Establishing SW1353 Chondrocytes as a Cellular Model of Chondrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
2.3. Cytotoxicity Assay
2.4. Annexin V–Fluorescein Isothiocyanate/Propidium Iodide Dual-Labelling Assay
2.5. Cell Cycle Assay
2.6. Lysate Preparation and Protein Determination
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Statistical Analysis
3. Results
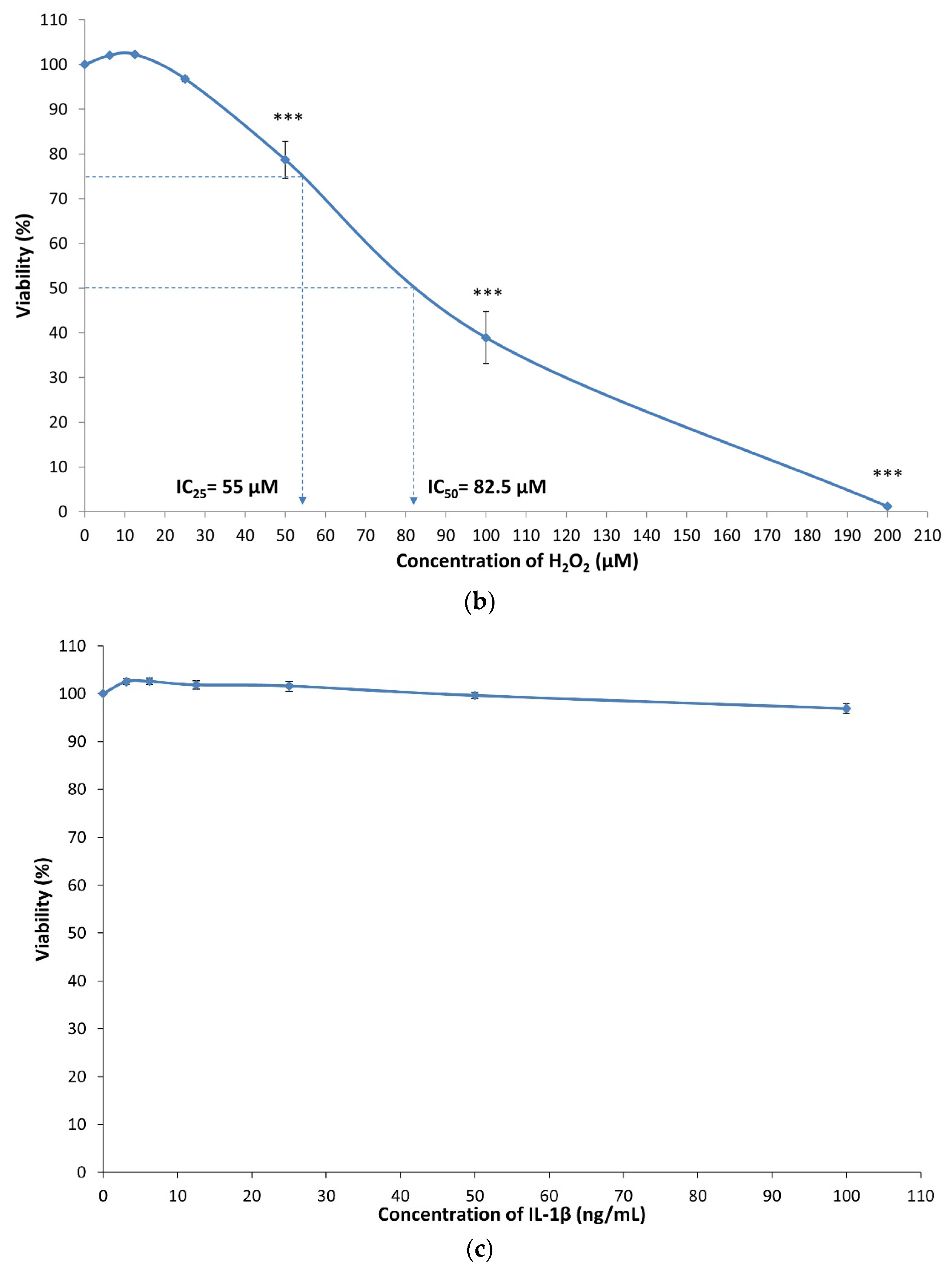
3.1. Cytotoxicity of MIA, H2O2 and IL-1β on SW1353 Chondrocytes
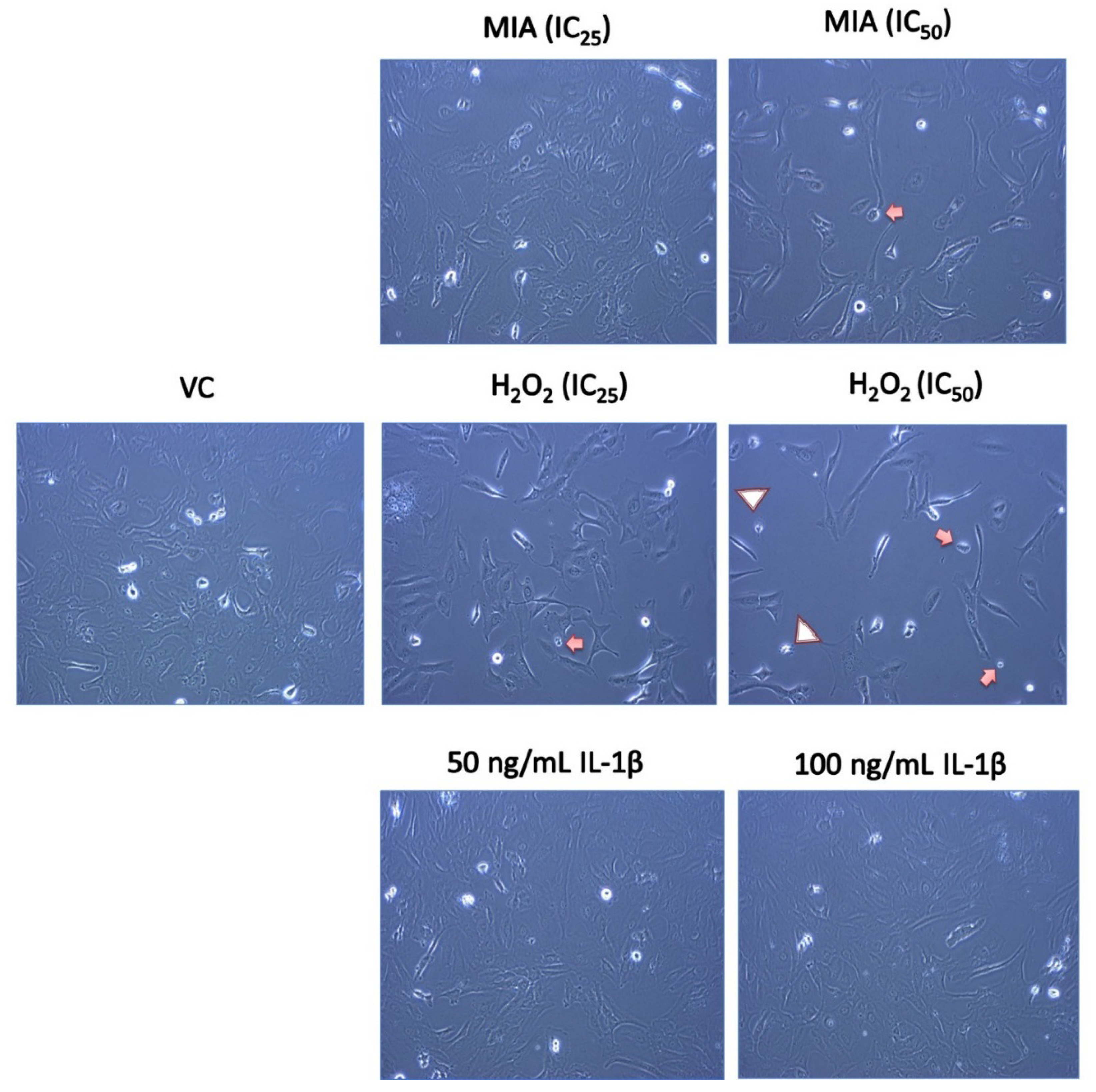
3.2. Morphological Changes of SW1353 Chondrocytes upon MIA, H2O2 and IL-1β Treatment
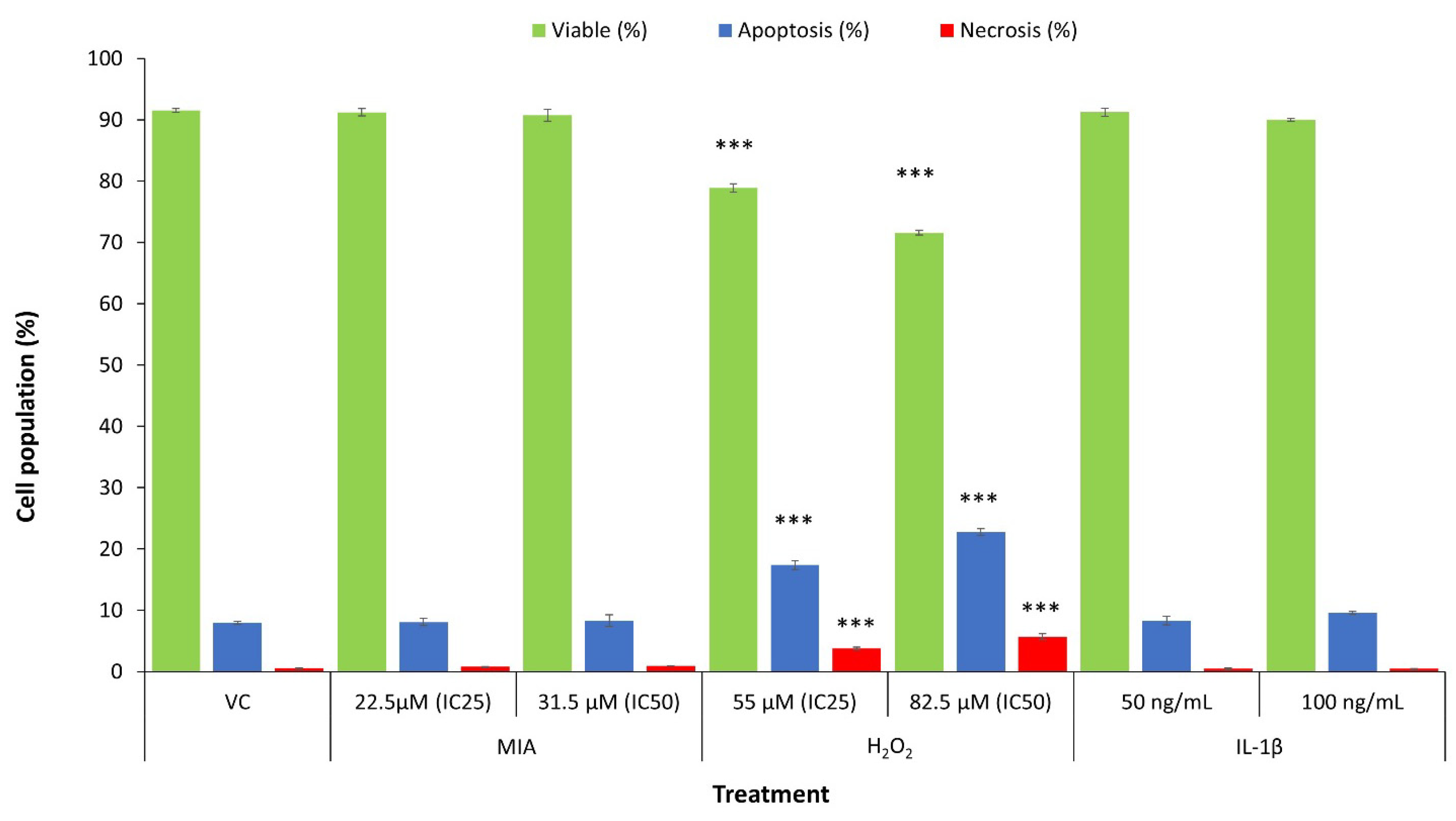
3.3. Mode of SW1353 Cell Death under MIA, H2O2 and IL-1β Treatment
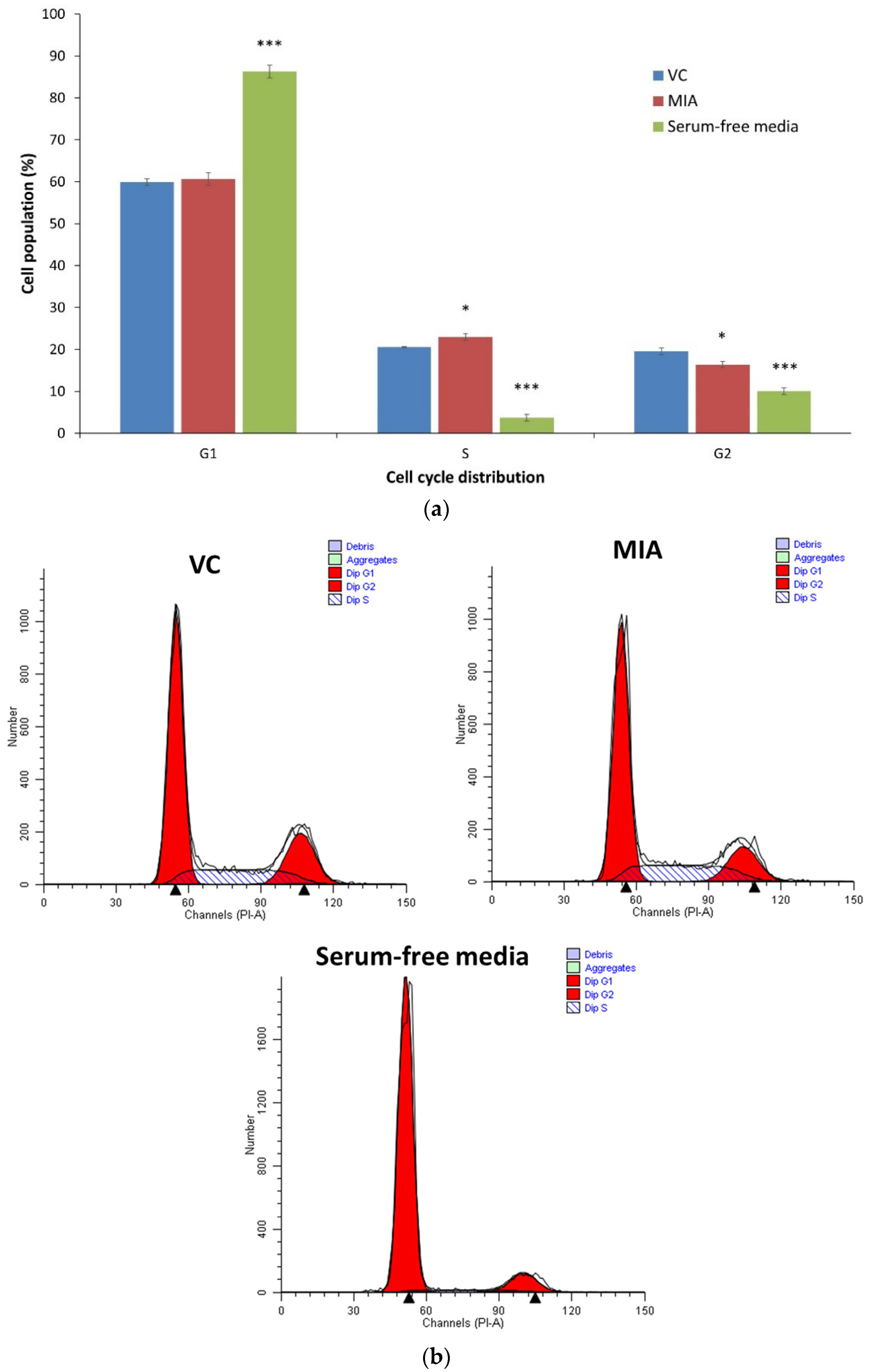
3.4. Cell Cycle Analysis
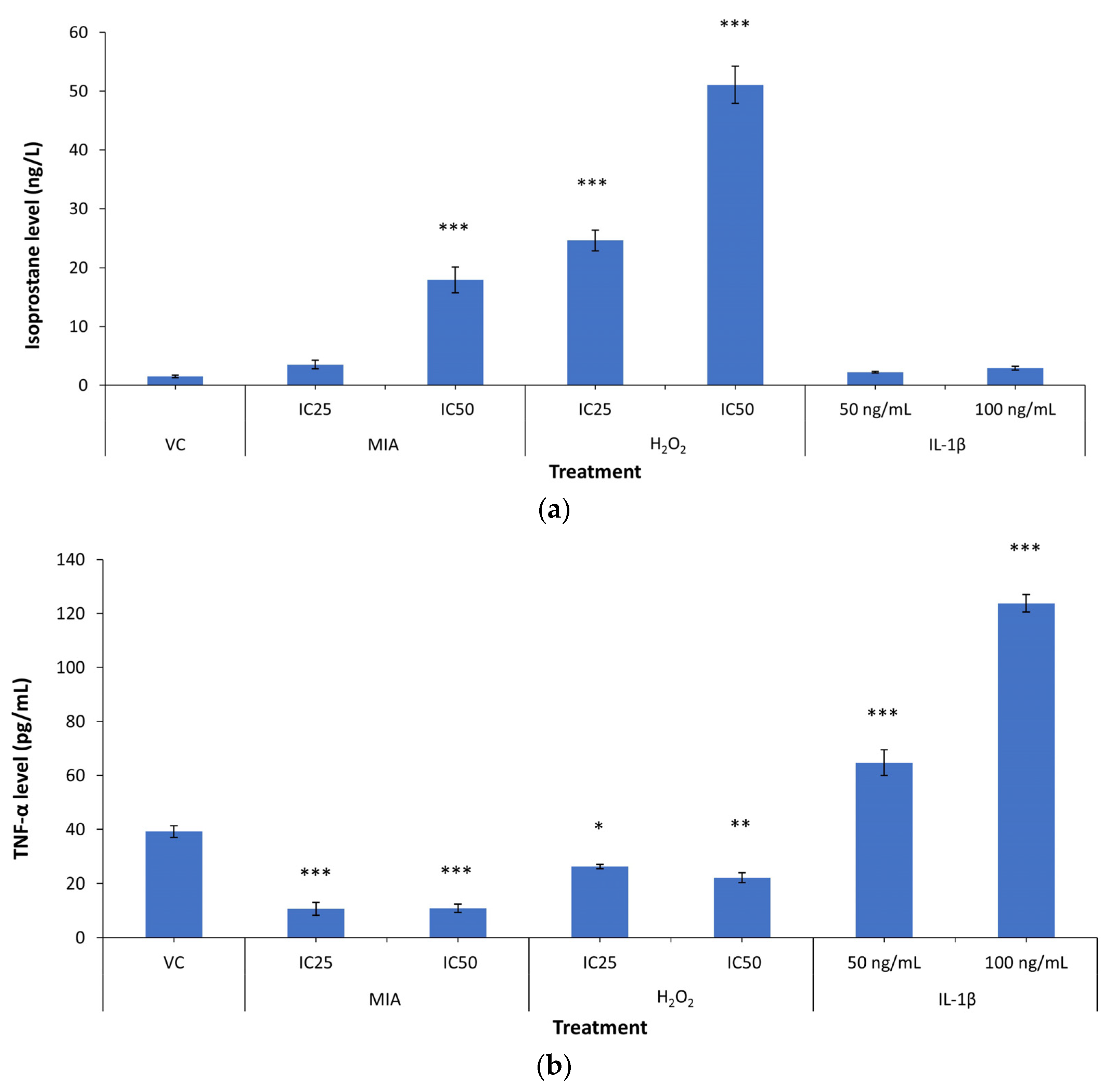
3.5. 8-Isoprostane F2-α and TNF-α Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heidari, B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Casp. J. Intern. Med. 2011, 2, 205–212. [Google Scholar]
- Grässel, S.; Muschter, D. Recent advances in the treatment of osteoarthritis. F1000Research 2020, 9, 325. [Google Scholar] [CrossRef]
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.-J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Zamli, Z.; Sharif, M. Chondrocyte apoptosis: A cause or consequence of osteoarthritis? Int. J. Rheum. Dis. 2011, 14, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.N.F.; Bastiaansen-Jenniskens, Y.; Narcisi, R.; Van Osch, G. Effect of inflammation on hypertrophy to human articular chondrocytes. Osteoarthr. Cartil. 2020, 28, S113–S114. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11, 111. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Pang, K.-L. Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrition 2017, 9, 1060. [Google Scholar] [CrossRef]
- van der Kraan, P.; Berg, W.V.D. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Samvelyan, H.J.; Hughes, D.; Stevens, C.; Staines, K.A. Models of Osteoarthritis: Relevance and New Insights. Calcif. Tissue Int. 2020. [Google Scholar] [CrossRef]
