing osteoblastic activity, local growth factor expression, protein secretion, and mineral deposition [26,27,28,29]. Therefore, we posit that the stimulatory effect of EMDs on osteoblasts counteracts the reduction in osteoblast function and differentiation caused by BPs. However, studies investigating the effects of EMD on osteoblasts under the influence of BPs and the potential of EMD in treating and preventing MRONJ remain scarce.
In this study, we aimed to investigate the effect of EMD on osteoblasts under the influence of BPs in vitro. Our evaluation focused on several key parameters, including cell viability, apoptosis, migration, and gene and protein expression. Through this comprehensive analysis, we aimed to elucidate the potential of EMD in mitigating the adverse effects of BPs on osteoblast function, thereby providing valuable insights into therapeutic strategies for the prevention and treatment of MRONJ in patients undergoing BP therapy.
2. Materials and Methods
2.1. Cell Cultures and Treatments
Human fetal osteoblast (hFOB) and osteosarcoma-derived immature osteoblast (MG63) cell lines were obtained from the American Type Culture Collection (Rockville, MD, USA). The hFOB cells were cultured in a 1:1 mixture of Ham’s F12 medium and Dulbecco’s modified Eagle’s medium (DMEM/F12; Gibco, Grand Island, NY, USA) without phenol red. The culture medium was supplemented with 2.5 mM L-glutamine, 0.3 mg/mL G418, penicillin (100 U/mL), streptomycin (100 µg/mL), and 10% fetal bovine serum (FBS; Gibco). MG63 cells were cultured in DMEM supplemented with penicillin (100 U/mL), streptomycin (100 µg/mL), and 10% FBS. Both cell lines were maintained as monolayers in plastic culture plates at 33 °C for hFOB and 37 °C for MG63 in a humidified atmosphere containing 5% CO2.
For the experiments, zoledronic acid/zoledronate (Zol) as a BP and EMD in a liquid formulation (Enamel matrix proteins in acetic acid at a concentration of 30 mg/mL; Straumann AG, Basel, Switzerland) were prepared and added to the wells in the culture plates with working concentrations of 25 μM and 30 μg/mL, respectively. The Zol concentration was determined based on the preliminary experiments conducted in this study (Figure 1). Since Zol decreased cell viability and demonstrated toxicity in a dose-dependent manner, an inhibitory concentration of 50% (EC50) as compared to the untreated control cells was used for analysis; thus, a Zol concentration of 25 μM was used for further experiments.
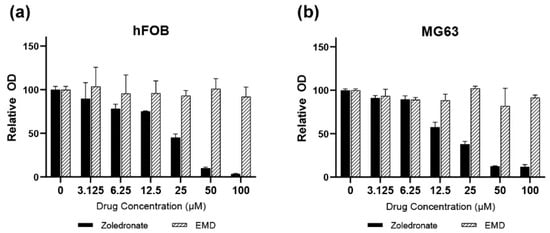
Figure 1.
(a) hFOB (b) MG63 cells. Assessment of hFOB and MG63 cell viability over 72 h using various concentrations of zoledronate and Emdogain (30 μg/mL). EMD, Emdogain.
After the cells were seeded onto appropriate plates and incubated for 24 h, they were supplemented with Zol and/or EMD based on the group assignment. The exposure period varied depending on the analytical method used. In the group treated with EMD alone, the cells were not exposed to Zol.
Four experimental groups were established:
- Group I (control), cells without Zol and EMD;
- Group II (Zol), cells treated with Zol only;
- Group III (Zol + EMD), cells treated with Zol and EMD;
- Group IV (EMD), cells treated with EMD only.
hFOB and MG63 cells were evaluated for viability, apoptosis, migration, and gene and protein expression. All experiments were performed in triplicate to ensure reproducibility and accuracy.
2.2. Cell Viability
Cell viability was assessed using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT; Sigma-Aldrich, St. Louis, MO, USA), which is a colorimetric method based on the metabolic activity of cells. Cells were seeded in 96-well plates at a density of 2 × 105 cells/cm2 and incubated for 24 h. Subsequently, Zol (25 μM) and/or EMD (30 μg/mL) were added to the wells based on the defined experimental groups. After 72 h of incubation, a stock solution of 5 mg/mL MTT dye was prepared in Dulbecco’s phosphate-buffered saline. Subsequently, 20 μL of the MTT dye was added to each well, followed by a 4 h incubation period. After removing the supernatant, 100 μL dimethyl sulfoxide (DMSO; Gibco) was added to solubilize the formed formazan crystals. The plate was then incubated for an additional 15 min. The resulting color was analyzed by measuring the absorbance at 570 nm (A570) using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA). Cell viability was quantified as a value relative to the control.
2.3. Cell Apoptosis Analysis
After 72 h of incubation with Zol and/or EMD, apoptosis was measured by staining the cells with annexin V fluorescein isothiocyanate (FITC; Sigma-Aldrich) and propidium iodide (PI; Sigma-Aldrich). The cells were suspended in binding buffer at a density of 1 × 106 cells/mL and stained with 5 μL of annexin V FITC and 5 μL PI. The cell suspension was then incubated in the dark at room temperature for 15 min. The stained cells were analyzed using a FACS CONTOIITM flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Different cell subpopulations were distinguished based on the following criteria:
- Q1: necrotic cells (annexin V-FITC-negative/PI-positive)
- Q2: late apoptotic cells (annexin V-FITC-positive/PI-positive)
- Q3: viable cells (annexin V-FITC-negative/PI-negative)
- Q4: early apoptotic cells (annexin V-FITC-positive/PI-negative)
The apoptosis rate was determined by the sum of the percentages of cells in Q2 and Q4.
