Abstract
The low recovery of fine chalcopyrite particles and the limited Cu/Fe selectivity with conventional thiol collectors prompted the evaluation of a Polystyrene–Divinylbenzene–Cetyltrimethylammonium Bromide–Potassium Amyl Xanthate (PS-DVB-CTAB-PAX) polymeric nanocollector. The copolymer was synthesized by emulsion polymerization and characterized using Total Organic Carbon (TOC) analysis, Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Particle Size Analysis, and contact angle measurement. Its performance was tested in a Hallimond cell (150 mL) using a synthetic industrial water solution (0.010 mol/L NaCl + 0.005 mol/L CaCl2) at a natural pH range of 6.0 to 8.0. PAX concentrations ranged from 0 to 16.19 mg L−1, and nanocollector doses equivalent to 0 to 45 mg g−1 of solid were tested. The nanocollector increased chalcopyrite recovery to 98 ± 1% for the −53 + 38 µm size fraction and maintained values greater than 95% in the coarse fractions, outperforming PAX across the entire dosage range. The PAX + nanocollector combination achieved the same recovery by reducing the total xanthate dosage by one-third, demonstrating a synergistic effect. TOC assays showed preferential adsorption of 96.6% on chalcopyrite versus 86.4% on pyrite, a difference that explains the observed Cu/Fe selectivity (pyrite floatability < 70%). The contact angle of chalcopyrite increased from 56.4° (water) to 86.5° in the presence of the nanocollector, demonstrating the generation of localized superhydrophobicity that reduces interfacial free energy and favors bubble–particle adhesion, whereas pyrite showed lower values of 51.1°, 58.3°, and 75.1°, confirming its more hydrophilic nature. These findings indicate that PS-DVB-CTAB-PAX enables optimized copper sulfide recovery, reduced thiol collector consumption, and improved metallurgical selectivity, making it a promising alternative for flotation circuits with high ionic strength water and for scaling up to pilot tests.
1. Introduction
The flotation of fine particles (<~20 μm) remains a significant challenge in mineral processing. As copper deposits present more complex and lower-grade mineralogy, the generation of fine particles increases after the comminution stages. Unfortunately, traditional thiol-type collectors (e.g., xanthates) show limited performance for this type of particle, where performance is hindered by low bubble–particle collision and adhesion efficiencies. To overcome these limitations, high collector doses are often required, which makes the operation more complex, increases costs, and reduces the quality of the concentrates, since excessive collector doses can induce increased gangue floatability. Numerous studies have shown that, in current flotation processes, a significant amount of fine copper sulfide particles is discharged into the tailings, as reported by []. These tailings, subsequently deposited in storage facilities (TSFs), contain a significant amount of copper that is not recovered. This phenomenon is particularly relevant in the current context of the depletion of conventional copper ores, which has led to considering tailings as a potential source of this metal. Suppes and Heuss-aßbichler [] estimated that TSFs contain approximately 0.13 gigatons of copper, representing 15% of current geological reserves, a figure also supported by [,].
In the last decade, the application of nanotechnology in flotation has emerged as a promising alternative. The search in Scopus using the keyword “nanoparticles flotation collectors” shows a steady increase in research on organic and inorganic nanoparticles as flotation collectors since the 2000s (Figure 1a), with a more pronounced growth in the last decade, particularly for polymer-based collectors (Figure 1b,c). According to Legawiec and Polowczyk [], these reagents are classified based on the properties of their core, which can be chemically modified through the incorporation of specific functional groups. Among the most studied nanocollectors are those based on polystyrene, cellulose, and inorganic materials (Figure 2), with the first two standing out for their effectiveness in modifying surface hydrophobicity. Although the functionalization of inorganic nanoparticles is a well-known procedure [], their application in flotation is recent and still limited due to their low affinity for mineral surfaces, which reduces hydrophobicity and process efficiency [,].
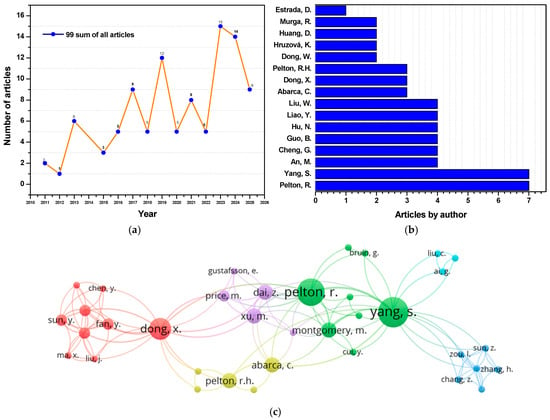
Figure 1.
