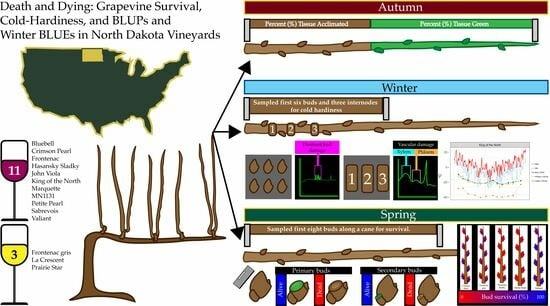
Death and Dying: Grapevine Survival, Cold Hardiness, and BLUPs and Winter BLUEs in North Dakota Vineyards
Abstract
1. Introduction
2. Materials and Methods
2.1. Planting Information
2.1.1. Absaraka, ND
2.1.2. Buffalo, ND
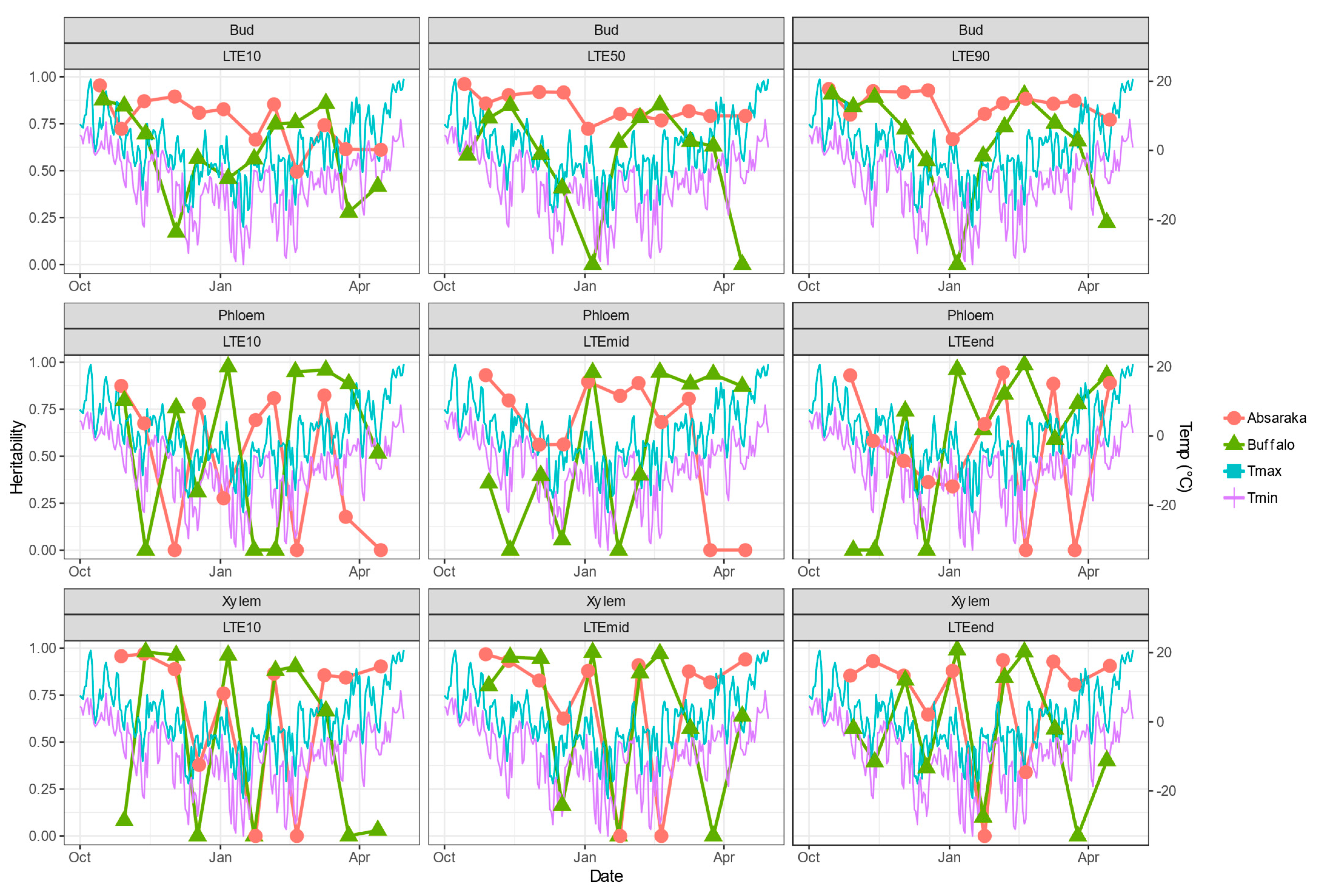
2.1.3. Environmental Conditions
2.2. Periderm Development and Cane Diameter
2.3. Differential Thermal Analysis
2.4. Dormant Bud Death
2.5. Statistical Analysis
3. Results
3.1. Periderm Development and Cane Diameter
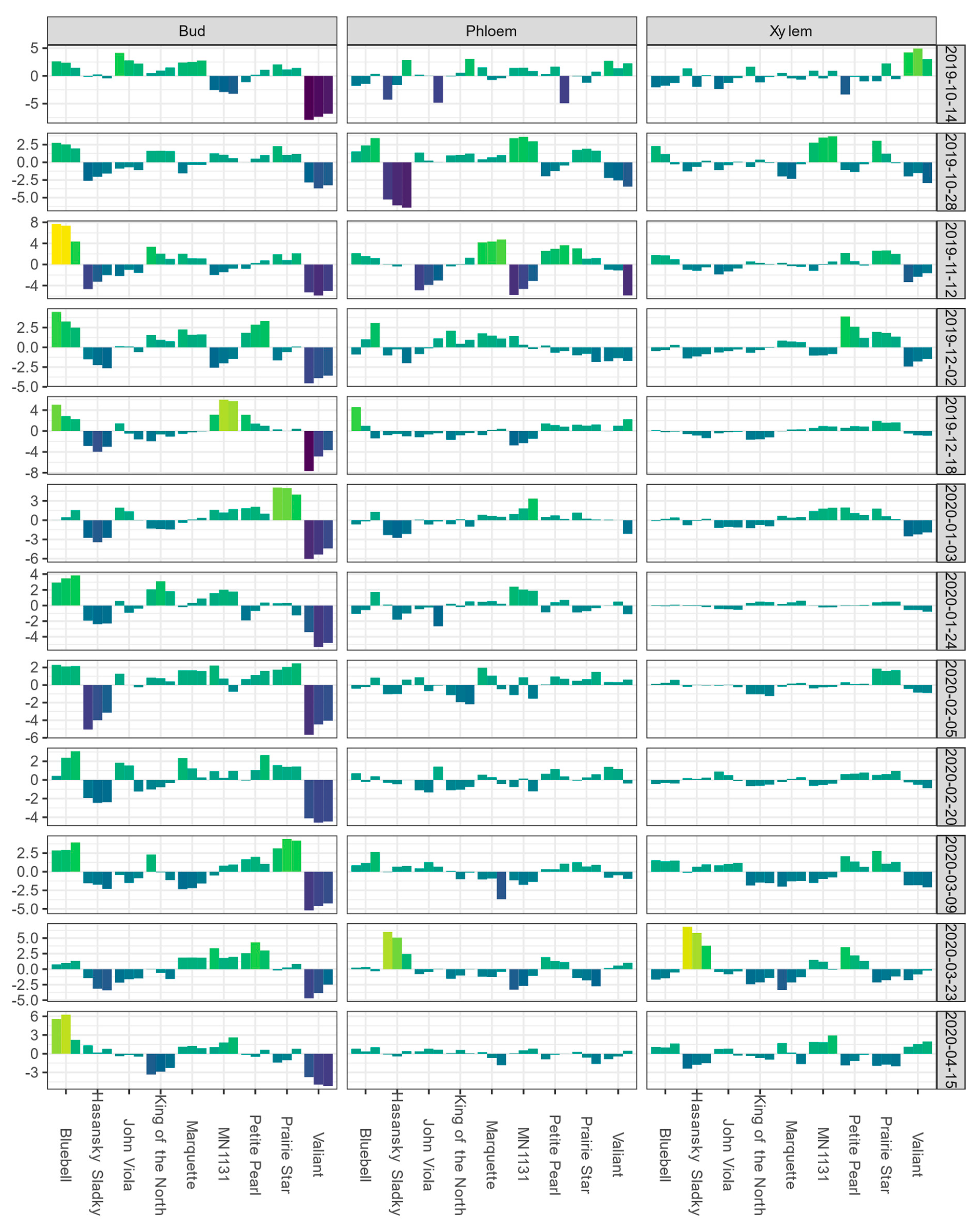
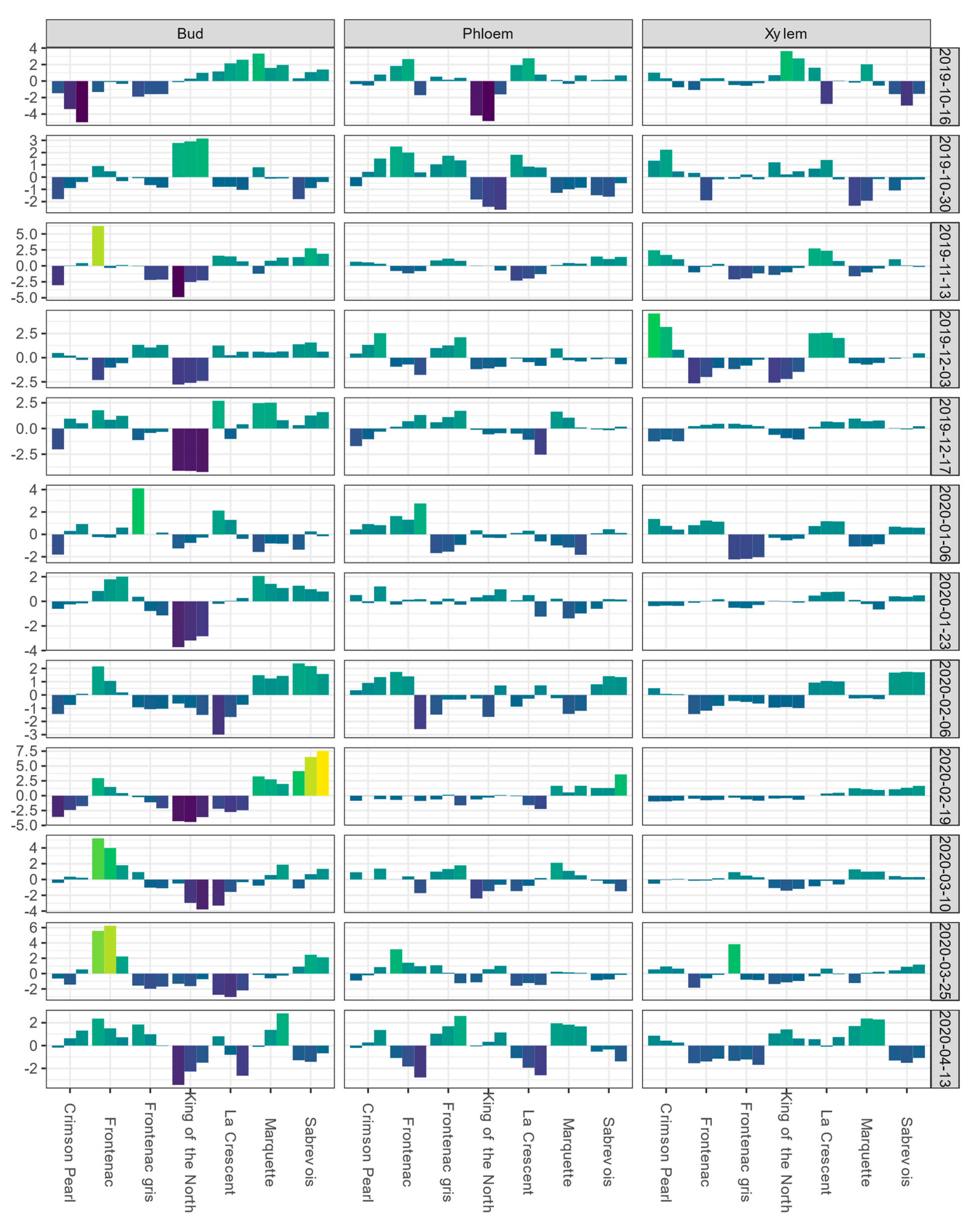
3.2. Winter BLUEs and Heritability of BLUPs for Low-Temperature Exotherms
3.3. Phenotypic and Genetic Correlations among Low-Temperature Exotherm Traits
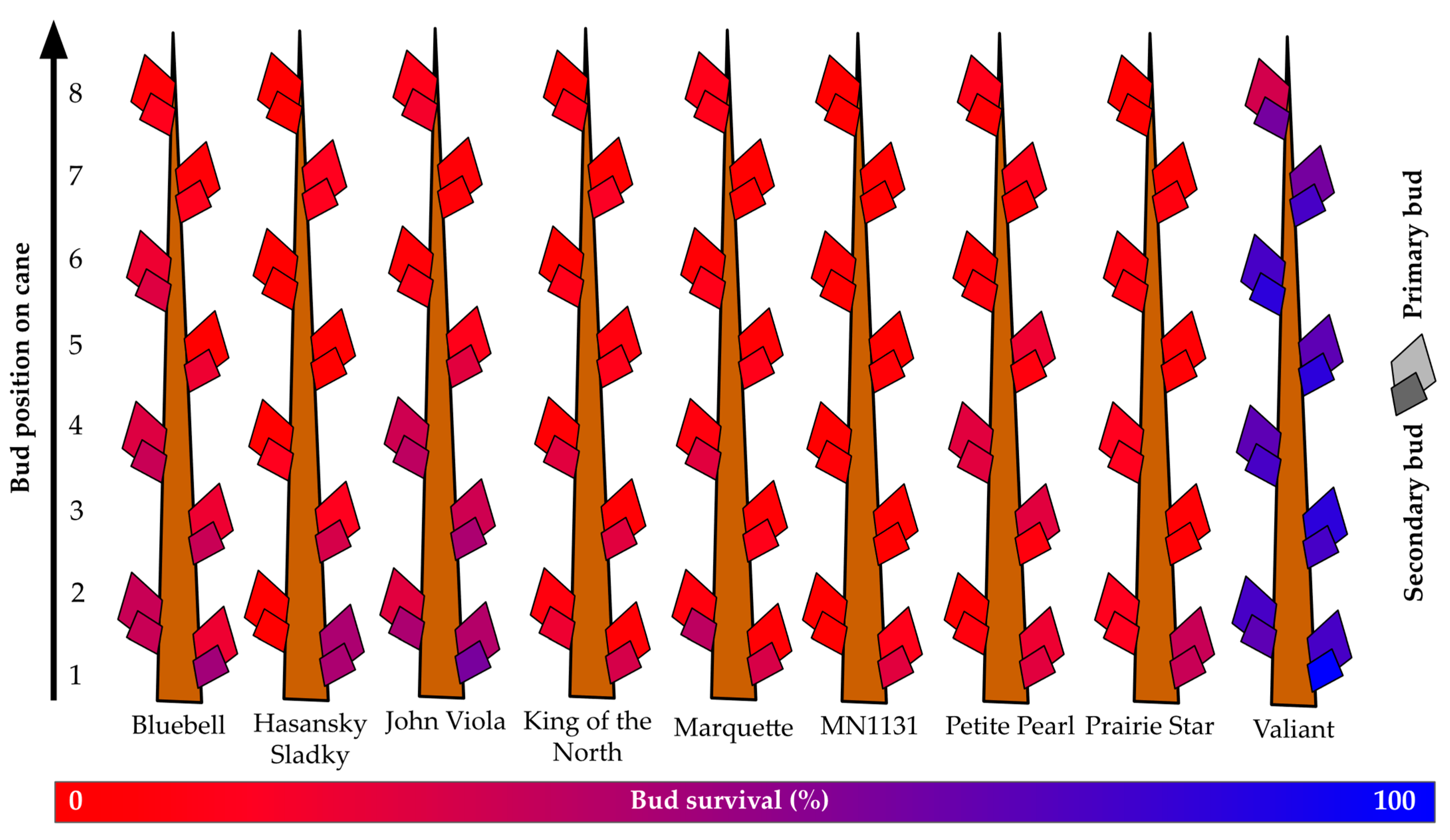
3.4. Dormant Bud Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fennell, A. Freezing Tolerance and Injury in Grapevines. J. Crop Improv. 2004, 10, 201–235. [Google Scholar] [CrossRef]
- Zabadal, T.J.; Dami, I.E.; Goffinet, M.C.; Martinson, T.E.; Chien, M.L. Winter Injury to Grapevines and Methods of Protection. Mich. State Univ. Ext. 2007, E-2930, 106. [Google Scholar]
- Svyantek, A.; Köse, B.; Stenger, J.; Auwarter, C.; Hatterman-Valenti, H. Cold-Hardy Grape Cultivar Winter Injury and Trunk Re-Establishment Following Severe Weather Events in North Dakota. Horticulturae 2020, 6, 75. [Google Scholar] [CrossRef]
- This, P.; Lacombe, T.; Thomas, M.R. Historical Origins and Genetic Diversity of Wine Grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.G. The Grapevine, Viticulture, and Winemaking: A Brief Introduction. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–29. ISBN 978-3-319-57706-7. [Google Scholar]
