Abstract
This paper introduces a variant of entropy measures based on vertex eccentricity and applies it to all graphs representing the isomers of octane. Taking into account the vertex degree as well (degree-ecc-entropy), we find a good correlation with the acentric factor of octane isomers. In particular, we compute the degree-ecc-entropy for three classes of dendrimer graphs.
1. Introduction
This paper introduces a new measure of graph entropy that takes into account the vertex degree and eccentricity. Each vertex is assigned a probability based on its degree and eccentricity. This assignment defines a probability distribution over the vertices of a graph to which Shannon’s entropy function is applied. The result is a numerical value called the eccentricity-entropy of the graph. The main goal of this paper is to study the properties of so-called dendrimer graphs [1,2] by means of eccentricity entropy; the principal result reported here is a good correlation between eccentricity-entropy and an important physical property of dendrimers.
In [1,2], we derived some topological information content of fullerenes. Since fullerenes are three regular graphs, the degree-ecc-entropy and the ecc-entropy explore the same topological information content of a graph. However, dendrimers are completely different. They are hyper-branched macromolecules, and thus, the degree of vertices is important in computing the degree-ecc-entropy. Since the degree-ecc-entropies are based on both the degree and eccentricity of vertices, we chose dendrimers. Here, we first obtain concrete expressions for the graph entropy measures on the defined classes of dendrimers. These results can be useful when applying the measures on the dendrimers for practical applications. Second, we generated numerical results to examine the correlations between the ecc-entropy and the acentric factor of octane isomers. We show that degree-ecc-entropy is highly correlated on dendrimers.
2. Definition and Examples
Let be a graph where V and E denote the sets of vertices and edges, respectively. The distance between two vertices u, v in G, denoted by , is defined as the length of a minimum path connecting them. The eccentricity of a vertex v, denoted by , is given by see [3,4].
A number of measures using Shannon’s entropy function have been introduced and investigated since the 1950s; see [5,6,7,8,9,10,11,12,13,14,15]. The discrete form of this well-known function is defined for a probability vector and has the form ; see [16]. Note that the logarithms used in this paper are all base two.
Measures such as the one introduced here typically define probability distributions over subsets of graph elements. In the present case, the subsets consist of single vertices. A precise definition of eccentricity entropy is as follows. For a given graph , let and , where and . The entropy is called the ecc-entropy based on where:
and . If we put , then we can reformulate Equation (1) as the following equation:
Example 1.
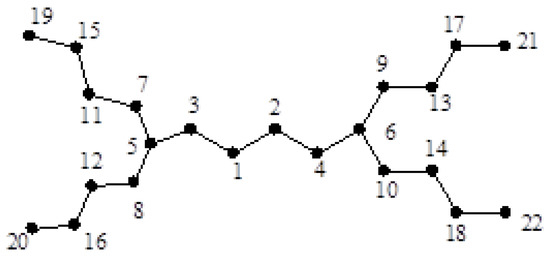
Consider the dendrimer graph shown in Figure 1. It is easy to see that:

Figure 1.
The dendrimer graph .
Furthermore, , , and the degree of other vertices is two. Hence,
and thus:
Consider now two dendrimer graphs and depicted in Figure 2 and Figure 3. Similar to the last example, by computing their eccentricities, one can see that:
and:
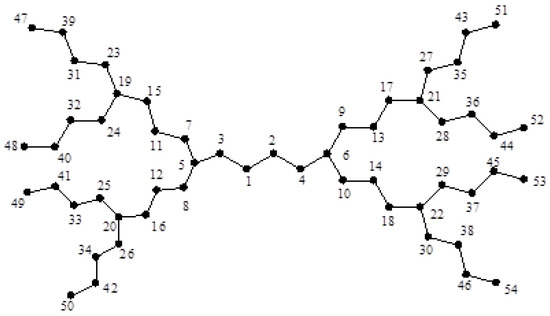
Figure 2.
The dendrimer graph .
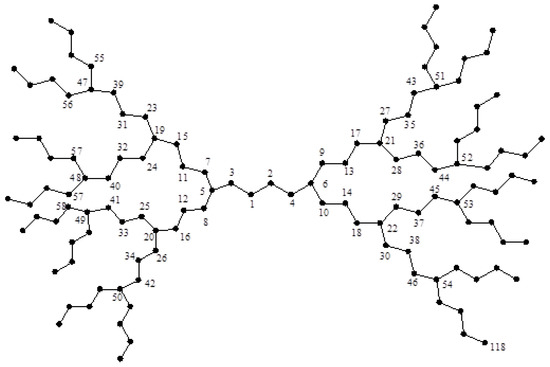
Figure 3.
The dendrimer graph .
Example 2.
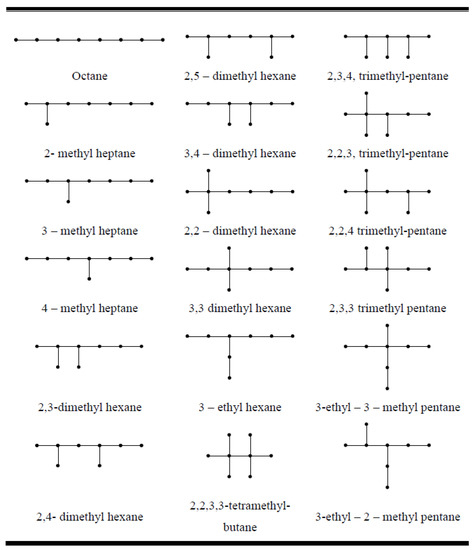