- Johnson, C.I.; Argyle, D.J.; Clements, D.N. In vitro models for the study of osteoarthritis. Vet. J. 2016, 209, 40–49. [Google Scholar] [CrossRef]
- Little, C.; Smith, M. Animal Models of Osteoarthritis. Curr. Rheumatol. Rev. 2008, 4, 175–182. [Google Scholar] [CrossRef]
- Muhammad, S.A.; Nordin, N.; Hussin, P.; Mehat, M.Z.; Tan, S.W.; Fakurazi, S. Optimization of Protocol for Isolation of Chondrocytes from Human Articular Cartilage. Cartilage 2019. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M.; Favero, M.; Dragomir, C.; El Hachem, K.; Hashimoto, K.; Plumb, D.A. Human chondrocyte cultures as models of cartilage-specific gene regulation. Methods Mol. Med. 2005, 107, 69–95. [Google Scholar] [CrossRef]
- Kim, E.-N.; Lee, H.-S.; Jeong, G.-S. Cudratricusxanthone O Inhibits H2O2-Induced Cell Damage by Activating Nrf2/HO-1 Pathway in Human Chondrocytes. Antioxidants 2020, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-W.; Huang, T.-C.; Hu, Y.-C.; Hsieh, B.-S.; Chiu, P.-R.; Cheng, H.-L.; Chang, K.-L. Zinc protects chondrocytes from monosodium iodoacetate-induced damage by enhancing ATP and mitophagy. Biochem. Biophys. Res. Commun. 2020, 521, 50–56. [Google Scholar] [CrossRef]
- Huang, T.-C.; Chang, W.-T.; Hu, Y.-C.; Hsieh, B.-S.; Cheng, H.-L.; Yen, J.-H.; Chiu, P.-R.; Chang, K.-L. Zinc Protects Articular Chondrocytes through Changes in Nrf2-Mediated Antioxidants, Cytokines and Matrix Metalloproteinases. Nutrition 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, J.; Ding, X.; Sun, Y.; Cui, Z.; Liu, W. Tanshinone I Inhibits IL-1β-Induced Apoptosis, Inflammation And Extracellular Matrix Degradation In Chondrocytes CHON-001 Cells And Attenuates Murine Osteoarthritis. Drug Des. Dev. Ther. 2019, 13, 3559–3568. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-D.; Kim, Y.-S.; Ko, S.-H.; Yoon, I.-J.; Cho, S.-G.; Chun, Y.-H.; Choi, B.-J.; Kim, E.-C. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J. Pineal Res. 2012, 53, 225–237. [Google Scholar] [CrossRef]