- Hou, L.; Zhang, G.; Zhao, F.; Zhu, D.; Fan, X.; Zhang, Z.; Liu, X. VvBAP1 Is Involved in Cold Tolerance in Vitis vinifera L. Front. Plant Sci. 2018, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Pierquet, P.; Stushnoff, C. Relationship of Low Temperature Exotherms to Cold Injury in Vitis Riparia Michx. Am. J. Enol. Vitic. 1980, 31, 1–6. [Google Scholar] [CrossRef]
- Pierquet, P.; Stushnoff, C.; Burke, M.J. Low Temperature Exotherms in Stem and Bud Tissues of Vitis Riparia Michx.1. J. Am. Soc. Hortic. Sci. 1977, 102, 54–55. [Google Scholar] [CrossRef]
- Howell, G.S. Grapevine Cold Hardiness: Mechanisms of Cold Acclimation, Mid-Winter Hardiness Maintenance, and Spring Deacclimation. In Proceedings of the ASEV 50th Anniversary Annual Meeting, Seattle, WA, USA, 19–23 June 2000; American Society for Enology and Viticulture, ASEV: Seattle, WA, USA, 2000; pp. 35–48. [Google Scholar]
- Dami, I.E.; Ennahli, S.; Zhang, Y. Assessment of Winter Injury in Grape Cultivars and Pruning Strategies Following a Freezing Stress Event. Am. J. Enol. Vitic. 2012, 63, 106–111. [Google Scholar] [CrossRef]
- Reynolds, A.G. Grapevine Breeding Programs for the Wine Industry; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-1-78242-080-4. [Google Scholar]
- Yilmaz, T.; Alahakoon, D.; Fennell, A. Freezing Tolerance and Chilling Fulfillment Differences in Cold Climate Grape Cultivars. Horticulturae 2021, 7, 4. [Google Scholar] [CrossRef]
- Stenger, J.E.; Hatterman-Valenti, H.M. Complex Plant Process Trait Evaluation Through Decomposition of Higher-Order Interaction: A Case Study in Acclimation Responses of Cold-Climate Hybrid Grapevine Through Bilinear and Multiway Methods. J. Am. Soc. Hortic. Sci. 2022, 147, 161–173. [Google Scholar] [CrossRef]