Scopus search results: (a) Number of documents on organic (polystyrene-based) and inorganic nanocollectors; (b) Leading authors who have used polystyrene-based nanocollectors; (c) Bibliographic map showing co-authorship among researchers who have worked with polystyrene-based nanocollectors.
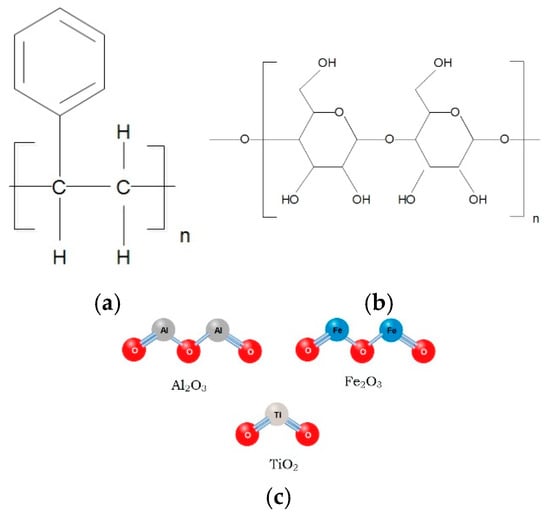
Figure 2.
Variety of cores used in the development of flotation reagents, according to Legawiec and Polowczyk []: (a) polystyrene-based core; (b) cellulose-based core; (c) inorganic nanoparticle-based cores.
In 2011, Yang et al. [] first reported a technology using hydrophobic polymeric nanocollectors designed to adsorb onto the surface of hydrophilic particles, rendering them hydrophobic and facilitating their attachment to air bubbles. In this pioneering study, ~46 nm cationic charged polystyrene nanoparticles (stabilized with CTAB) were able to coat 43 μm glass spheres (simulating mineral particles) and promote their almost complete flotation. Interestingly, a coverage of only ~5% of the glass surface with nanoparticles was sufficient to achieve high efficiencies, in contrast to conventional molecular collectors, which typically require coverage of at least 25% of the surface to achieve acceptable recoveries []. The adsorption of these hydrophobic nanocollectors dramatically increased the particle–bubble adhesion force: the force required to detach a coated particle increased from ~0.0086 μN (for a clean particle) to ~1.9 μN with the nanoparticle coating. These results demonstrate the enormous potential of hydrophobic nanoparticles as collectors, by inducing localized superhydrophobicity at the mineral surface and ensuring stable contact with bubbles, even for microscopic particles [].
Since then, multiple investigations have delved into the development of functionalized polymeric nanocollectors for metal sulfide flotation. Yang et al. [] expanded the concept by evaluating polystyrene nanoparticles in various contexts. They demonstrated that the effectiveness of nanocollectors correlates with their surface hydrophobicity, identifying that a minimum water–nanofilm contact angle of ~50–85° is required to achieve high recoveries. Following Yang et al., the contact angle is determined on nanoparticle-coated surfaces or films formed by the nanoparticles, which allows evaluating the collectively induced hydrophobicity rather than that of isolated particles. They also found that nanoparticles with softer polymer shells (styrene copolymers with n-butyl acrylate, which have a lower glass transition temperature) adhere more firmly to mineral surfaces, acting as more efficient collectors than rigid nanoparticles []. This is attributed to the fact that “soft” nanocollectors can deform slightly upon adsorption, creating larger contact areas and stronger van der Waals forces with the substrate. Similarly, decreasing the size of nanoparticles below ~50 nm tends to improve their collector performance, possibly by increasing the coverage density and penetration at the air-water interface. In parallel, Dong et al. [] investigated the effect of morphology (including anisotropic Janus-type particles) and surface adhesion, concluding that smaller, non-spherical, soft-shelled nanocollector particles achieve higher binding forces and better flotation performance [].