- Afifah, E.; Mozef, T.; Sandra, F.; Arumwardana, S.; Rihibiha, D.D.; Nufus, H.; Rizal, R.; Amalia, A.; Bachtiar, I.; Murti, H.; et al. Induction of Matrix Metalloproteinases in Chondrocytes by Interleukin IL-1β as an Osteoarthritis Model. J. Math. Fundam. Sci. 2019, 51, 103–111. [Google Scholar] [CrossRef]
- Finger, F.; Schörle, C.; Zien, A.; Gebhard, P.; Goldring, M.B.; Aigner, T. Molecular phenotyping of human chondrocyte cell lines T/C-28a2, T/C-28a4, and C-28/I2. Arthritis Rheum. 2003, 48, 3395–3403. [Google Scholar] [CrossRef]
- Claassen, H.; Schicht, M.; Brandt, J.; Reuse, K.; Schädlich, R.; Goldring, M.B.; Guddat, S.S.; Thate, A.; Paulsen, F. C-28/I2 and T/C-28a2 chondrocytes as well as human primary articular chondrocytes express sex hormone and insulin receptors—Useful cells in study of cartilage metabolism. Ann. Anat.-Anat. Anz. 2011, 193, 23–29. [Google Scholar] [CrossRef]
- Huang, L.; Cao, J.; Cao, L.; Gao, L.; Yang, Y.; Xu, L. Puerarin induces cell apoptosis in human chondrosarcoma cell line SW1353 via inhibition of the PI3K/Akt signaling pathway. Oncol. Lett. 2017, 14, 5585–5590. [Google Scholar] [CrossRef][Green Version]
- Merluzzi, V.J.; Faanes, R.B.; Czajkowski, M.; Last-Barney, K.; Harrison, P.C.; Kahn, J.; Rothlein, R. Membrane-associated interleukin 1 activity on human U937 tumor cells: Stimulation of PGE2 production by human chondrosarcoma cells. J. Immunol. 1987, 139, 166–168. [Google Scholar]
- Gebauer, M.; Saas, J.; Sohler, F.; Haag, J.; Söder, S.; Pieper, M.; Bartnik, E.; Beninga, J.; Zimmer, R.; Aigner, T. Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1β. Osteoarthr. Cartil. 2005, 13, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Cecen, B.; Keles, D.; Oktay, G.; Kozaci, L.D. Effects of simvastatin on matrix metalloproteinase regulation in IL-1β-induced SW1353 cells. Chem. Interact. 2019, 310, 108730. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Tu, Y.; Ma, T.; Liu, X.; Wen, T.; Cai, M.; Xia, Z.; Mei, J. Lactoferrin Inhibits IL-1β-Induced Chondrocyte Apoptosis Through AKT1-Induced CREB1 Activation. Cell. Physiol. Biochem. 