- Stenger, J.; Hatterman-Valenti, H. Contrasting Responses to Environmental Conditions by Three Cold-Climate Winegrape Cultivars Grown in the United States Northern Plains Region. Acta Hortic. 2017, 1188, 173–180. [Google Scholar] [CrossRef]
- Karimi, R. Potassium-Induced Freezing Tolerance Is Associated with Endogenous Abscisic Acid, Polyamines and Soluble Sugars Changes in Grapevine. Sci. Hortic. 2017, 215, 184–194. [Google Scholar] [CrossRef]
- Sarikhani, H.; Haghi, H.; Ershadi, A.; Esna-Ashari, M.; Pouya, M. Foliar Application of Potassium Sulphate Enhances the Cold-Hardiness of Grapevine (Vitis vinifera L.). J. Hortic. Sci. Biotechnol. 2014, 89, 141–146. [Google Scholar] [CrossRef]
- Lucau-Danila, A.; Toitot, C.; Goulas, E.; Blervacq, A.S.; Hot, D.; Bahrman, N.; Sellier, H.; Lejeune-Hénaut, I.; Delbreil, B. Transcriptome Analysis in Pea Allows to Distinguish Chilling and Acclimation Mechanisms. Plant Physiol. Biochem. 2012, 58, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Wilwerth, J.; Ker, K.; Inglis, D. Best Management Practices for Reducing Winter İnjury in Grapevines; Brock University: St. Catharines, ON, Canada, 2014. [Google Scholar]
- Hatterman-Valenti, H.M.; Auwarter, C.P.; Stenger, J.E. Evaluation of Cold-Hardy Grape Cultivars for North Dakota and the North Dakota State University Germplasm Enhancement Project. Acta Hortic. 2016, 1115, 13–22. [Google Scholar] [CrossRef]
- Wisniewski, M.; Nassuth, A.; Teulières, C.; Marque, C.; Rowland, J.; Cao, P.B.; Brown, A. Genomics of Cold Hardiness in Woody Plants. Crit. Rev. Plant Sci. 2014, 33, 92–124. [Google Scholar] [CrossRef]