More recently, research has emerged on polymeric nanocollectors functionalized with specific active groups, seeking to improve selectivity and performance in more complex systems. For example, Yang et al. [] explored polystyrene nanocollectors modified with vinyl imidazole groups, demonstrating that they can chelate Ni2+ ions on the surface of pentlandite and promote its selective flotation []. Indeed, these imidazolium-functional nanocollectors achieved specific adsorption on pentlandite, even in the presence of other, more negatively charged particles (e.g., glasses or silicates), thereby displacing slime coatings and detaching impurities from the surface of the valuable mineral. He et al. [] synthesized polystyrene nanoparticles bearing thiazole groups by emulsion polymerization and reported that these sulfur- and nitrogen-functional nanocollectors significantly increased the recovery of ultrafine chalcopyrite. For their part, Abarca et al. [] proposed a ‘nanoparticle library’ approach to choose optimal collectors, and established three key physicochemical characteristics that an effective nanocollector must meet: (1) high surface hydrophobicity, sufficient to adhere to air bubbles; (2) colloidal stability in solution, avoiding aggregation between nanoparticles; (3) adsorption selectivity, so that nanoparticles are preferentially deposited on the valuable mineral (chalcopyrite) and not on the gangue (pyrite or others).
Murga et al. [] developed imidazole-functionalized polystyrene nanoparticles (called St-CTAB-VI) as collectors for chalcopyrite. Their flotation tests showed that incorporating the imidazolium group into the nanocollector substantially improves Cu recovery compared to similar unfunctionalized nanoparticles, which was attributed to specific interactions with Cu–Fe sites on the chalcopyrite surface. An et al. [] extended the use of hydrophobic nanocollectors to the coal sector, demonstrating that the deposition of polystyrene nanoparticles on the carbonaceous surface increased its hydrophobicity and surface roughness, improving bubble capture. Even natural nanomaterials have been explored: Choi et al. [] used hydrophobic talc (a layered silicate) nanoparticles as a collector for fine malakite (Cu2CO3(OH)2), significantly increasing its floatability without using traditional collectors. On the other hand, Hrůzová et al. [] introduced micro- and nanoparticles of organosolv lignin—a wood-derived biopolymer—as a sustainable and biodegradable collector for Cu-Ni-Zn sulfides. Interestingly, these lignin nanocollectors achieved recoveries of more than 80% of chalcopyrite from a Cu-Ni ore. When applied to a complex Cu-Pb-Zn ore, they enabled a 50% reduction in xanthate dosage, yielding final recoveries of up to 91% Cu, 85% Pb, and 98% Zn, surpassing the results achieved with xanthate alone.
Despite the advances described, these reagents have not yet been applied on an industrial scale. First, most studies to date have been conducted in simplified laboratory systems (microflotation tests in deionized water with pure minerals). The performance of nanocollectors needs to be evaluated in more complex environments, for example, using industrial water with high ionic strength (simulating seawater or recycled process water) and the presence of dissolved impurities. Colloidal stability and collector effectiveness can be drastically altered under these conditions due to the compression of the electrical double layer and the possible aggregation of nanoparticles. Second, there is a notable lack of research focused on mixed mineralogical systems. In real-world contexts, chalcopyrite must be selectively floated from pyrite and other gangues; however, most work on nanocollectors has been limited to single-mineral systems. The simultaneous interaction of nanocollectors with multiple sulfides (e.g., a chalcopyrite–pyrite mixture) has not been sufficiently studied. Aspects such as the preferential adsorption of the nanocollector on chalcopyrite versus pyrite, the possible hydrophobic co-aggregation between valuable particles induced by the nanoparticles, or the competition for adsorption sites in the presence of clay coatings remain open questions. Similarly, the compatibility of nanocollectors with traditional reagents (frothers, pH modifiers, depressants) in a complete flotation system remains to be explored. That is, how do nanocollectors behave in a conventional cell with intense agitation, bubbles generated by aeration, and in combination with commercial frothers?
Considering the above, this work hypothesizes that it is possible to improve the recovery of fine chalcopyrite particles under industrial water conditions (water with a certain salinity level) by using functionalized polymeric nanocollectors that maximize adsorption on chalcopyrite and confer robust hydrophobicity resistant to detachment. To verify this hypothesis, the objectives of the study include: (1) synthesize polymeric nanoparticles functionalized with specific groups with affinity towards chalcopyrite, (2) characterize their adsorption on chalcopyrite and pyrite surfaces (evaluating parameters such as zeta potential, contact angle), (3) compare the flotation performance of these nanoparticles versus traditional collectors in microflotation tests, and (4) analyze the physicochemical mechanisms involved in the nanocollector–mineral interaction (role of surface charge, hydrophobic interactions and colloidal structure at the solid/liquid interface).