2015, 36, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Gu, W. Effects of miR-146a-5p on chondrocyte interleukin-1β-induced inflammation and apoptosis involving thioredoxin interacting protein regulation. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Xu, F.; Song, Y.; Guo, A. Anti-Apoptotic Effects of Docosahexaenoic Acid in IL-1β-Induced Human Chondrosarcoma Cell Death through Involvement of the MAPK Signaling Pathway. Cytogenet. Genome Res. 2019, 158, 17–24. [Google Scholar] [CrossRef]
- Yasuhara, R.; Miyamoto, Y.; Akaike, T.; Akuta, T.; Nakamura, M.; Takami, M.; Morimura, N.; Yasu, K.; Kamijo, R. Interleukin-1β induces death in chondrocyte-like ATDC5 cells through mitochondrial dysfunction and energy depletion in a reactive nitrogen and oxygen species-dependent manner. Biochem. J. 2005, 389, 315–323. [Google Scholar] [CrossRef]
- Zahan, O.-M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef]
- Bhatti, F.-U.-R.; Mehmood, A.; Wajid, N.; Rauf, M.; Khan, S.N.; Riazuddin, S. Vitamin E protects chondrocytes against hydrogen peroxide-induced oxidative stress in vitro. Inflamm. Res. 2013, 62, 781–789. [Google Scholar] [CrossRef]
- Asada, S.; Fukuda, K.; Nishisaka, F.; Matsukawa, M.; Hamanisi, C. Hydrogen peroxide induces apoptosis of chondrocytes; involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm. Res. 2001, 50, 19–23. [Google Scholar] [CrossRef]
- Chang, Z.; Huo, L.; Li, P.; Wu, Y.; Zhang, P. Ascorbic acid provides protection for human chondrocytes against oxidative stress. Mol. Med. Rep. 2015, 12, 7086–7092. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Na, J.-Y.; Song, K.-B.; Choi, D.-S.; Kim, J.-H.; Kwon, Y.-B.; Kwon, J.-K. Protective Effect of Ginsenoside Rb1on Hydrogen Peroxide-induced Oxidative Stress in Rat Articular Chondrocytes. J. Ginseng Res. 2012, 36, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Hong, S.H.; Shin, S.S.; Lee, D.-S.; Han, M.H.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; Park, E.K.; et al. Activation of the Nrf2/HO-1 Signaling Pathway Contributes to the Protective Effects of Sargassum serratifolium Extract against Oxidative Stress-Induced DNA Damage and Apoptosis in SW1353 Human Chondrocytes. Int. J. Environ. Res. Public Health 2018, 15, 1173. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-W.; Choi, E.O.; Kwon, D.H.; Kim, B.H.; Hwang, H.J.; Kim, B.W.; Choi, Y.H. Cytoprotective Effects of Schisandrin A against Hydrogen Peroxide-induced Oxidative Stress in SW1353 Human Chondrocytes. J. Life Sci. 2017, 27, 1070–1077. [Google Scholar] [CrossRef]
- Yudoh, K.; Van Trieu, N.; Nakamura, H.; Hongo-Masuko, K.; Kato, T.; Nishioka, K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: Oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res. 2005, 7, R380–R391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Makosa, D.; Miller, B.F.; Griffin, T.M. Glutathione as a mediator of cartilage oxidative stress resistance and resilience during aging and osteoarthritis. Connect. Tissue Res. 2020, 61, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Shin, J.; Cho, Y.; Kim, H.-S.; Gu, Y.-R.; Kim, H.; You, K.T.; Chang, M.J.; Chang, C.B.; Kang, S.-B.; et al. Stress-activated miR-204 governs senescent phenotypes of chondrocytes to promote osteoarthritis development. Sci. Transl. Med. 2019, 11, eaar6659. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.-R.; Hu, Y.-C.; Huang, T.-C.; Hsieh, B.-S.; Yeh, J.-P.; Cheng, H.-L.; Huang, L.-W.; Chang, K.-L. Vitamin C Protects Chondrocytes against Monosodium Iodoacetate-Induced Osteoarthritis by Multiple Pathways. Int. J. Mol. Sci. 2016, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.-R.; Eo, S.-H.; Abbas, Q.; Ahmed, M.; Kim, S.J. Applications of Chondrocyte-Based Cartilage Engineering: An Overview. BioMed Res. Int. 2016, 2016, 1879837. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dringen, R. Differential effects of iodoacetamide and iodoacetate on glycolysis and glutathione metabolism of cultured astrocytes. Front. Neuroenergetics 2009, 1. [Google Scholar] [CrossRef]
- Jiang, L.; Li, L.; Geng, C.; Gong, D.; Jiang, L.; Ishikawa, N.; Kajima, K.; Zhong, L. Monosodium iodoacetate induces apoptosis via the mitochondrial pathway involving ROS production and caspase activation in rat chondrocytes in vitro. J. Orthop. Res. 2012, 31, 364–369. [Google Scholar] [CrossRef]