- Kalberer, S.R.; Wisniewski, M.; Arora, R. Deacclimation and Reacclimation of Cold-Hardy Plants: Current Understanding and Emerging Concepts. Plant Sci. 2006, 171, 3–16. [Google Scholar] [CrossRef]
- Fennell, A.; Hoover, E. Photoperiod Influences Growth, Bud Dormancy, and Cold Acclimation in Vitis Labruscana and V. Riparia. J. Am. Soc. Hortic. Sci. 1991, 116, 270–273. [Google Scholar] [CrossRef]
- Wake, C.M.F.; Fennell, A. Morphological, Physiological and Dormancy Responses of Three Vitis Genotypes to Short Photoperiod. Physiol. Plant. 2000, 109, 203–210. [Google Scholar] [CrossRef]
- Wisniewski, M.; Bassett, C.; Gusta, L.V. An Overview of Cold Hardiness in Woody Plants: Seeing the Forest Through the Trees. HortScience 2003, 38, 952–959. [Google Scholar] [CrossRef]
- Stushnoff, C. Breeding and Selection Methods for Cold Hardiness in Deciduous Fruit Crops1. HortScience 1972, 7, 10–13. [Google Scholar] [CrossRef]
- Grant, T.N.L.; Gargrave, J.; Dami, I.E. Morphological, Physiological, and Biochemical Changes in Vitis Genotypes in Response to Photoperiod Regimes. Am. J. Enol. Vitic. 2013, 64, 466–475. [Google Scholar] [CrossRef]
- Piepho, H.P.; Möhring, J.; Melchinger, A.E.; Büchse, A. BLUP for Phenotypic Selection in Plant Breeding and Variety Testing. Euphytica 2008, 161, 209–228. [Google Scholar] [CrossRef]
- Soh, A.C. Ranking Parents by Best Linear Unbiased Prediction (BLUP) Breeding Values in Oil Palm. Euphytica 1994, 76, 13–21. [Google Scholar] [CrossRef]
- Searle, S.R.; Casella, G.; McCulloch, C.E. Variance Components; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-0-470-31769-3. [Google Scholar]