2.4. Cell Migration
2.4.1. Scratch Wound Healing Assay
hFOB cells were initially seeded in 6-well plates at a density of 3 × 105 per well in DMEM/F12 supplemented with 10% FBS. After 12 h of seeding, the cells were exposed to Zol (25 μM) and/or EMD (30 μg/mL) for 48 h. A scratch was then made in the cell monolayer; the closing of the scratch wound was observed at 24 and 48 h and the size of the gap was calculated using ImageJ software (version 1.54g).
2.4.2. Boyden Chamber Assay
The migration assay was conducted in an AP48-modified Boyden chamber (NeuroProbe, Cabin John, MD, USA). Briefly, the migration chamber consisted of an upper and lower compartment with a porous membrane in between them. The upper compartment was free of chemoattractants, whereas the lower compartment was filled with 0.1% bovine serum albumin (Sigma-Aldrich) as a chemoattractant. Cells were seeded in DMEM/F12 supplemented with 10% FBS at a density of 3 × 105 per well into the upper compartment of the migration assay setup. They were then exposed to Zol (25 μM) and/or EMD (30 μg/mL) and allowed to migrate through a porous membrane to the lower chamber.
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
qRT-PCR was used to analyze the expression of genes encoding osteogenic and osteoclastogenesis-stimulating markers. Cells were seeded in 60 cm2 culture flasks and incubated with Zol (25 μM) and/or EMD (30 μg/mL) for 72 h. Total RNA was extracted using the TRI reagent (Molecular Research Center, Cincinnati, OH, USA) and quantified using a Nanodrop spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA). Primer sequences for genes encoding alkaline phosphatase (ALP), osteocalcin (OC), type 1 collagen (COL1), bone sialoprotein (BSP), macrophage colony-stimulating factor (M-CSF), receptor activator of nuclear factors κB ligand (RANKL), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are listed in Table 1.

Table 1.
PCR primers designed for genes encoding alkaline phosphatase (ALP), osteocalcin (OC), receptor activator of nuclear factor kappa-B ligand (RANKL), macrophage colony stimulating factor (M-CSF), bone sialoprotein (BSP), type 1 collagen (COL1).
For cDNA synthesis, reverse transcription was performed with TOPscriptTM RT DryMIX (Enxymonics, Daejeon, Republic of Korea). PowerUp SYBR Green Master Mix (ThermoFisher Scientific) was used for PCR reactions. The 2−ΔΔCT method was used to determine RNA expression levels [30], and the expression levels were normalized against GAPDH mRNA levels.
2.6. Western Blotting
hFOB cells were incubated for 72 h with Zol and/or EMD, then harvested and lysed in cold lysis buffer (Sigma) for 30 min. The lysates were centrifuged at 4 °C, and the supernatant was collected. Protein content was quantified using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). Thirty micrograms of protein from each sample was separated using 6–20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane via wet electroblotting. The membranes were blocked with a solution containing 5% dried skimmed milk powder in Tris-buffered saline with Tween® 20 buffer (Bio-Rad Laboratories). They were then incubated overnight at 4 °C with the primary antibodies targeting ALP, BSP, M-CSF, RANKL (Cat. No. ab83259, ab52128, ab9693, and ab9957, respectively; Abcam, Cambridge, UK), OCN, COL1A2, and β-actin (Cat. No. sc-365797, sc-166865, and sc-47778, respectively; Santa Cruz Biotechnology, Dallas, TX, USA). The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (Bio-Rad Laboratories). The immunoreactive proteins were determined using an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech, Amersham, United Kingdom).
2.7. Statistical Analysis
One-way analysis of variance (ANOVA), followed by the Tukey’s post-hoc test, was used for normally distributed data. For non-normally distributed data, the Kruskal–Wallis test and Mann–Whitney U test with Bonferroni corrections were applied. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS software (version 26.0; IBM Corp., Armonk, NY, USA).
3. Results
3.1. Cell Viability
A dose-dependent suppressive effect of Zol on cell viability was observed in both hFOB and MG63 cells after 72 h (Figure 1). Cell viability decreased to <50% when the drug concentration reached 25 μM. In hFOB cells treated with Zol, cell viability significantly decreased to 44% compared to the control (p < 0.001; Figure 2). However, the addition of EMD significantly mitigated this effect by increasing cell viability (p = 0.011), although it remained significantly lower than that of the control (p = 0.023). A similar trend was observed in MG63 cells, in which Zol treatment led to a significant decrease in cell viability. However, although the addition of EMD improved cell viability compared to that of the Zol-treated group, this improvement was not statistically significant (p = 0.091). In both cell types, supplementation with EMD reversed the negative effects of Zol on cell viability.
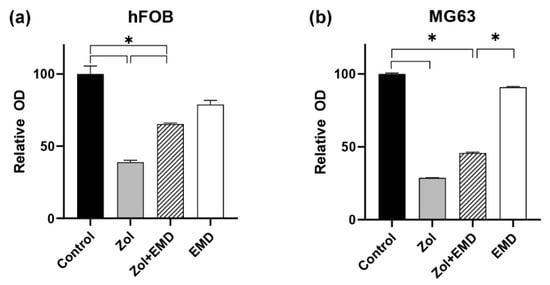
Figure 2.
Assessment of cell viability according to the combination of zoledronate and Emdogain. (a) hFOB (b) MG63 cells. Asterisks (*) indicate statistical significance compared to the control, and the error bars represent standard deviations. Zol, zoledronate; EMD, Emdogain.
3.2. Cell Apoptosis
Treatment with Zol markedly increased the apoptosis rate of hFOB cells to 93.2% from 6.5% in the control group. However, in the group treated with both Zol and EMD, the apoptosis rate decreased to 64.7%. In MG63 cells, the Zol-treated group exhibited an increased apoptosis rate of 67.3% compared to 5.3% in the control group. Similar to what was observed in hFOB cells, the apoptosis rate of MG63 cells treated with both Zol and EMD was reduced to 18.1% (Figure 3).