2. Materials and Methods
2.1. Materials
The chalcopyrite (Durango, Mexico) and pyrite (Huanzala, Peru) samples correspond to primary minerals purchased from Ward’s Science. Using pure minerals ensured controlled mineralogy and reliable evaluation of nanocollector mechanisms under industrial water conditions. Future studies will extend this approach to industrial tailings. Each mineral was crushed separately in a Retsch BB250XL jaw crusher to a size of less than 2 mm (approximately #10 mesh). The retained material (>2 mm) was pre-classified by first removing ferromagnetic impurities using a hand-held magnet and then removing non-magnetic particles under a stereoscopic magnification. The cleaned fraction was homogenized and divided into 12 representative subsamples using a Labtech Herbro rotary divider, which were then combined in pairs to obtain six final batches of each mineral.
Each batch was further reduced using an eight-tube Rotary Micro Riffler, enhancing statistical representativeness. To verify the mineralogical composition, a 5 g subsample of each mineral was ground to <75 µm (200 mesh) in a Retsch RM200 automatic mortar and analyzed by X-ray diffraction (Bruker D8 Advance). The remaining portions were vacuum sealed in polyethylene bags and stored at 4 °C to minimize surface oxidation.
Before microflotation experiments, each batch was ground to the specified particle size fractions (−150 +90, −90 +75, −75 +53, and −53 +38 µm), ensuring consistency between experiments. This comminution and subsampling protocol guaranteed the purity, homogeneity, and traceability required for the procedures described in Section 2.2.
Potassium amyl xanthate (PAX, 97% p.a.; Mathiesen, Santiago, Chile) was used as the reference collector in the micro-flotation assays. Styrene (99%, Sigma-Aldrich), previously deinhibited by alkaline washing and distillation, was used for the synthesis of the nanocollector. The remaining reagents were used without further treatment: sodium hydroxide (NaOH, ≥98%), sodium dodecyl sulfate (SDS, ≥99%), potassium persulfate (KPS, ≥99%), cetyltrimethylammonium bromide (CTAB, ≥98%), and divinylbenzene (DVB, 80%), all supplied by Sigma-Aldrich. All reagents were of analytical grade, stored in amber bottles, and maintained at 4 °C to preserve their purity and reactivity until use.
2.2. Methods
2.2.1. Zeta Potential Measurements
The zero-point charge (ZPC) of chalcopyrite and pyrite samples was determined in a synthetic industrial water solution (SIW) by zeta potential measurements, following the protocol of Valdivieso et al. []. To promote colloidal stability, the −53 + 38 µm fraction was used. In each test, 2 g of minerals were dispersed in 150 mL of SIW, and the pH was adjusted by the gradual addition of HCl or NaOH (0.1 M and 0.01 M). The suspensions were conditioned for 15 min at 25 °C under an air atmosphere. A 0.4 mL sample was subsequently extracted, transferred to an Omega® cuvette, and analyzed in a Litesizer 500® operated in dynamic light scattering mode. Each condition was measured in triplicate for 3 min, and the average value was recorded as the zeta potential. The procedure was replicated identically for both minerals, ensuring comparability and reproducibility. The data obtained allowed for tracing the evolution of the surface charge with pH and locating the point of zero charge, critical information for interpreting the collector-mineral interaction tests described in Section 3.2.
2.2.2. Preparation of Synthetic Industrial Water (SIW)
All micro-flotation experiments were performed at natural pH using the previously described particle fractions and a synthetic industrial water solution (SIW) prepared daily. The SIW was obtained by dissolving 1.17 g of NaCl and 1.109 g of CaCl2 in 2000 mL of deionized (DI) water (18.2 MΩ cm), yielding final concentrations of 0.010 mol/L NaCl and 0.005 mol/L CaCl2. The synthetic industrial water (SIW) employed in this study was prepared following the formulation reported by Nieto et al. [,], which reproduces the ionic strength of industrial media. Accordingly, only Na+ and Ca2+ were included as the dominant cations, while other ions typically present in process waters (e.g., K+, Mg2+, Cu2+, Fe2+/3+, Zn2+, SO42−, NO3−) were not considered in this formulation, to maintain a simplified and controlled system for comparative purposes. Future work should address these additional species to simulate real industrial conditions more accurately.
The physico-chemical properties of the SIW, determined at 23 °C and 1 atm, are summarized in Table 1 and include pH, density, electrical conductivity, and salinity. These parameters were used as a reference to interpret the flotation results obtained under strictly controlled conditions.

Table 1.
Information about the chemical properties of SIW at 23 °C and 1 atm, according to Nieto et al. [].