- Hill, R.R., Jr.; Rosenberger, J.L. Methods for Combining Data from Gemrplasm Evaluation Trials1. Crop Sci. 1985, 25, 467–470. [Google Scholar] [CrossRef]
- Tatar, I. Comparison of Two Single Curtain and Two Double Curtain Trellis Systems with Marquette and Petite Pearl Wine Grapes. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2020. [Google Scholar]
- Stenger, J.E. Factors Affecting Grapevine Establishment in Northern Production Regions. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2011. [Google Scholar]
- Alvarado, G.; López, M.; Vargas, M.; Pacheco, Á.; Rodríguez, F.; Burgueño, J.; Crossa, J. META-R (Multi Environment Trail Analysis with R for Windows), Version 6.04. CIMMYT Research Data & Software Repository Network. 2015. Available online: https://hdl.handle.net/11529/10201 (accessed on 17 January 2024).
- Alvarado, G.; Rodríguez, F.M.; Pacheco, A.; Burgueño, J.; Crossa, J.; Vargas, M.; Pérez-Rodríguez, P.; Lopez-Cruz, M.A. META-R: A Software to Analyze Data from Multi-Environment Plant Breeding Trials. Crop J. 2020, 8, 745–756. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H.; Chang, W. “Package ‘Ggplot2’.” Create Elegant Data Visualisations Using the Grammar of Graphics. 2016. Available online: https://rdrr.io/cran/ggplot2/man/ggplot2-package.html (accessed on 17 January 2024).
- Google Sheets: Free Online Spreadsheet Editor|Google Workspace. Available online: https://docs.google.com/ (accessed on 21 April 2022).
- Google Slides: Free Online Slideshow Maker|Google Workspace. Available online: https://docs.google.com/ (accessed on 21 April 2022).