- Martin, G.; Andriamanalijaona, R.; Mathy-Hartert, M.; Henrotin, Y.; Pujol, J.-P. Comparative effects of IL-1β and hydrogen peroxide (H2O2) on catabolic and anabolic gene expression in juvenile bovine chondrocytes. Osteoarthr. Cartil. 2005, 13, 915–924. [Google Scholar] [CrossRef]
- Fauzi, S.; Rajab, N.; Leong, L.; Pang, K.; Nawi, N.; Nasir, N.; Lorin, F.; Yusof, F. Apoptosis and cell cycle effect of Lignosus rhinocerus extract on HCT 116 human colorectal cancer cells. Int. J. Pharm. Sci. Rev. Res. 2015, 33, 13–17. [Google Scholar]
- Hasan, W.N.W.; Ghafar, N.A.; Chin, K.-Y.; Ima-Nirwana, S. Annatto-derived tocotrienol stimulates osteogenic activity in preosteoblastic MC3T3-E1 cells: A temporal sequential study. Drug Des. Dev. Ther. 2018, 12, 1715–1726. [Google Scholar] [CrossRef]
- Fadok, V.A.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Leong, L.M.; Chan, K.M.; Hamid, A.; Latip, J.; Rajab, N.F. Herbal Formulation C168 Attenuates Proliferation and Induces Apoptosis in HCT 116 Human Colorectal Carcinoma Cells: Role of Oxidative Stress and DNA Damage. Evid.-Based Complement. Altern. Med. 2016, 2016, 2091085. [Google Scholar] [CrossRef]
- Pang, K.-L.; Chin, K.-Y. Comment on: Food for Bone: Evidence for a Role for Delta-Tocotrienol in the Physiological Control of Osteoblast Migration. Int. J. Mol. Sci. 2020, 21, 6674. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z. Critical Aspects in Analysis of Cellular DNA Content. Curr. Protoc. Cytom. 2010, 52, 7.2.1–7.2.8. [Google Scholar] [CrossRef] [PubMed]
- BT LAB Bioassay Technology Laboratory. Human 8-Isoprostane F2 Alpha ELISA Kit. Available online: http://www.bt-laboratory.com/product/human-8-isoprostane-f2-alpha-elisa-kit/ (accessed on 15 January 2021).
- Elabscience. Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit. Available online: https://www.elabscience.com/p-human_tnf_alpha_tumor_necrosis_factor_alpha_elisa_kit-17972.html (accessed on 15 January 2021).