2.2.3. Preparation of Stock Solutions (PAX and Nanocollector)
The potassium amyl xanthate (PAX) stock solution was prepared by dissolving 0.075 g of collector in 100 mL of deionized water (18.2 MΩ cm), yielding a concentration of 750 mg.L−1. Aliquots were then taken from this solution to achieve final concentrations of 0, 2.02, 4.05, 8.09, 12.14, and 16.19 mg.L−1 in microflotation assays.
The PS-DVB-CTAB-PAX nanocollector was independently prepared at a concentration of 20 g/L by dissolving 2 g of nanoparticles in 100 mL of DI water. In each test, 0–4.5 mL of this suspension was added to the microflotation cell containing 2 g of mineral, corresponding to 0–45 mg g−1 of solid, following the methodology of Murga et al. []. Table 2 summarizes the dosed volumes of PAX and nanocollector, as well as the total volumes and resulting concentrations.

Table 2.
Volumes and concentrations of PAX–Nanocollector mixtures.
Both solutions were prepared immediately before use, stored in amber bottles, and shaken gently to prevent aggregation or degradation, ensuring reproducibility and chemical stability throughout the experiments.
2.2.4. Synthesis of Nanoparticles by Emulsion Polymerization
The PS-DVB-CTAB-PAX copolymer was synthesized by emulsion polymerization (Figure 3a) using a three-necked flask equipped with a condenser, continuous argon purge, and magnetic stirring (Daihan LabTech). Initially, 0.40 g of NaOH and 0.24 g of SDS were dissolved in 100 mL of DI water to generate the micelles. The solution was then transferred to a 250 mL flask, and 3.30 g of styrene, 0.20 g of DVB, 0.20 g of CTAB, and 0.30 g of PAX were added sequentially (Table 3). After 10 min of moderate stirring under an inert atmosphere, the reaction was initiated by the addition of 0.10 g of potassium persulfate (KPS, 0.73 mM) as an initiator. The system was maintained at 70°C for 5 h, controlling the influx of O2 to avoid radical inhibition [,,,].
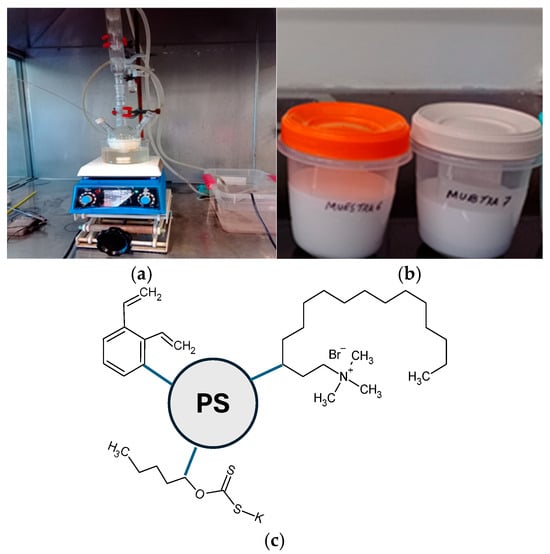
Figure 3.
(a) Experimental setup for batch emulsion polymerization. (b) Obtained polystyrene nanoparticles. Schematic representation of the core–shell structure of the PS-DVB-CTAB-PAX nanocollector. (c) Schematic representation of the core–shell structure of the PS-DVB-CTAB-PAX nanocollector.

Table 3.
Composition of reagents used in nanoparticle synthesis.
After the polymerization, the emulsion was poured into a beaker, and a saturated NaCl solution was added to destabilize it. The nanoparticles were recovered by centrifugation and washed three times with DI water to remove residual surfactants and monomers. The resulting solid was washed in ultrapure water and stored at 4 °C in amber bottles until characterization (Figure 3b) and subsequent use in the micro-flotation tests described in Section 3.
The synthesized copolymer PS-DVB-CTAB-PAX exhibits a core–shell structure (Figure 3c). The polystyrene (PS) forms the hydrophobic core, providing mechanical stability and anchoring sites for functional groups. At the same time, divinylbenzene (DVB) acts as a cross-linking agent, enhancing rigidity and reducing the dissolution of the nanocollector. The cetyltrimethylammonium bromide (CTAB) molecules are oriented toward the particle surface, imparting a positive charge that promotes electrostatic attraction to chalcopyrite, which is slightly negatively charged in synthetic seawater (SIW). Finally, the potassium amyl xanthate (PAX) groups are incorporated on the periphery as specific functional moieties capable of forming complexes with Cu–S sites on the chalcopyrite surface.