- Paroschy, J.H.; Meiering, A.G.; Peterson, R.L.; Hostetter, G.; Neff, A. Mechanical Winter Injury in Grapevine Trunks. Am. J. Enol. Vitic. 1980, 31, 227–232. [Google Scholar] [CrossRef]
- Andrews, P.K.; Sandidge, C.R.; Toyama, T.K. Deep Supercooling of Dormant and Deacclimating Vitis Buds. Am. J. Enol. Vitic. 1984, 35, 175–177. [Google Scholar] [CrossRef]
- Quamme, H.A. Mechanism of Supercooling in Overwintering Peach Flower Buds1. J. Am. Soc. Hortic. Sci. 1978, 103, 57–61. [Google Scholar] [CrossRef]
- Falconer, D.S. Introduction to Quantitative Genetics; Pearson Education: London, UK, 1996; ISBN 978-81-317-2740-9. [Google Scholar]
- Lynch, M.; Walsh, B. Genetics and Analysis of Quantitative Traits; Sinauer: Sunderland, MA, USA, 1998; ISBN 978-0-87893-481-2. [Google Scholar]
- Neyhart, J.L.; Lorenz, A.J.; Smith, K.P. Multi-Trait Improvement by Predicting Genetic Correlations in Breeding Crosses. G3 Genes|Genomes|Genet. 2019, 9, 3153–3165. [Google Scholar] [CrossRef]
- Bernardo, R.N. Breeding for Quantitative Traits in Plants; Stemma Press: Woodbury, MN, USA, 2020; ISBN 978-0-9720724-3-4. [Google Scholar]
- Piepho, H.-P.; Möhring, J. Computing Heritability and Selection Response from Unbalanced Plant Breeding Trials. Genetics 2007, 177, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.; Hartung, J.; Rath, J.; Piepho, H.-P. Estimating Broad-Sense Heritability with Unbalanced Data from Agricultural Cultivar Trials. Crop Sci. 2019, 59, 525–536. [Google Scholar] [CrossRef]
- Larkin, D.L.; Lozada, D.N.; Mason, R.E. Genomic Selection—Considerations for Successful Implementation in Wheat Breeding Programs. Agronomy 2019, 9, 479. [Google Scholar] [CrossRef]
- De Rosa, V.; Vizzotto, G.; Falchi, R. Cold Hardiness Dynamics and Spring Phenology: Climate-Driven Changes and New Molecular Insights Into Grapevine Adaptive Potential. Front. Plant Sci. 2021, 12, 644528. [Google Scholar] [CrossRef] [PubMed]
- Londo, J.P.; Kovaleski, A.P. Characterization of Wild North American Grapevine Cold Hardiness Using Differential Thermal Analysis. Am. J. Enol. Vitic. 2017, 68, 203–212. [Google Scholar] [CrossRef]
- Londo, J.P.; Johnson, L.M. Variation in the Chilling Requirement and Budburst Rate of Wild Vitis Species. Environ. Exp. Bot. 2014, 106, 138–147. [Google Scholar] [CrossRef]
- Kovaleski, A.P.; Reisch, B.I.; Londo, J.P. Deacclimation Kinetics as a Quantitative Phenotype for Delineating the Dormancy Transition and Thermal Efficiency for Budbreak in Vitis Species. AoB Plants 2018, 10, ply066. [Google Scholar] [CrossRef]
- Or, E. Grape Bud Dormancy Release—The Molecular Aspect. In Grapevine Molecular Physiology & Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 1–29. ISBN 978-90-481-2305-6. [Google Scholar]
- Kovaleski, A.P.; Londo, J.P. Tempo of Gene Regulation in Wild and Cultivated Vitis Species Shows Coordination between Cold Deacclimation and Budbreak. Plant Sci. 2019, 287, 110178. [Google Scholar] [CrossRef]
- Aipperspach, A.; Hammond, J.; Hatterman-Valenti, H. Utilizing Pruning and Leaf Removal to Optimize Ripening of Vitis Riparia-Based ‘Frontenac Gris’ and ‘Marquette’ Wine Grapes in the Northern Great Plains. Horticulturae 2020, 6, 18. [Google Scholar] [CrossRef]
- Olson, B.K.; Brooke, M.; Wang, Z.; Svyantek, A.; Stenger, J.; Hatterman-Valenti, H. ‘Frontenac’ Grape Response to Canopy Management in North Dakota. Horticulturae 2021, 7, 288. [Google Scholar] [CrossRef]
- Olson, B.K. Frontenac’ Response to Leaf Removal and Training Systems and a Microvinification and Deacidification Bioassay of Interspecific Hybrids (Vitis spp.). Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2016. [Google Scholar]

| Genotype | Location | Periderm Node (%) | Cane Diameter (mm) |
|---|---|---|---|
| Bluebell | Absaraka, ND | 79 ± 4 z | 4.63 ± 0.11 |