Consider all isomers of octane as shown in Figure 4. The acentric factor and the entropy of the octane isomers are given in Table 1. The ecc-entropy of these isomers can be directly derived from the definition of eccentricity and Equation (1). These values are reported in Table 1. By comparing the values of Table 1, one can see easily that there is a correlation between ecc-entropy and the acentric factor of octane isomers and a correlation between ecc-entropy and S. However, by putting , we achieve a new version of entropy, namely degree-ecc-entropy, in which the correlation between degree-ecc-entropy and the acentric factor of octane isomers increases sharply to . This shows that important physical properties of these molecules can be determined by computing degree-ecc-entropy. Degree-ecc-entropy is a very specialized measure, and of course, other entropy measures might capture other properties of these molecules.

Figure 4.
All octane isomers.

Table 1.
AcenFac, S, ecc-entropy, anddeg-ecc-entropy of the octane isomers.
3. General Results on the Ecc-Entropy of Dendrimers
Two vertices of a graph are said to be similar if one can be mapped into the other by an automorphism. This is important here since it is well known that similar vertices have the same eccentricity. Hence, in a vertex-transitive graph, for all sequences , we have:
As a special case, if for all , then
Proposition 1
([9]). Suppose G is a graph and are all orbits of on . Then:
In addition, if , then:
where
Dendrimers constructed by hyper-branched macromolecules can be arranged in either convergent or divergent form. A dendrimer graph is a molecular graph associated with a dendrimer molecule. The aim of this section is to determine the ecc-entropy of three classes of dendrimers. The first class consists of connected, acyclic graphs, namely trees.
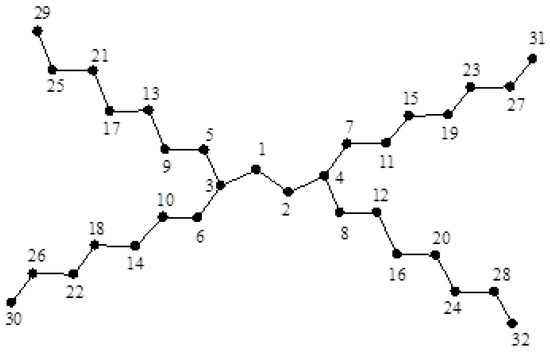
Let be the dendrimer graph shown in Figure 5 with vertices. Note that is isomorphic to the path . Clearly,
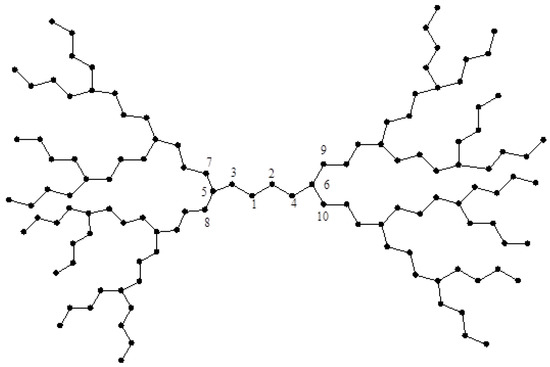
Figure 5.
The dendrimer graph .
Hence, we have the following theorem.
Theorem 1.
For graph , if for then,
where and If in addition , then:
where:
Proof.