The proposed mechanism involves an initial electrostatic adsorption, where the CTAB headgroups interact with the negatively charged chalcopyrite surface, facilitating the attachment of the nanocollector. Subsequently, a specific interaction occurs as PAX groups coordinate with exposed Cu(I) centers, forming stable Cu-xanthate bonds that strengthen the attachment. The formation of localized superhydrophobic domains follows, as the PS–DVB matrix creates a nanometric hydrophobic coating that reduces interfacial energy and increases the contact angle. Finally, Cu/Fe selectivity arises because pyrite presents a lower density of reactive sites and more oxidized surface species, which limit both electrostatic attraction and PAX complexation, resulting in the lower adsorption observed experimentally (TOC and flotation tests).
Overall, the design of PS-DVB-CTAB-PAX reflects a synergistic combination of chemical affinity (PAX–Cu) and electrostatic adsorption (CTAB–charged surface), leading to a hybrid collector with enhanced efficiency toward fine chalcopyrite particles.
2.2.5. Micro-Flotation Experiments
The floatability of chalcopyrite and pyrite was evaluated in a 150 mL glass Hallimond cell. Different concentrations of PAX and nanocollector were tested. Recovery was investigated as a function of particle size, collector/nanocollector dosage, and the use of synthetic industrial water solution (SIW), maintaining the natural pH between 6 and 8.
Each test consisted of two stages: conditioning and flotation. Approximately 2 g of mineral was placed in 150 mL of SIW with the required reagent dosage. The pulp was agitated for 15 min at 535 rpm, and the pH was monitored using a HANNA HI 2221 m. The suspension was then transferred to the Hallimond cell, maintained at 214 rpm, and N2 was injected at a rate of 30 mL min−1 for 3 min (Table 4). After the flotation, the concentrate and tailings were filtered, dried, and weighed. The tests were performed in duplicate, and the differences between replicates were consistently low (<3%–5%), which is a standard and acceptable range in flotation studies. Metallurgical recovery, R (%), was calculated from the masses of concentrate and tailings, ensuring reproducibility before the kinetic experiments described in Section 3. In the Hallimond cell microflotation tests, no frother was used to isolate the effect of the collector (PAX) and the nanocollector on mineral recovery.
where and are the masses of the concentrate and tailings, respectively.

Table 4.
Operational conditions of the micro-flotation tests.
2.2.6. Total Organic Carbon (TOC) Analysis
The adsorption of the PS-DVB-CTAB-PAX nanocollector on the particle surfaces was evaluated by TOC measurements. A reference test was first conducted without minerals to quantify the nanocollector concentration in solution (since no adsorption occurs in this case), and the results were then compared with those from tests performed in the presence of minerals to determine the adsorbed fraction. A stock solution of the nanocollector was then prepared at 20 g L−1 (2.0 g of nanoparticles in 100 mL of DI water, 18.2 MΩ cm).
Assays without minerals. 4.5 mL of the stock solution was added to 145.5 mL of SIW (total volume 150 mL), and the mixture was analyzed directly. An identical aliquot was filtered through a 0.45 µm PTFE membrane and reanalyzed to separate the soluble fraction from the nanoparticle-associated fraction.
Assays with minerals. About 3 g of chalcopyrite or pyrite (−53 + 38 µm) were suspended in 145.5 mL of SIW and stirred for 5 min. Then, 4.5 mL of stock solution was added, and stirring was continued for an additional 3 min to promote adsorption. The resulting suspension was filtered (0.45 µm, PTFE), and the filtrate was analyzed.
All determinations were performed in duplicate at 23 °C and 1 atm using a Shimadzu TOC-L analyzer. Results were expressed in mg/L as C and used to close reagent balances and estimate the adsorbed fraction, information discussed in Section 3.
2.2.7. Contact Angle Measurement
A polished chalcopyrite pellet (Ø 33 mm) was embedded in epoxy resin and cleaned with absolute ethanol (≥99.8%, analytical grade, Sigma-Aldrich) to remove surface contaminants. The contact angle was measured using the sessile drop method with an Attension Theta Lite Optical Tensiometer (Biolin Scientific AB, Göteborg, Sweden) equipped with a USB3 digital camera and controlled through OneAttension software (version 2.4.1).Three systems were evaluated: (i) DI water (reference), (ii) PAX solution, and (iii) PS-DVB-CTAB-PAX nanocollector emulsion. In each test, a 5 µL drop of the corresponding liquid phase was carefully deposited on the previously conditioned mineral surface, and images were recorded at 25 °C. The software automatically calculated the contact angle through contour adjustment, and manual correction was applied as needed. At least five independent measurements were taken by condition, and the average value was reported. The wettability results were subsequently correlated with microflotation recoveries (Section 3) to evaluate the comparative efficiency of the tested collectors.