| Crimson Pearl | Buffalo, ND | 79 ± 4 | 5.52 ± 0.14 |
| Hasansky Sladky | Absaraka, ND | 63 ± 5 | 4.63 ± 0.15 |
| Frontenac | Buffalo, ND | 81 ± 4 | 5.87 ± 0.16 |
| Frontenac gris | Buffalo, ND | 79 ± 2 | 5.68 ± 0.08 |
| John Viola | Absaraka, ND | 66 ± 5 | 6.94 ± 0.27 |
| King of the North | Absaraka, ND | 54 ± 4 | 5.08 ± 0.08 |
| Buffalo, ND | 57 ± 5 | 6.73 ± 0.19 | |
| La Crescent | Buffalo, ND | 84 ± 4 | 5.85 ± 0.23 |
| Marquette | Absaraka, ND | 91 ± 2 | 6.02 ± 0.11 |
| Buffalo, ND | 87 ± 3 | 6.22 ± 0.17 | |
| MN1131 | Absaraka, ND | 82 ± 3 | 6.19 ± 0.17 |
| Petite Pearl | Absaraka, ND | 82 ± 4 | 5.75 ± 0.18 |
| Prairie Star | Absaraka, ND | 72 ± 4 | 4.99 ± 0.13 |
| Sabrevois | Buffalo, ND | 35 ± 4 | 4.48 ± 0.12 |
| Valiant | Absaraka, ND | 72 ± 3 | 5.97 ± 0.15 |
| Mean | Absaraka, ND | 75 | 6.02 |
| Buffalo, ND | 74 | 5.57 | |
| Overall | 75 | 5.81 | |
| CV (%) | Absaraka, ND | 41 | 24.88 |
| Buffalo, ND | 44 | 20.44 | |
| Overall | 42 | 23.43 |
| Statistic | Bud LTE10 1 | Bud LTE50 | Bud LTE90 | Phloem LTE10 | Phloem LTEmid | Phloem LTEend | Xylem LTE10 | Xylem LTEmid | Xylem LTEend |
|---|---|---|---|---|---|---|---|---|---|
| Heritability | 0.936 | 0.955 | 0.965 | 0.000 | 0.050 | 0.335 | 0.658 | 0.591 | 0.684 |
| Genotypic variance | 4.914 | 5.199 | 4.353 | 0.000 | 0.013 | 0.159 | 0.463 | 0.232 | 0.246 |
| Env. variance | 4.471 | 4.552 | 5.515 | 13.668 | 17.300 | 30.361 | 30.911 | 27.644 | 19.373 |
| Geno. × Env. variance | 1.795 | 1.417 | 0.810 | 1.574 | 1.518 | 1.518 | 1.914 | 1.305 | 0.850 |
| Residual variance | 7.572 | 5.216 | 3.710 | 3.830 | 2.405 | 3.901 | 1.467 | 0.923 | 0.794 |
| Mean | −19.77 | −23.151 | −25.80 | −19.54 | −22.77 | −28.17 | −36.36 | −38.76 | −40.75 |
| CV | 13.917 | 9.865 | 7.466 | 10.016 | 6.811 | 7.010 | 3.331 | 2.479 | 2.186 |
| p-value | |||||||||
| Genotype | <0.0001 2 | <0.0001 | <0.0001 | 0.9999 | 0.9218 | 0.4099 | 0.0179 | 0.0542 | 0.0102 |
| Env. | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0066 | <0.0001 | <0.0001 | <0.0001 |
| Geno. × Env. | <0.0001 | <0.0001 | 0.0002 | 0.0048 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Statistic | Bud LTE10 1 | Bud LTE50 | Bud LTE90 | Phloem LTE10 | Phloem LTEmid | Phloem LTEend | Xylem LTE10 | Xylem LTEmid | Xylem LTEend |
|---|---|---|---|---|---|---|---|---|---|
| Heritability | 0.815 | 0.838 | 0.839 | 0.476 | 0.618 | 0.581 | 0.499 | 0.747 | 0.732 |
| Genotypic variance | 1.775 | 1.448 | 1.153 | 0.111 | 0.161 | 0.252 | 0.174 | 0.324 | 0.164 |
| Env. variance | 10.399 | 9.310 | 8.346 | 13.320 | 14.998 | 25.517 | 30.034 | 25.816 | 18.958 |
| Geno. × Env. variance | 2.089 | 1.470 | 1.372 | 0.376 | 0.191 | 0.593 | 0.777 | 0.675 | 0.242 |
| Residual variance | 9.402 | 6.400 | 4.216 | 1.930 | 1.800 | 2.812 | 2.287 | 1.060 | 0.839 |
| Mean | −19.92 | −23.50 | −25.87 | −19.93 | −22.83 | −27.89 | −36.50 | −38.86 | −40.80 |
| CV | 15.393 | 10.764 | 7.936 | 6.970 | 5.876 | 6.014 | 4.143 | 2.650 | 2.245 |
| p-value | |||||||||
| Genotype | 0.0005 2 | 0.0001 | 0.0001 | 0.2458 | 0.0753 | 0.1081 | 0.2091 | 0.0063 | 0.0096 |
| Env. | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Geno. × Env. | 0.0010 | 0.0007 | <0.0001 | 0.1794 | 0.4359 | 0.1487 | 0.0338 | 0.0008 | 0.0632 |
| Bud LTE10 | Bud LTE50 | Bud LTE90 | Phloem LTE10 | Phloem LTEmid | Phloem LTEend | Xylem LTE10 | Xylem LTEmid | Xylem LTEend | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bud LTE10 | 0.9876 | <0.0001 | 0.9518 | <0.0001 | 0.2492 | 0.5179 | 0.1121 | 0.7739 | 0.5266 | 0.1453 | 0.5916 | 0.0934 | 0.6710 | 0.0479 | 0.7485 | 0.0203 | ||
| Bud LTE50 | 0.9999 | 0.0001 | 0.9856 | <0.0001 | 0.3201 | 0.4010 | 0.2069 | 0.5933 | 0.5928 | 0.0925 | 0.6793 | 0.0442 | 0.7511 | 0.0196 | 0.8120 | 0.0078 | ||
| Bud LTE90 | 0.9830 | 0.0001 | 0.9959 | 0.0001 | 0.4004 | 0.2856 | 0.3092 | 0.4182 | 0.6410 | 0.0628 | 0.7208 | 0.0285 | 0.7939 | 0.0106 | 0.8552 | 0.0033 | ||