Applying our method for graph and in Example 1, we obtain the eccentricity values of vertices in Table 2. It is easy to see that every vertex of has degree 1, 2, or 3. Since has n orbits, there are n types of vertices in and . Computing the eccentricity of each vertex as reported in Table 2, the assertion follows. □

Table 2.
Eccentricity of every vertex of in general.
Example 3.
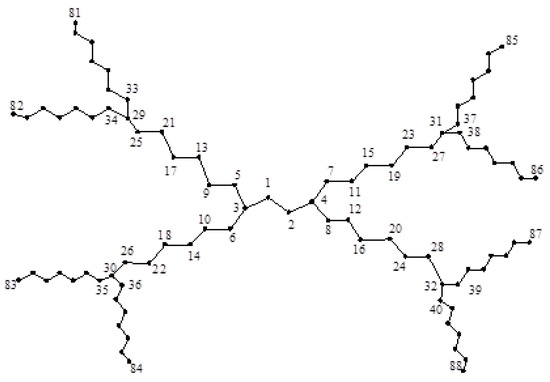
Consider the dendrimer graph as depicted in Figure 6. It is easy to see that:

Figure 6.
The dendrimer graph .
Furthermore, , , and the degree of other vertices is two. Hence,
and thus:

Figure 7.
The dendrimer graph .

Figure 8.
The dendrimer graph .
In general, the number of vertices of is:
Hence, we conclude the following theorem.
Theorem 2.
Proof.

As in the proof of Theorem 1, since there are n orbits for this graph, we can divide the vertices of the graph into n distinct types. Using Table 3, the proof is straightforward. □

Table 3.
Eccentricity of every vertex of where .
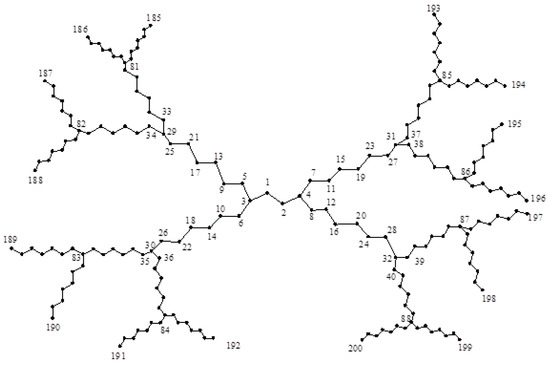
Finally, we are ready to compute the entropy of dendrimer graph shown in Figure 9, Figure 10 and Figure 11. First, we assume ; see Figure 9. It is not difficult to see that:
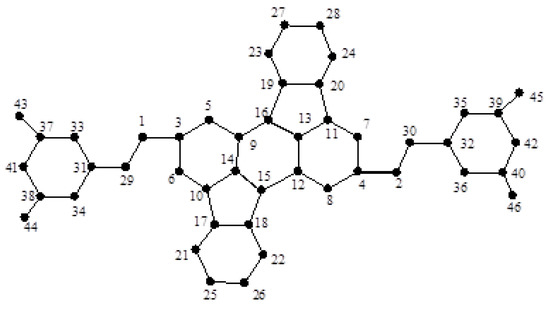
Figure 9.
The dendrimer graph .
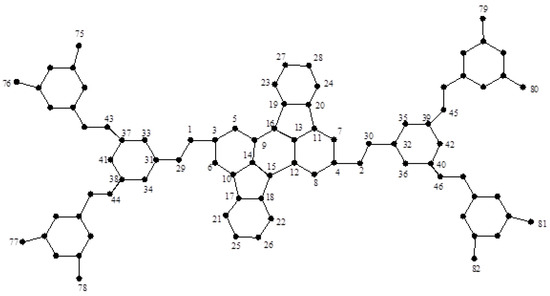
Figure 10.
The dendrimer graph .
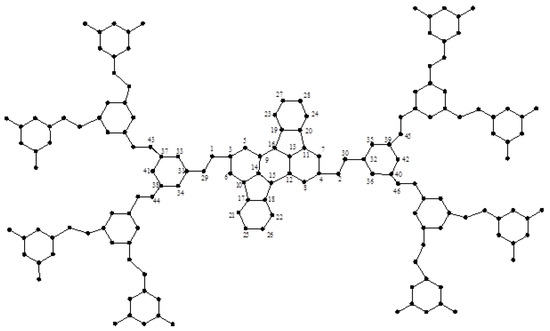
Figure 11.
The dendrimer graph .
Furthermore, , , and the degree of the other vertices is two. Hence,
and thus:
Similarly, we can compute these values for the cases in graph , which is depicted in Figure 10. A direct computation shows that:
Now, consider Table 4 and Figure 11. As found in the previous theorems, there are n types of vertices in graph , and their eccentricities are reported in Table 4. In general, the number of vertices of is:

Table 4.
Eccentricity of every vertex of in the total case.
Hence, we have the following theorem:
Theorem 3.
4. Numerical Results
One method to estimate the complexity of a network is to measure the entropy of network invariants, such as eccentricity or degree sequences; see [17]. On the other hand, the main application of the Shannon entropy is the information content of graphs. In [18], it was shown how the entropy of different graph properties can produce divergent values of entropy for exactly the same graph.
The findings presented in this section show that the correlation between ecc and degree-ecc-entropy measures of three dendrimers is approximately , see Figure 12, Figure 13 and Figure 14. This means that the ecc and degree-ecc-entropy measures might be interesting for further investigations in predicting the physico-chemical properties of molecules [12,14,18,19,20,21,22,23,24]. The reasons for choosing these molecular descriptors is that the dendrimers are hyper-branched molecules the degree and eccentricity of vertices of which are important.
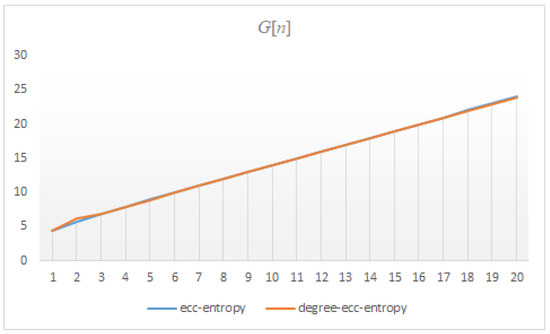
Figure 12.
The correlation between the ecc and degree-ecc-entropies of .
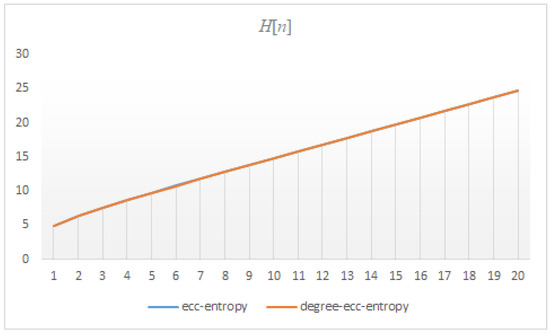
Figure 13.
The correlation between the ecc and degree-ecc-entropies of .
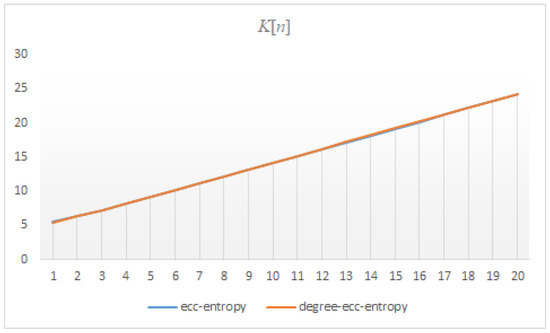
Figure 14.
The correlation between the ecc and degree-ecc-entropies of .
5. Conclusions
In this paper, we studied the mathematical description of chemical structures using information- entropy-based structural descriptors. In other words, the present paper established important correlations between ecc and degree-ecc-entropies of three dendrimers , , and . In [1], the authors focused on fullerenes and computed some results on these structures. Since fullerenes are regular graphs and the degree-ecc-entropies are based on both the degree and eccentricity of vertices, we chose dendrimers, which are highly branched molecules. In future work, we plan to apply our methods to other classes of molecular graphs.
Author Contributions
M.G., M.D., S.Z., A.M., and F.E.-S. wrote the paper.
Funding
Matthias Dehmer thanks the Austrian Science Funds for supporting this work (project P30031).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ghorbani, M.; Dehmer, M.; Zangi, S. Graph operations based on using distance-based graph entropies. Appl. Math. Comput. 2018, 333, 547–555. [Google Scholar] [CrossRef]
- Ghorbani, M.; Dehmer, M.; Zangi, S. On certain aspects of graph entropies of fullerenes. MATCH Commun. Math. Comput. Chem. 2019, 81, 163–174. [Google Scholar]
- Ashrafi, A.R.; Ghorbani, M. Eccentric Connectivity Index of Fullerenes. In Novel Molecular Structure Descriptors–Theory and Applications II; Gutman, I., Furtula, B., Eds.; MCM: Kragujevac, Serbia, 2008; pp. 183–192. [Google Scholar]
- Bonchev, D.; Trinajestić, N. Information theory, distance matrix and molecular branching. J. Chem. Phys. 1977, 67, 4517–4533. [Google Scholar] [CrossRef]
- Dehmer, M.; Emmert-Streib, F.; Shi, Y. Graph distance measures based on topological indices revisited. Appl. Math. Comput. 2015, 266, 623–633. [Google Scholar] [CrossRef]