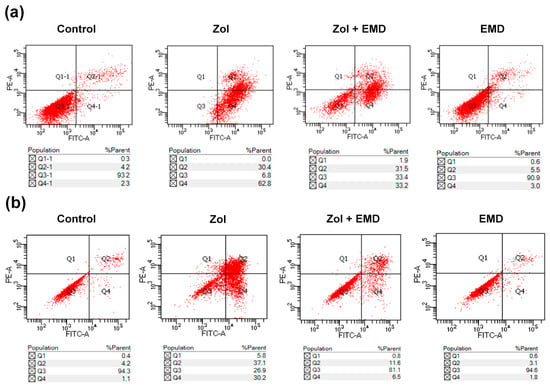
Figure 3.
Subpopulation analysis of stained cells with Annexin V-FITC/PI. (a) hFOB (b) MG63 cells. Necrotic, late apoptotic, viable, and early apoptotic cells appeared in the top left quadrant (Q1), top right quadrant (Q2), bottom left quadrant (Q3) and bottom right quadrant (Q4), respectively. The apoptotic rate is determined as the percentage of cells in Q2 + Q4.
3.3. Cell Migration
The wound scratch assay results for hFOB cells showed that the wound healing process was notably slowed over time in the Zol-treated group. However, in the group treated with both Zol and EMD, the effect of Zol was not profound, and the width of the scratched wound was similar to that of the control (Figure 4).
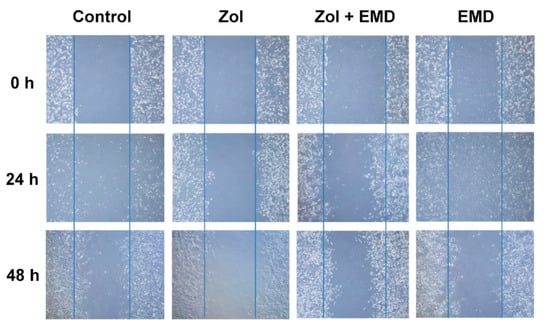
Figure 4.
Wound scratch assay of hFOB cells treated with zoledronate and Emdogain over 24 and 48 h. Zol, zoledronate; EMD, Emdogain.
The fold change in cell migration was measured using the Boyden chamber assay and normalized to that of the control group (set to 1; Figure 5). In hFOB cells, cell migration was significantly decreased in the Zol-treated group compared to that of the control group (p < 0.001). However, in the group treated with both Zol and EMD, cell migration significantly increased (p = 0.005) and became statistically similar to that of the control. Similarly, in MG63 cells, cell migration was significantly decreased by Zol treatment alone (p < 0.001). Conversely, the addition of EMD significantly enhanced cell migration, which was approximately double that of the Zol-treated group (p = 0.007).
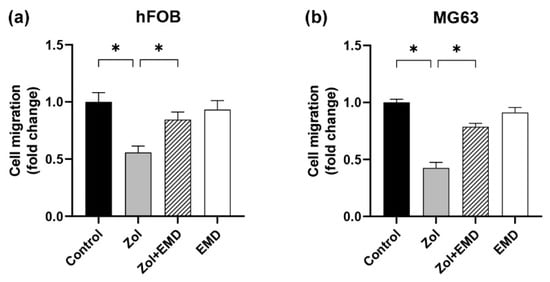
Figure 5.
Cell migration assessed using the Boyden chamber assay for (a) hFOB and (b) MG63 cells. Asterisks (*) indicate statistical significance compared to the control. The error bars represent standard deviations. Zol, zoledronate; EMD, Emdogain.
3.4. Gene Expression
Figure 6 and Figure 7 show the qRT-PCR analysis results for gene expression levels in hFOB and MG63 cells. In hFOB cells, Zol treatment alone significantly reduced the expression of ALP; however, ALP expression levels were significantly upregulated with the co-addition of EMD compared with that of the Zol-treated group (p = 0.047). Zol treatment did not reduce M-CSF, BSP, and COL1 expression levels, whereas the co-addition of EMD significantly increased their expression. In MG63 cells, treatment with Zol resulted in decreased expression of ALP, M-CSF, and BSP, whereas co-treatment with EMD and Zol led to a significant increase in the expression of ALP and BSP. Additionally, while the expression of OC, RANKL, and COL1 was significantly increased compared to that of the control, these markers also showed a significant increase when treated with Zol alone.
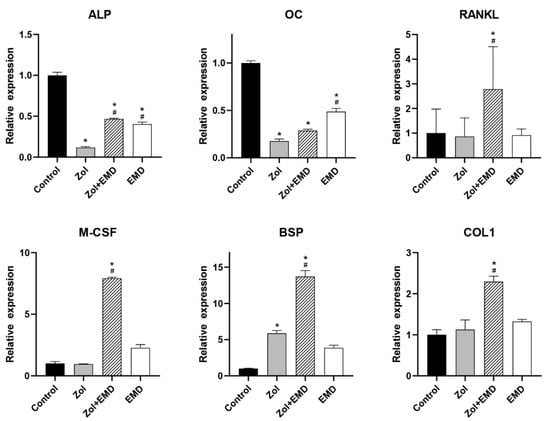
Figure 6.
qRT-PCR analysis results for hFOB cells. Gene expression levels were normalized to those of the control. Asterisks (*) indicate statistical significance compared to the control group, while hash signs (#) denote significance compared to the Zol group. The error bars indicate the standard deviation. ALP, alkaline phosphatase; OC, osteocalcin; RANKL, receptor activator of nuclear factor kappa-B ligand; M-CSF, macrophage colony-stimulating factor; BSP, bone sialoprotein; COL1, type 1 collagen; Zol, zoledronate; EMD, Emdogain.