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- van Engeland, M.; Nieland, L.J.W.; Ramaekers, F.C.S.; Schutte, B.; Reutelingsperger, C.P.M. Annexin V-Affinity assay: A re-view on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- White, E.Z.; Pennant, N.M.; Carter, J.R.; Hawsawi, O.; Odero-Marah, V.; Hinton, C.V. Serum deprivation initiates adaptation and survival to oxidative stress in prostate cancer cells. Sci. Rep. 2020, 10, 12505. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P. Chapter 19—Measurement of Biomarkers of Oxidative Stress and Airway Inflammation in Exhaled Breath Condensate: Methodology and Potential Applications in Patients with COPD and Healthy Smokers. In Volatile Biomarkers; Amann, A., Smith, D., Eds.; Elsevier: Boston, MA, USA, 2013; pp. 360–381. [Google Scholar] [CrossRef]
- Das-Bradoo, S.; Bielinsky, A. DNA Replication and Checkpoint Control in S Phase. Nat. Educ. 2010, 3, 50. [Google Scholar]
- Xia, T.; Gao, R.; Zhou, G.; Liu, J.; Li, J.; Shen, J. Trans-Cinnamaldehyde Inhibits IL-1β-Stimulated Inflammation in Chondrocytes by Suppressing NF-κB and p38-JNK Pathways and Exerts Chondrocyte Protective Effects in a Rat Model of Osteoarthritis. BioMed Res. Int. 2019, 2019, 4039472. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, T.; Sel, S.; Hütten, H.; Röpke, M.; Roessner, A.; Nass, N. Curcumin Blocks Interleukin-1 Signaling in Chondrosarcoma Cells. PLoS ONE 2014, 9, e99296. [Google Scholar] [CrossRef]
- Barksby, H.E.; Hui, W.; Wappler, I.; Peters, H.H.; Milner, J.M.; Richards, C.D.; Cawston, T.E.; Rowan, A.D. Interleukin-1 in combination with oncostatin M up-regulates multiple genes in chondrocytes: Implications for cartilage destruction and repair. Arthritis Rheum. 2006, 54, 540–550. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Jayakumar, T.; Duann, Y.-F.; Chou, Y.-C.; Hsieh, C.-Y.; Yu, S.-Y.; Sheu, J.-R.; Hsiao, G. Chondroprotective Role of Sesamol by Inhibiting MMPs Expression via Retaining NF-κB Signaling in Activated SW1353 Cells. J. Agric. Food Chem. 2011, 59, 4969–4978. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.D.; He, A.S.; Fu, M.; Sheng, P.; Liao, W.M. Sinomenine suppresses expression of interleukin-1beta-induced matrix metalloproteinases in human osteoarthritic chondrocytes. J. Med. Plants Res. 2010, 4, 1830–1836. [Google Scholar] [CrossRef]
- Ha, Y.-J.; Choi, Y.S.; Kang, E.H.; Shin, K.; Kim, T.K.; Song, Y.W.; Lee, Y.J. SOCS1 suppresses IL-1β-induced C/EBPβ expression via transcriptional regulation in human chondrocytes. Exp. Mol. Med. 2016, 48, e241. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.J.; Choi, S.-I.; Choi, B.-R.; Lee, Y.-S.; Lee, D.Y.; Kim, G.S. Cartilage protective and anti-analgesic effects of ALM16 on monosodium iodoacetate induced osteoarthritis in rats. BMC Complement. Altern. Med. 2019, 19, 325. [Google Scholar] [CrossRef] [PubMed]
- Park, J.U.; Kim, S.-J.; Na, C.-S.; Choi, C.-H.; Seo, C.S.; Son, J.-K.; Kang, B.Y.; Kim, Y.R. Chondroprotective and anti-inflammatory effects of ChondroT, a new complex herbal medication. BMC Complement. Altern. Med. 2016, 16, 213. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Chen, G.; Li, R.; Rong, X.; Zhou, G.; Zhong, Y. Ginsenoside Rb1 Reduces Nitric Oxide Production via Inhibition of Nuclear Factor-κB Activation in Interleukin-1β- Stimulated SW1353 Chondrosarcoma Cells. Trop. J. Pharm. Res. 2014, 13, 1071–1078. [Google Scholar] [CrossRef]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef]
- Schaefer, J.F.; Millham, M.L.; De Crombrugghe, B.; Buckbinder, L. FGF signaling antagonizes cytokine-mediated repression of Sox9 in SW1353 chondrosarcoma cells. Osteoarthr. Cartil. 2003, 11, 233–241. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Zhong, Y.; Zhang, S.; Zhou, L.; Shang, S. Fuyuan Decoction Enhances SOX9 and COL2A1 Expression and Smad2/3 Phosphorylation in IL-1β-Activated Chondrocytes. Evid.-Based Complement. Altern. Med. 2015, 2015, 821947. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, C.; Jeong, J.-W.; Lee, D.-S.; Yim, M.-J.; Lee, J.M.; Han, M.H.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; Park, E.K.; et al. Sargassum serratifolium Extract Attenuates Interleukin-1β-Induced Oxidative Stress and Inflammatory Response in Chondrocytes by Suppressing the Activation of NF-κB, p38 MAPK, and PI3K/Akt. Int. J. Mol. Sci. 2018, 19, 2308. [Google Scholar] [CrossRef]
- Liu, C.-C.; Zhang, Y.; Dai, B.-L.; Ma, Y.-J.; Zhang, Q.; Wang, Y.; Yang, H. Chlorogenic acid prevents inflammatory responses in IL-1β-stimulated human SW-1353 chondrocytes, a model for osteoarthritis. Mol. Med. Rep. 2017, 16, 1369–1375. [Google Scholar] [CrossRef]
- Kühn, K.; Hashimoto, S.; Lotz, M. IL-1β Protects Human Chondrocytes from CD95-Induced Apoptosis. J. Immunol. 2000, 164, 2233–2239. [Google Scholar] [CrossRef]
- Pirkmajer, S.; Chibalin, A.V. Serum starvation: Caveat emptor. Am. J. Physiol. Physiol. 2011, 301, C272–C279. [Google Scholar] [CrossRef] [PubMed]