2.2.8. Scanning Electron Microscopy (SEM-EDX) Analysis
To characterize the synthesized nanoparticles, a Hitachi SU 3500 Scanning Electron Microscope (Hitachi High-Tech Corporation, Tokyo, Japan) was used. Before SEM observation, the nanoparticle samples underwent a metallization process to enhance electrical conductivity and image contrast. This procedure was essential to ensure accurate observation of surface morphology and nanoparticle dimensions, as well as to prevent potential damage to the equipment.
2.2.9. Transmission Electron Microscopy (TEM) Analysis
Additionally, a Talos F200C G2 Transmission Electron Microscope (Thermo Fisher Scientific, Waltham, MA, USA) was employed to obtain a more detailed characterization of the nanoparticles. TEM images were captured, and particle size was manually measured using ImageJ software (version number v1.54p) to perform a quantitative analysis of particle dimensions. The data obtained were subsequently processed and plotted using the Python programming language and Minitab statistical software (version number 3.14.0), allowing for a comparative analysis among the different particle size distributions obtained.
2.2.10. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
For the polystyrene-based nanoparticles, FTIR spectra were recorded in KBr disks using a Perkin Elmer FTIR spectrophotometer model 1600 (Perkin Elmer, Waltham, MA, USA), within the range of 4000 to 450 cm−1.
3. Results and Discussions
3.1. Mineralogical Characterization of Chalcopyrite and Pyrite
X-ray diffraction (XRD) confirmed the presence of the main sulfide phases and quantified the pre-sorting efficiency. The mineral purity values reported in Table 5 and Table 6 were determined by quantitative phase analysis of the XRD patterns obtained before and after magnetic and manual pre-cleaning, using a Bruker D8 Advance diffractometer (Bruker AXS GmbH, Karlsruhe, Germany). Before treatment, chalcopyrite showed mass fractions of 53.3% (−53 + 38 µm) and 49.6% (−150 + 90 µm), accompanied by pyrite (22.7%), magnetite (8.8%), and isocubanite (9.5%) (Table 5, Figure 4a). After magnetic cleaning and manual inspection, the mineral purity increased to 91.3% for chalcopyrite and 94.0% for pyrite (Table 6, Figure 4b), with the virtual disappearance of the secondary phases in the powder patterns (Figure 5).

Table 5.
Crystalline phases identified in chalcopyrite samples before preclassification.

Table 6.
Crystalline phases of chalcopyrite and pyrite after preclassification.
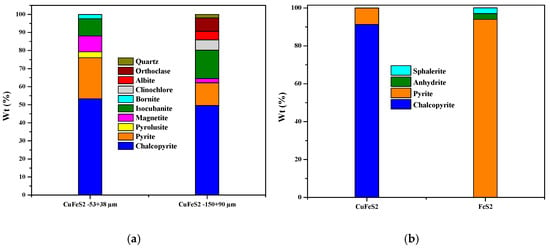
Figure 4.
Modal mineralogy (a) Crystalline phases detected in chalcopyrite before preclassification. (b) crystalline phases of chalcopyrite and pyrite after preclassification.
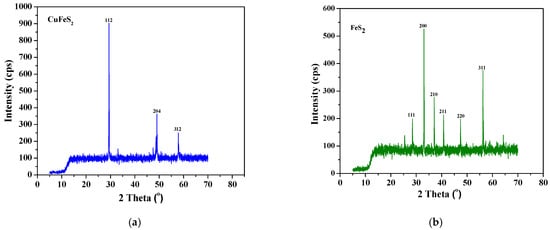
Figure 5.
X-ray Diffraction patterns of (a) chalcopyrite and (b) pyrite.