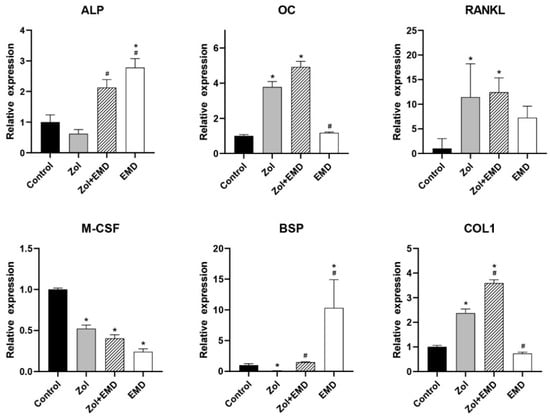
Figure 7.
qRT-PCR analysis results for MG63 cells. Gene expression levels were normalized to those of the control. Asterisks (*) indicate statistical significance compared to the control group, while hash signs (#) denote significance compared to the Zol group. The error bars indicate the standard deviation. ALP, alkaline phosphatase; OC, osteocalcin; RANKL, receptor activator of nuclear factor kappa-B ligand; M-CSF, macrophage colony-stimulating factor; BSP, bone sialoprotein; COL1, type 1 collagen; Zol, zoledronate; EMD, Emdogain.
3.5. Protein Expression
Compared to that of the control, the expression levels of ALP, OC, M-CSF, and COL1 were decreased in the Zol-treated group, with significant decreases observed for ALP, OC, and COL1 (Figure 8). Although the addition of EMD to Zol treatment resulted in an increased expression of all proteins, only the results for OC, RANKL, M-CSF, BSP, and COL1 were statistically significant compared to those of the Zol-only group. Decreases in RANKL and BSP levels were not notable after Zol treatment; however, their levels increased when EMD and Zol were administered together, as compared to those of the control group.
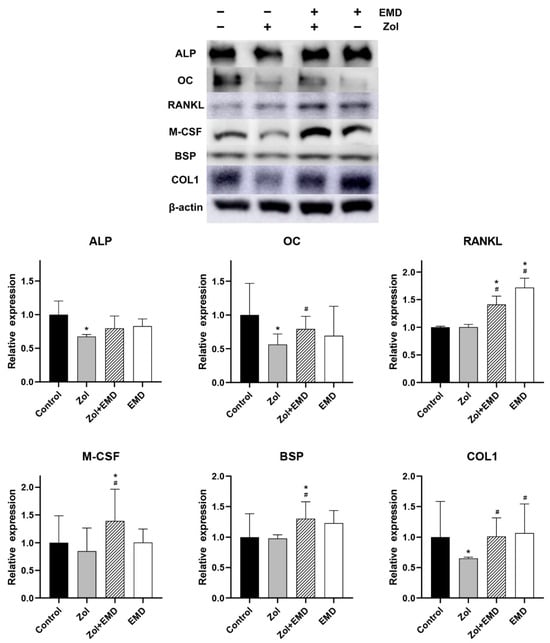
Figure 8.
Western blot analysis results of protein expression in hFOB cells. Levels were normalized to those of the control. Asterisks (*) indicate statistical significance compared to the control group, while hash signs (#) denote significance compared to the Zol group. The error bars indicate the standard deviation. ALP, alkaline phosphatase; OC, osteocalcin; RANKL, receptor activator of nuclear factor kappa-B ligand; M-CSF, macrophage colony-stimulating factor; BSP, bone sialoprotein; COL1, type 1 collagen; Zol, zoledronate; EMD, Emdogain.
4. Discussion
Although the exact pathophysiology of MRONJ remains unclear, it is now considered a multifactorial disease [11]. One of the contributing factors is the suppressive effect of BPs on various bone cells [31,32]. The most common type of BPs are nitrogen-containing BPs (N-BPs), which inhibit farnesyl diphosphate synthase in the mevalonate pathway (MVP) [33]. Several enzymes within the MVP are potential targets of N-BPs and inhibiting them leads to the loss of prenylation of small GTP-binding proteins, including Ras, Rho, Rab, Arf, and Ran [34,35,36]. Combined with their high affinity for mineral tissue [37,38], N-BPs exhibit a long-lasting detrimental effect on the bone environment [39]. Suppressive effects of BPs are not limited to osteoclast-mediated bone resorption. They also reduce bone formation by osteoblasts, thereby leading to impaired bone remodeling. This mechanism may potentially contribute to MRONJ [31,40].
Numerous studies have investigated the suppressive effects of BPs on osteoblasts, particularly on osteogenesis [8,13,14,15,16]. These studies indicate that BPs inhibit osteoblast viability and proliferation, induce apoptosis, and downregulate the expression of proteins involved in osteoblast differentiation, leading to decreased mineralization [8,13,14,15,16]. Our findings align with those of previous studies [8,9,13,14,15,16,17,18], demonstrating that Zol suppressed cell viability, induced apoptosis, inhibits cell migration, and downregulated the gene and protein expression associated with cell differentiation and bone mineralization.
EMD refers to the purified extract of the naturally developing enamel matrix proteins that are present during the secretory stage of dental crown development [25]. The application of EMD stimulates local growth factor expression, extracellular matrix deposition, mineral deposition, and wound healing, similar to the early processes of tooth development and alveolar bone formation [41,42]. Therefore, EMD is considered to have regenerative potential, supporting bone formation as an osteo-promotive agent [27]. EMD alone is not osteogenic, meaning it does not produce ectopic bone formation; however, it was shown to have an osteo-promotive effect on bone regeneration, especially when combined with bone graft materials [43]. Furthermore, EMD induces the proliferation of microvascular endothelial cells and promotes angiogenesis, which is crucial for bone tissue wound healing [44].