- Dehmer, M.; Mehler, A. A new method of measuring similarity for a special class of directed graphs. Tatra Mt. Math. Publ. 2007, 36, 39–59. [Google Scholar]
- Dehmer, M.; Mowshowitz, A. Generalized graph entropies. Complexity 2011, 17, 45–50. [Google Scholar] [CrossRef]
- Dehmer, M.; Mowshowitz, A.; Emmert-Streib, F. Connections between classical and parametric network entropies. PLoS ONE 2011, 6, e15733. [Google Scholar] [CrossRef] [PubMed]
- Dehmer, M.; Sivakumar, L.; Varmuza, K. Uniquely discriminating molecular structures using novel eigenvalue-based descriptors. MATCH Commun. Math. Comput. Chem. 2012, 67, 147–172. [Google Scholar]
- Dehmer, M. Strukturelle Analyse web-basierter Dokumente. In Multimedia und Telekooperation, Deutscher Universitäts Verlag; Springer: Wiesbaden, Germany, 2006. [Google Scholar]
- Dehmer, M.; Varmuza, K.; Borgert, S.; Emmert-Streib, F. On entropy-based molecular descriptors: Statistical analysis of real and synthetic chemical structures. J. Chem. Inf. Model. 2009, 49, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Gutman, I.; Furtula, B.; Katanić, V. Randić index and information. AKCE Int. J. Graphs Comb. 2018, 18. [Google Scholar] [CrossRef]
- Kazemi, R. Entropy of weighted graphs with the degree-based topological indices as weights. MATCH Commun. Math. Comput. Chem. 2016, 76, 69–80. [Google Scholar]
- Li, X.; Qin, Z.; Wei, M.; Gutman, I. Novel inequalities for generalized graph entropies graph energies and topological indices. Appl. Math. Comput. 2015, 259, 470–479. [Google Scholar] [CrossRef]
- Dehmer, M.; Emmert-Streib, F.; Shi, Y. Interrelations of graph distance measures based on topological indices. PLoS ONE 2014, 9, e94985. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Morzy, M.; Kajdanowicz, T.; Kazienko, P. On Measuring the Complexity of Networks: Kolmogorov Complexity versus Entropy. Complexity 2017, 2017, 3250301. [Google Scholar] [CrossRef]
- Zenil, H.; Kiani, N.A.; Tegnér, J. Low-algorithmic-complexity entropy-deceiving graphs. Phys. Rev. E 2017, 96, 012308. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.; Nadeem, M.F.; Zahid, Z.; Zafar, S.; Gao, W. Computing certain topological indices of the line graphs of subdivision graphs of some rooted product graphs. Mathematics 2019, 7, 393. [Google Scholar] [CrossRef]
- Aslam, A.; Ahmad, S.; Binyamin, M.A.; Gao, W. Calculating topological indices of certain OTIS Interconnection networks. Open Chem. 2019, 17, 220–228. [Google Scholar] [CrossRef]
- Bonchev, D.; Buck, G.A. Quantitative measures of network complexity. In Complexity in Chemistry, Biology, and Ecology; Springer: New York, NY, USA, 2005; pp. 191–235. [Google Scholar]
- Škrekovski, R.; Dimitrov, D.; Zhong, J.M.; Wu, H.L.; Gao, W. Remarks on multiplicative atom-bond connectivity index. IEEE Access 2019, 7, 76806–76811. [Google Scholar] [CrossRef]
- Zenil, H.; Kiani, N.A.; Tegnér, J. A Review of graph and network complexity from an algorithmic information perspective. Entropy 2018, 20, 551. [Google Scholar] [CrossRef]
- Zenil, H.; Kiani, N.A.; Shang, M.; Tegnér, J. Algorithmic complexity and reprogrammability of chemical structure networks. Parallel Process. Lett. 2018, 28, 1850005. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).