- Iula, L.; Keitelman, I.A.; Sabbione, F.; Fuentes, F.; Guzman, M.; Galletti, J.G.; Gerber, P.P.; Ostrowski, M.; Geffner, J.R.; Jancic, C.C.; et al. Autophagy Mediates Interleukin-1β Secretion in Human Neutrophils. Front. Immunol. 2018, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-Q.; Liu, D.; Li, H.; Jiang, L.-S.; Dai, L.-Y. Interleukin-1β enhances the effect of serum deprivation on rat annular cell apoptosis. Apoptosis 2007, 12, 2155–2161. [Google Scholar] [CrossRef]
- Li, B.; Sun, C.; Sun, J.; Yang, M.-H.; Zuo, R.; Liu, C.; Lan, W.-R.; Liu, M.-H.; Huang, B.; Zhou, Y. Autophagy mediates serum starvation-induced quiescence in nucleus pulposus stem cells by the regulation of P27. Stem Cell Res. Ther. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.H.; Kim, H.K.; Anh, N.T.T.; Kim, N.; Ko, K.S.; Rhee, B.D.; Han, J. Time-dependent proteomic and genomic alterations in Toll-like receptor-4-activated human chondrocytes: Increased expression of lamin A/C and annexins. Korean J. Physiol. Pharmacol. 2017, 21, 531–546. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef]
- McDermott, B.T.; Peffers, M.J.; McDonagh, B.; Tew, S.R. Translational regulation contributes to the secretory response of chondrocytic cells following exposure to interleukin-1β. J. Biol. Chem. 2019, 294, 13027–13039. [Google Scholar] [CrossRef]
- Lo, Y.Y.C.; Cruz, T.F. Involvement of Reactive Oxygen Species in Cytokine and Growth Factor Induction of c-fos Expression in Chondrocytes. J. Biol. Chem. 1995, 270, 11727–11730. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, K. Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS). Dose-Response 2014, 12, 288–341. [Google Scholar] [CrossRef]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Chapter 4: Reactive Oxygen Species, Oxidative Damage and Cell Death. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 45–55. [Google Scholar] [CrossRef]
- Jiang, L.P.; Li, L.J.; Gong, D.Z.; Geng, C.Y.; Jiang, L.J.; Zhong, L.F. Monosodium iodoacetate induces apoptosis of primary rat chondrocytes. Chin. J. Tissue Eng. Res. 2013, 17, 247–253. [Google Scholar] [CrossRef]
- ThermoFisher Scientific. Cell Culture Media Formulation Tool. Available online: https://www.thermofisher.com/my/en/home/life-science/cell-culture/mammalian-cell-culture/classical-media/gibco-media-formulation-tool.html (accessed on 1 February 2021).
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrition 2018, 10, 1564. [Google Scholar] [CrossRef]
- Jagtap, J.C.; Chandele, A.; Chopde, B.; Shastry, P. Sodium pyruvate protects against H(2)O(2) mediated apoptosis in human neuroblastoma cell line-SK-N-MC. J. Chem. Neuroanat. 2003, 26, 109–118. [Google Scholar] [CrossRef]
- Babich, H.; Liebling, E.J.; Burger, R.F.; Zuckerbraun, H.L.; Schuck, A.G. Choice of DMEM, formulated with or without pyruvate, plays an important role in assessing the in vitro cytotoxicity of oxidants and prooxidant nutraceuticals. In Vitro Cell. Dev. Biol.-Anim. 2009, 45, 226–233. [Google Scholar] [CrossRef]
- Yen, Y.-W.; Lai, Y.-J.; Kong, Z.-L. Apoptosis associated with osteoarthritis inhibited by Shiikuwasha extract via down-regulating JAK2/SATA3 pathway in SW1353 cells. Int. J. Food Sci. Nutr. 2019, 4, 68–76. [Google Scholar]
- Zhuang, C.; Xu, N.-W.; Gao, G.-M.; Ni, S.; Miao, K.-S.; Li, C.-K.; Wang, L.-M.; Xie, H.-G. Polysaccharide from Angelica sinensis protects chondrocytes from H2O2-induced apoptosis through its antioxidant effects in vitro. Int. J. Biol. Macromol. 2016, 87, 322–328. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lu, N.; Zhou, Z. Cellular and nuclear degradation during apoptosis. Curr. Opin. Cell Biol. 2009, 21, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, C.F.; Broek, N.J.F.V.D.; Postma, P.; Berger, R.; Brenkman, A.B. A Protocol for Quantifying Lipid Peroxidation in Cellular Systems by F2-Isoprostane Analysis. PLoS ONE 2013, 8, e80935. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, K.-L.; Chow, Y.Y.; Leong, L.M.; Law, J.X.; Ghafar, N.A.; Soelaiman, I.N.; Chin, K.-Y. Establishing SW1353 Chondrocytes as a Cellular Model of Chondrolysis. Life 2021, 11, 272. https://doi.org/10.3390/life11040272
Pang K-L, Chow YY, Leong LM, Law JX, Ghafar NA, Soelaiman IN, Chin K-Y. Establishing SW1353 Chondrocytes as a Cellular Model of Chondrolysis. Life. 2021; 11(4):272. https://doi.org/10.3390/life11040272
Chicago/Turabian StylePang, Kok-Lun, Yoke Yue Chow, Lek Mun Leong, Jia Xian Law, Norzana Abd Ghafar, Ima Nirwana Soelaiman, and Kok-Yong Chin. 2021. "Establishing SW1353 Chondrocytes as a Cellular Model of Chondrolysis" Life 11, no. 4: 272. https://doi.org/10.3390/life11040272
APA StylePang, K.-L., Chow, Y. Y., Leong, L. M., Law, J. X., Ghafar, N. A., Soelaiman, I. N., & Chin, K.-Y. (2021). Establishing SW1353 Chondrocytes as a Cellular Model of Chondrolysis. Life, 11(4), 272. https://doi.org/10.3390/life11040272