| Phloem LTE10 | 0.2723 | 0.4784 | 0.4611 | 0.2116 | 0.6278 | 0.0702 | 0.9431 | 0.0001 | 0.8267 | 0.0060 | 0.4866 | 0.1841 | 0.4493 | 0.2250 | 0.4601 | 0.2127 | ||
| Phloem LTEmid | 0.1636 | 0.6741 | 0.3829 | 0.3091 | 0.5606 | 0.1164 | 0.9999 | 0.0001 | 0.7982 | 0.0099 | 0.4602 | 0.2125 | 0.4204 | 0.2599 | 0.3902 | 0.2992 | ||
| Phloe LTEend | 0.9999 | 0.0001 | 0.9999 | 0.0001 | 0.9999 | 0.0001 | 0.9999 | 0.0001 | 0.9999 | 0.0001 | 0.5854 | 0.0977 | 0.5543 | 0.1214 | 0.5547 | 0.1211 | ||
| Xylem LTE10 | 0.5204 | 0.1509 | 0.5904 | 0.0942 | 0.6465 | 0.0599 | 0.5692 | 0.1097 | 0.4417 | 0.2339 | 0.9999 | 0.0001 | 0.9861 | <0.0001 | 0.9370 | 0.0002 | ||
| Xylem LTEmid | 0.5864 | 0.0970 | 0.6472 | 0.0595 | 0.7042 | 0.0342 | 0.5439 | 0.1301 | 0.3818 | 0.3106 | 0.9999 | 0.0001 | 0.9992 | 0.0001 | 0.9758 | <0.0001 | ||
| Xylem LTEend | 0.6846 | 0.0419 | 0.7178 | 0.0294 | 0.7649 | 0.0163 | 0.6820 | 0.0430 | 0.3909 | 0.2982 | 0.9999 | 0.0001 | 0.9749 | 0.0001 | 0.9964 | 0.0001 | ||
| Bud LTE10 | Bud LTE50 | Bud LTE90 | Phloem LTE10 | Phloem LTEmid | Phloem LTEend | Xylem LTE10 | Xylem LTEmid | Xylem LTEend | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bud LTE10 | 0.8905 | 0.0072 | 0.8353 | 0.0193 | 0.7498 | 0.0523 | 0.6841 | 0.0901 | 0.1054 | 0.8220 | 0.1883 | 0.6859 | 0.2522 | 0.5854 | 0.4274 | 0.3388 | ||
| Bud LTE50 | 0.8883 | 0.0075 | 0.9880 | <0.0001 | 0.7240 | 0.0658 | 0.5635 | 0.1878 | 0.3758 | 0.4062 | 0.3468 | 0.4460 | 0.4241 | 0.3430 | 0.6236 | 0.1345 | ||
| Bud LTE90 | 0.8116 | 0.0267 | 0.9999 | 0.0001 | 0.6620 | 0.1052 | 0.4762 | 0.2801 | 0.3489 | 0.4430 | 0.4675 | 0.2901 | 0.5342 | 0.2167 | 0.7136 | 0.0717 | ||
| Phloem LTE10 | 0.9999 | 0.0001 | 0.9624 | 0.0005 | 0.9158 | 0.0038 | 0.7668 | 0.0443 | 0.4561 | 0.3037 | −0.1391 | 0.7661 | −0.2067 | 0.6565 | −0.0224 | 0.9620 | ||
| Phloem LTEmid | 0.6951 | 0.0829 | 0.6434 | 0.1190 | 0.5649 | 0.1864 | 0.7600 | 0.0474 | 0.5323 | 0.2187 | −0.0887 | 0.8500 | −0.3120 | 0.4958 | −0.1184 | 0.8004 | ||
| Phloem LTEend | −0.5284 | 0.2228 | 0.2911 | 0.5265 | 0.4206 | 0.3475 | 0.3323 | 0.4666 | 0.6187 | 0.1385 | −0.1501 | 0.7481 | −0.2927 | 0.5241 | −0.1004 | 0.8305 | ||
| Xylem LTE10 | −0.0735 | 0.8755 | 0.3392 | 0.4567 | 0.5842 | 0.1684 | −0.5312 | 0.2199 | −0.1932 | 0.6780 | 0.4265 | 0.3399 | 0.8856 | 0.0080 | 0.8453 | 0.0166 | ||
| Xylem LTEmid | 0.1164 | 0.8038 | 0.4162 | 0.3530 | 0.5973 | 0.1567 | −0.4866 | 0.2682 | −0.4903 | 0.2640 | −0.4435 | 0.3189 | 0.9551 | 0.0008 | 0.9671 | 0.0004 | ||
| Xylem LTEend | 0.4397 | 0.3235 | 0.6908 | 0.0857 | 0.8176 | 0.0247 | −0.2428 | 0.5999 | −0.3416 | 0.4533 | −0.5093 | 0.2430 | 0.8846 | 0.0082 | 0.9556 | 0.0008 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köse, B.; Svyantek, A.; Kadium, V.R.; Brooke, M.; Auwarter, C.; Hatterman-Valenti, H. Death and Dying: Grapevine Survival, Cold Hardiness, and BLUPs and Winter BLUEs in North Dakota Vineyards. Life 2024, 14, 178. https://doi.org/10.3390/life14020178
Köse B, Svyantek A, Kadium VR, Brooke M, Auwarter C, Hatterman-Valenti H. Death and Dying: Grapevine Survival, Cold Hardiness, and BLUPs and Winter BLUEs in North Dakota Vineyards. Life. 2024; 14(2):178. https://doi.org/10.3390/life14020178
Chicago/Turabian StyleKöse, Bülent, Andrej Svyantek, Venkateswara Rao Kadium, Matthew Brooke, Collin Auwarter, and Harlene Hatterman-Valenti. 2024. "Death and Dying: Grapevine Survival, Cold Hardiness, and BLUPs and Winter BLUEs in North Dakota Vineyards" Life 14, no. 2: 178. https://doi.org/10.3390/life14020178
APA StyleKöse, B., Svyantek, A., Kadium, V. R., Brooke, M., Auwarter, C., & Hatterman-Valenti, H. (2024). Death and Dying: Grapevine Survival, Cold Hardiness, and BLUPs and Winter BLUEs in North Dakota Vineyards. Life, 14(2), 178. https://doi.org/10.3390/life14020178