Although the exact cellular mechanisms by which EMD influences osteoblast behavior remain unclear, several studies have indicated that EMD can stimulate the secretion and activation of growth factors such as TGF-β and BMP [27,45,46,47]. TGF-β plays a critical role in bone remodeling, including matrix protein synthesis, and directly affects osteoblasts through processes such as proliferation or differentiation, depending on their osteoblastic cell phenotypes or differentiation stages [48]. Schwartz et al. showed that the effect of EMD on osteoblasts varies depending on the maturation stage of the osteoblastic lineage [49]. Other studies have demonstrated stage-specific effects of EMD on osteoblast differentiation, suggesting that EMD may be particularly effective in driving osteoblast differentiation in less mature or more undifferentiated cells [50,51].
This study demonstrated an elevated apoptosis rate in hFOB and MG63 cells following treatment with Zol, which was reversed by the addition of EMD. Tumor necrosis factor alpha (TNF-α), an inflammatory cytokine, mediates apoptosis in response to infection or injury [52], and a study demonstrated that EMD could reduce TNF-α-induced apoptosis similarly to the effects seen with TGF-β [53]. Therefore, EMD may stimulate proliferation and prevent apoptosis of osteoblasts under the influence of BP, thereby enhancing bone remodeling.
Our results suggest that EMD has a stimulatory effect on cell migration, which mitigates the negative effects of BPs. Although one study indicated that EMD has a minimal effect on cell migration [54], other studies suggested that EMD promoted the migration of human umbilical vein endothelial cells in both the wound-healing and Boyden chamber assays [44,55]. The exact mechanism underlying this increased migration remains unclear; however, it has been hypothesized that EMD accelerates cell migration through chemotaxis [55,56].
Several experimental studies have also demonstrated that EMD enhances the expression of genes associated with osteoblast differentiation and mineralization [49,57,58,59,60,61,62]. EMD influences osteoblast differentiation across various cell models, including MG-63, MC3T3-E1, Kusa/A 1, and 2T9 cells [49,57,58,59,60,61], and it promotes osteogenesis by upregulating bone-associated genes such as ALP, OCN, and BSP, thereby stimulating mineral nodule formation [62]. In our study, the decreased gene expression of ALP due to BPs was reversed by the co-addition of EMD in both of the cell lines examined. This trend was also observed for OC and RANKL expression in the hFOB cells. Although the suppressive effects on M-CSF, BSP, and COL1 genes were not profound, the addition of EMD further increased their expression. However, there were discrepancies in the responses of several genes across different cell lines. Several factors can contribute to these variations, including differences in cell line characteristics, the stage of differentiation, and heterogeneity within each cell line. Different cell lines exhibit unique behaviors and characteristics owing to genetic variations, mutations, or adaptations that occur during isolation and culture [63]. Consequently, their responses to external stimuli, such as drug treatments, can vary significantly. There was also a trend indicating that BP treatment suppressed protein expressions, and the addition of EMD to the Zol treatment facilitated the recovery of the expression levels of several measured proteins. However, the gene expression levels were not entirely consistent. Considering that BPs inhibit the prenylation of proteins [34,35,36], it is possible that gene expression does not always correlate with protein expression during Zol treatment.
EMD is widely used in periodontal therapy and has demonstrated effectiveness in tissue regeneration [64,65,66]. Building on previously proven effects, this study suggests that EMD mitigates the effects of Zol, resulting in the recovery of cell survival, migration, and gene and protein expression. However, the behavior of the osteoblasts was not fully restored, and further studies are necessary to confirm their effects at the cellular level. Moreover, the exact mechanism of action of EMD remains unknown, and results may vary in in vivo conditions. Different EMD concentrations may lead to better results, suggesting that optimizing the dosage could enhance its therapeutic effectiveness. Nevertheless, EMD does not require general administration, and no negative effects on bone physiology have been reported. Therefore, if dentoalveolar surgery such as tooth extraction, periodontal, or implant surgery is planned for patients with a history of BP therapy or those currently undergoing such therapy, the concomitant use of EMD during the surgical procedure may reduce the risk of MRONJ development or implant failure. Additionally, it can be applied in the surgical treatment of MRONJ with the expectation of reducing the recurrence of osteonecrosis. Pentoxifylline or teriparatide have been reported as preventive and adjuvant treatment strategies for MRONJ through pharmacological approaches [67,68]. However, these treatments require systemic administration, whereas EMD can be applied locally to adjacent teeth and bony surfaces during surgery. EMD is already commercially available, indicating its potential for broader clinical applications. However, further preclinical studies and clinical trials are essential to confirm the efficacy and safety of EMD and to develop an optimal strategy for its application.
Author Contributions
Conceptualization, J.J.; Methodology, J.J. and J.-Y.O.; Validation, J.J., J.-Y.O. and Y.-D.K.; Formal Analysis, M.K. and M.C.; Investigation, M.K. and M.C.; Writing—Original Draft Preparation, M.K. and J.J.; Writing—Review and Editing, J.J. and J.-Y.O.; Visualization, M.K. and J.J.; Supervision, J.J., J.-Y.O. and Y.-D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The EMD-liquid was provided by Straumann AG (Basel, Switzerland).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kawahara, M.; Kuroshima, S.; Sawase, T. Clinical considerations for medication-related osteonecrosis of the jaw: A comprehensive literature review. Int. J. Implant. Dent. 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.P.; Ward, L.C.; Stewart, G.O.; Will, R.K.; Criddle, R.A.; Prince, R.L.; Stuckey, B.G.; Dhaliwal, S.S.; Bhagat, C.I.; Retallack, R.W.; et al. A randomized clinical trial comparing oral alendronate and intravenous pamidronate for the treatment of Paget’s disease of bone. Bone 2004, 34, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R. Zoledronic acid to prevent skeletal complications in cancer: Corroborating the evidence. Cancer Treat. Rev. 2005, 31 (Suppl. 3), 19–25. [Google Scholar] [CrossRef]
- Coleman, R. Metastasis prevention with bone-targeted agents: A complex interaction between the microenvironment and tumour biology. J. Bone Min. Metab. 2023, 41, 290–300. [Google Scholar] [CrossRef]
- Mollica, V.; Rizzo, A.; Rosellini, M.; Marchetti, A.; Ricci, A.D.; Cimadamore, A.; Scarpelli, M.; Bonucci, C.; Andrini, E.; Errani, C.; et al. Bone Targeting Agents in Patients with Metastatic Prostate Cancer: State of the Art. Cancers 2021, 13, 546. [Google Scholar] [CrossRef]
- Fleisch, H. The role of bisphosphonates in breast cancer: Development of bisphosphonates. Breast Cancer Res. 2001, 4, 30. [Google Scholar] [CrossRef]
- Walter, C.; Klein, M.; Pabst, A.; Al-Nawas, B.; Duschner, H.; Ziebart, T. Influence of bisphosphonates on endothelial cells, fibroblasts, and osteogenic cells. Clin. Oral Investig. 2010, 14, 35–41. [Google Scholar] [CrossRef]
- Koch, F.P.; Yekta, S.S.; Merkel, C.; Ziebart, T.; Smeets, R. The impact of bisphosphonates on the osteoblast proliferation and Collagen gene expression in vitro. Head Face Med. 2010, 6, 12. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral. and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral. and Maxillofacial Surgeons’ Position. Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Pozzi, S.; Vallet, S.; Mukherjee, S.; Cirstea, D.; Vaghela, N.; Santo, L.; Rosen, E.; Ikeda, H.; Okawa, Y.; Kiziltepe, T. High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties. Clin. Cancer Res. 2009, 15, 5829–5839. [Google Scholar] [CrossRef]
- Basso, F.G.; Silveira Turrioni, A.P.; Hebling, J.; de Souza Costa, C.A. Zoledronic acid inhibits human osteoblast activities. Gerontology 2013, 59, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.J.; Niehoff, P.; Kimmig, B.; Wiltfang, J.; Açil, Y. Expression profile and synthesis of different collagen types I, II, III, and V of human gingival fibroblasts, osteoblasts, and SaOS-2 cells after bisphosphonate treatment. Clin. Oral Investig. 2010, 14, 51–58. [Google Scholar] [CrossRef]
- Patntirapong, S.; Singhatanadgit, W.; Chanruangvanit, C.; Lavanrattanakul, K.; Satravaha, Y. Zoledronic acid suppresses mineralization through direct cytotoxicity and osteoblast differentiation inhibition. J. Oral Pathol. Med. 2012, 41, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; Rojas, J.; Greig, I.R.; van’t Hof, R.J.; Ralston, S.H. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif. Tissue Int. 2008, 82, 191–201. [Google Scholar] [CrossRef]
- Giannasi, C.; Niada, S.; Farronato, D.; Lombardi, G.; Manfredi, B.; Farronato, G.; Brini, A.T. Nitrogen Containing Bisphosphonates Impair the Release of Bone Homeostasis Mediators and Matrix Production by Human Primary Pre-Osteoblasts. Int. J. Med. Sci. 2019, 16, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.B.; Herlofson, B.B.; Landin, M.A.; Reseland, J.E. Alendronate alters osteoblast activities. Acta Odontol. Scand. 2016, 74, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Komai, M.; Itoh, T.; Imano, M.; Sakamoto, K.; Shimaoka, H.; Takeda, T.; Ogawa, N.; Mashimo, K.; Fujiwara, D.; et al. Nitrogen-containing bisphosphonates inhibit RANKL- and M-CSF-induced osteoclast formation through the inhibition of ERK1/2 and Akt activation. J. Biomed. Sci. 2014, 21, 10. [Google Scholar] [CrossRef]
- Ibanez, L.; Nacher-Juan, J.; Terencio, M.C.; Ferrandiz, M.L.; Alcaraz, M.J. Osteostatin Inhibits M-CSF+RANKL-Induced Human Osteoclast Differentiation by Modulating NFATc1. Int. J. Mol. Sci. 2022, 23, 8551. [Google Scholar] [CrossRef]
- Jung, J.; Shim, G.-J.; Kim, M.; Yoon, Y.; Kim, J.-E.; Jue, S.-S.; Al-Nawas, B.; Kwon, Y.-D. Effect and timing of parathyroid hormone analog administration for preventing medication-related osteonecrosis of the jaws in a murine model. J. Cranio-Maxillofac. Surg. 2021, 49, 719–725. [Google Scholar]
- Ziebart, T.; Koch, F.; Klein, M.; Guth, J.; Adler, J.; Pabst, A.; Al-Nawas, B.; Walter, C. Geranylgeraniol–a new potential therapeutic approach to bisphosphonate associated osteonecrosis of the jaw. Oral Oncol. 2011, 47, 195–201. [Google Scholar] [CrossRef]
- Jung, J.; Park, J.S.; Chun, J.; Al-Nawas, B.; Ziebart, T.; Kwon, Y.D. Geranylgeraniol Application in Human Osteoblasts and Osteoclasts for Reversal of the Effect of Bisphosphonates. Life 2023, 13, 1353. [Google Scholar] [CrossRef]
- Stout, B.M.; Alent, B.J.; Pedalino, P.; Holbrook, R.; Gluhak-Heinrich, J.; Cui, Y.; Harris, M.A.; Gemperli, A.C.; Cochran, D.L.; Deas, D.E.; et al. Enamel matrix derivative: Protein components and osteoinductive properties. J. Periodontol. 2014, 85, e9–e17. [Google Scholar] [CrossRef]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35, 87–105. [Google Scholar] [CrossRef]
- Miron, R.J.; Chandad, F.; Buser, D.; Sculean, A.; Cochran, D.L.; Zhang, Y. Effect of Enamel Matrix Derivative Liquid on Osteoblast and Periodontal Ligament Cell Proliferation and Differentiation. J. Periodontol. 2016, 87, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, Y.; Xia, Q.; Meng, M.; Ye, Z.; Tang, Z.; Feng, H.; Chen, X.; Chen, H.; Zeng, X.; et al. Enamel matrix derivative (EMD) enhances the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs). Bioengineered 2021, 12, 7033–7045. [Google Scholar] [CrossRef]
- Song, Z.C.; Li, S.; Dong, J.C.; Sun, M.J.; Zhang, X.L.; Shu, R. Enamel matrix proteins regulate hypoxia-induced cellular biobehavior and osteogenic differentiation in human periodontal ligament cells. Biotech. Histochem. 2017, 92, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Park, J.S.; Righesso, L.; Pabst, A.M.; Al-Nawas, B.; Kwon, Y.D.; Walter, C. Effects of an oral bisphosphonate and three intravenous bisphosphonates on several cell types in vitro. Clin. Oral Investig. 2018, 22, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Misso, G.; Porru, M.; Stoppacciaro, A.; Castellano, M.; De Cicco, F.; Leonetti, C.; Santini, D.; Caraglia, M. Evaluation of the in vitro and in vivo antiangiogenic effects of denosumab and zoledronic acid. Cancer Biol. Ther. 2012, 13, 1491–1500. [Google Scholar] [CrossRef]
- Rodan, G.A.; Reszka, A.A. Bisphosphonate mechanism of action. Curr. Mol. Med. 2002, 2, 571–577. [Google Scholar] [CrossRef]
- Yang, Z. Small GTPases: Versatile signaling switches in plants. Plant Cell 2002, 14, S375–S388. [Google Scholar] [CrossRef]
- Zafar, S.; Coates, D.; Cullinan, M.; Drummond, B.; Milne, T.; Seymour, G. Effects of zoledronic acid and geranylgeraniol on the cellular behaviour and gene expression of primary human alveolar osteoblasts. Clin. Oral Investig. 2016, 20, 2023–2035. [Google Scholar] [CrossRef] [PubMed]
- Dunford, J.E.; Rogers, M.J.; Ebetino, F.H.; Phipps, R.J.; Coxon, F.P. Inhibition of protein prenylation by bisphosphonates causes sustained activation of Rac, Cdc42, and Rho GTPases. J. Bone Miner. Res. 2006, 21, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.; Xia, Z.; Barnett, B.; Triffitt, J.; Phipps, R.; Dunford, J.; Locklin, R.; Ebetino, F.; Russell, R. Differences between bisphosphonates in binding affinities for hydroxyapatite. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 92, 149–155. [Google Scholar] [CrossRef]
- Otto, S.; Pautke, C.; Opelz, C.; Westphal, I.; Drosse, I.; Schwager, J.; Bauss, F.; Ehrenfeld, M.; Schieker, M. Osteonecrosis of the jaw: Effect of bisphosphonate type, local concentration, and acidic milieu on the pathomechanism. J. Oral Maxillofac. Surg. 2010, 68, 2837–2845. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Z.; Wang, L.; Jin, X.; Shen, Y.; Nan, C.; Liu, H. Minimally effective concentration of zoledronic acid to suppress osteoclasts in vitro. Exp. Ther. Med. 2018, 15, 5330–5336. [Google Scholar] [PubMed]
- Walter, C.; Pabst, A.; Ziebart, T.; Klein, M.; Al-Nawas, B. Bisphosphonates affect migration ability and cell viability of HUVEC, fibroblasts and osteoblasts in vitro. Oral Dis. 2011, 17, 194–199. [Google Scholar] [CrossRef]
- Sculean, A.; Windisch, P.; Keglevich, T.; Fabi, B.; Lundgren, E.; Lyngstadaas, P. Presence of an enamel matrix protein derivative on human teeth following periodontal surgery. Clin. Oral Investig. 2002, 6, 183–187. [Google Scholar] [CrossRef]
- Lyngstadaas, S.P.; Lundberg, E.; Ekdahl, H.; Andersson, C.; Gestrelius, S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J. Clin. Periodontol. 2001, 28, 181–188. [Google Scholar] [CrossRef]
- Miron, R.J.; Wei, L.; Bosshardt, D.D.; Buser, D.; Sculean, A.; Zhang, Y. Effects of enamel matrix proteins in combination with a bovine-derived natural bone mineral for the repair of bone defects. Clin. Oral Investig. 2014, 18, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Chen, C.L.; Lin, M.T. Enamel matrix derivative exhibits angiogenic effect in vitro and in a murine model. J. Clin. Periodontol. 2003, 30, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nagano, T.; Yamakoshi, Y.; Gomi, K.; Arai, T.; Fukae, M.; Katagiri, T.; Oida, S. Enamel matrix derivative gel stimulates signal transduction of BMP and TGF-β. J. Dent. Res. 2005, 84, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Kulakauskiene, R.; Aukstakalnis, R.; Sadzeviciene, R. Enamel matrix derivate induces periodontal regeneration by activating growth factors: A review. Stomatologija 2020, 22, 49–53. [Google Scholar] [PubMed]
- Wyganowska-Swiatkowska, M.; Urbaniak, P.; Nohawica, M.M.; Kotwicka, M.; Jankun, J. Enamel matrix proteins exhibit growth factor activity: A review of evidence at the cellular and molecular levels. Exp. Ther. Med. 2015, 9, 2025–2033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonewald, L.; Mundy, G. Role of transforming growth factor-beta in bone remodeling. Clin. Orthop. Relat. Res. 1990, 250, 261–276. [Google Scholar] [CrossRef]
- Schwartz, Z.; Carnes, D.L., Jr.; Pulliam, R.; Lohmann, C.H.; Sylvia, V.L.; Liu, Y.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Porcine fetal enamel matrix derivative stimulates proliferation but not differentiation of pre-osteoblastic 2T9 cells, inhibits proliferation and stimulates differentiation of osteoblast-like MG63 cells, and increases proliferation and differentiation of normal human osteoblast NHOst cells. J. Periodontol. 2000, 71, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Caluseru, O.M.; Guillemette, V.; Zhang, Y.; Gemperli, A.C.; Chandad, F.; Sculean, A. Influence of enamel matrix derivative on cells at different maturation stages of differentiation. PLoS ONE 2013, 8, e71008. [Google Scholar]
- Miron, R.J.; Saulacic, N.; Buser, D.; Iizuka, T.; Sculean, A. Osteoblast proliferation and differentiation on a barrier membrane in combination with BMP2 and TGFβ1. Clin. Oral Investig. 2013, 17, 981–988. [Google Scholar] [CrossRef]
- Zeldich, E.; Koren, R.; Dard, M.; Nemcovsky, C.; Weinreb, M. Enamel matrix derivative protects human gingival fibroblasts from TNF-induced apoptosis by inhibiting caspase activation. J. Cell. Physiol. 2007, 213, 750–758. [Google Scholar] [CrossRef]
- He, J.; King, Y.; Jiang, J.; Safavi, K.E.; Spångberg, L.S.; Zhu, Q. Enamel matrix derivative inhibits TNF-α–induced apoptosis in osteoblastic MC3T3-E1 cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 761–767. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Zhang, Y.; Caballé-Serrano, J.; Shirakata, Y.; Bosshardt, D.D.; Buser, D.; Sculean, A. Osteogain improves osteoblast adhesion, proliferation and differentiation on a bovine-derived natural bone mineral. Clin. Oral Implant. Res. 2017, 28, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Bertl, K.; An, N.; Bruckmann, C.; Dard, M.; Andrukhov, O.; Matejka, M.; Rausch-Fan, X. Effects of enamel matrix derivative on proliferation/viability, migration, and expression of angiogenic factor and adhesion molecules in endothelial cells in vitro. J. Periodontol. 2009, 80, 1622–1630. [Google Scholar] [CrossRef]
- Karima, M.M.; Van Dyke, T.E. Enamel matrix derivative promotes superoxide production and chemotaxis but reduces matrix metalloproteinase-8 expression by polymorphonuclear leukocytes. J. Periodontol. 2012, 83, 780–786. [Google Scholar] [CrossRef]
- Hagewald, S.; Pischon, N.; Jawor, P.; Bernimoulin, J.P.; Zimmermann, B. Effects of enamel matrix derivative on proliferation and differentiation of primary osteoblasts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 243–249. [Google Scholar] [CrossRef]
- He, J.; Jiang, J.; Safavi, K.E.; Spangberg, L.S.; Zhu, Q. Emdogain promotes osteoblast proliferation and differentiation and stimulates osteoprotegerin expression. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, P.; Bernimoulin, J.P.; Trackman, P.; Hagewald, S. Stimulation of osteoblasts with Emdogain increases the expression of specific mineralization markers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 304–308. [Google Scholar] [CrossRef]
- Yoneda, S. The effects of enamel matrix derivative (EMD) on osteoblastic cells. Kokubyo Gakkai Zasshi 2002, 69, 207–214. [Google Scholar] [CrossRef]
- Miron, R.J.; Hedbom, E.; Ruggiero, S.; Bosshardt, D.D.; Zhang, Y.; Mauth, C.; Gemperli, A.C.; Iizuka, T.; Buser, D.; Sculean, A. Premature osteoblast clustering by enamel matrix proteins induces osteoblast differentiation through up-regulation of connexin 43 and N-cadherin. PLoS ONE 2011, 6, e23375. [Google Scholar] [CrossRef]
- Lossdörfer, S.; Sun, M.; Götz, W.; Dard, M.; Jäger, A. Enamel matrix derivative promotes human periodontal ligament cell differentiation and osteoprotegerin production in vitro. J. Dent. Res. 2007, 86, 980–985. [Google Scholar] [CrossRef]
- Gillet, J.P.; Varma, S.; Gottesman, M.M. The clinical relevance of cancer cell lines. J. Natl. Cancer Inst. 2013, 105, 452–458. [Google Scholar] [CrossRef]
- Aimetti, M.; Stasikelyte, M.; Mariani, G.M.; Cricenti, L.; Baima, G.; Romano, F. The flapless approach with and without enamel matrix derivatives for the treatment of intrabony defects: A randomized controlled clinical trial. J. Clin. Periodontol. 2024, 51, 1112–1121. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Tamagnone, L.; Roffredo, A.; Costanzo, L. The Use of Enamel Matrix Derivative to Modulate Wound Healing of Peri-implant Soft Tissues. Int. J. Periodontics Restor. Dent. 2024, 44, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Koronna, I.; Schacher, B.; Dahmer, I.; Nickles, K.; Sonnenschein, S.K.; Kim, T.S.; Eickholz, P.; Petsos, H. Long-term stability of infrabony defects treated with enamel matrix derivative alone: A retrospective two-centre cohort study. J. Clin. Periodontol. 2023, 50, 996–1009. [Google Scholar] [CrossRef]
- Sim, I.W.; Borromeo, G.L.; Tsao, C.; Hardiman, R.; Hofman, M.S.; Papatziamos Hjelle, C.; Siddique, M.; Cook, G.J.R.; Seymour, J.F.; Ebeling, P.R. Teriparatide Promotes Bone Healing in Medication-Related Osteonecrosis of the Jaw: A Placebo-Controlled, Randomized Trial. J. Clin. Oncol. 2020, 38, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Colapinto, G.; Goker, F.; Nocini, R.; Albanese, M.; Nocini, P.F.; Sembronio, S.; Argenta, F.; Robiony, M.; Del Fabbro, M. Outcomes of a Pharmacological Protocol with Pentoxifylline and Tocopherol for the Management of Medication-Related Osteonecrosis of the Jaws (MRONJ): A Randomized Study on 202 Osteoporosis Patients. J. Clin. Med. 2023, 12, 4662. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).