3.2. Zeta Potential Measurements
Figure 4 compares the evolution of the zeta potential (ζ) of chalcopyrite (Cpy) and pyrite (Py) between pH 4–10 in distilled water (DW) and in the SIW. As shown in Figure 6, in DW, both sulfides maintain a net negative charge throughout the studied range, with magnitudes of approximately −35 mV for pyrite and ≈ approximately −30 mV for chalcopyrite at pH 4. In contrast, the presence of electrolytes in SIW compresses the electrical double layer, shifting the potential towards less negative values (−10 to −15 mV), which brings the point of apparent zero charge (PZC) closer to pH ≈ 3.5–4 for Cpy and ≈4.5 for Py.
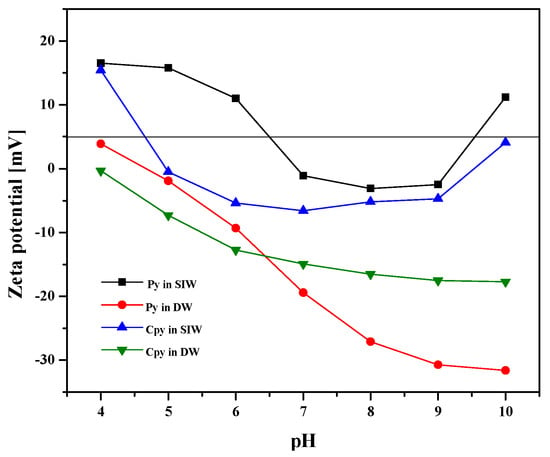
Figure 6.
Zeta potential variation in chalcopyrite and pyrite as a function of pH in SIW Solution and Distilled Water.
Two synergistic phenomena explain this shift: (i) Ionic screening and compression of the double layer. Na+ and, especially, Ca2+ cations reduce the thickness of the diffuse layer (κ−1), decreasing the magnitude of ζ as the threshold of 0.01 M in ionic strength is exceeded []; (ii) Specific adsorption of Ca2+. Calcium exhibits a greater affinity for sulfur-containing and oxo-hydroxylated sites, neutralizing charges, and increasing the potential density in the Stern layer [].
The lower absolute value of ζ under saline conditions has two direct implications for flotation with nanocollectors: (i) Reduction in particle–bubble electrostatic repulsion, favoring bubble adhesion and, therefore, metallurgical recovery; (ii) Facilitating the adsorption of collectors and nanocollectors, since the lower charge density favors short-distance hydrophilic interactions [].
The zeta potential measurements were conducted to validate the electrokinetic behavior of chalcopyrite and pyrite under the specific conditions of this study (synthetic industrial water versus distilled water) and to provide a direct link with the flotation results. Although the general trend of reduced ζ-potential in saline media has been previously reported, our results confirm this effect for the studied system and demonstrate its relevance to nanocollector adsorption. The lower absolute ζ-potential values facilitate the attachment of collectors and nanocollectors, explaining the improved chalcopyrite recovery discussed in Section 3.3.
In SIW, Na+ and Ca2+ ions compress the electrical double layer, reducing the absolute ζ-potential values. This favors collector and nanocollector adsorption by decreasing particle–bubble electrostatic repulsion. The effect was experimentally validated through triplicate zeta potential measurements across a wide pH range and further supported by TOC adsorption and contact angle results, which confirmed preferential affinity toward chalcopyrite.
3.3. Flotation Experiments
In SIW at natural pH, chalcopyrite flotation exhibits two distinct zones (Figure 7a). In the first, between 0 and 4 ppm PAX, all fractions improve sharply: the −150 + 90 µm and −90 + 75 µm particles reach ≈97%–99% at only 2–4 ppm, while the −75 + 53 µm fraction reaches 90% at 8 ppm. This rapid increase reflects the fact that xanthate adsorption kinetics is very efficient for particles ≥ 53 µm: a single monolayer is sufficient to ensure stable bubble–particle collision and adhesion.
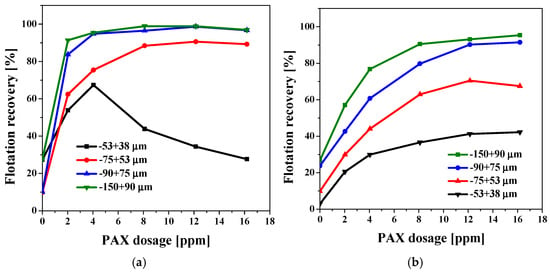
Figure 7.
Flotation recovery of (a) chalcopyrite and (b) pyrite as a function of PAX dosage and particle size in SIW at Natural pH.
The second zone appears when the dosage exceeds the optimum (~4 ppm). The medium and coarse fractions maintain a plateau (≤3 points of variation), but the finest fraction, ≈53 + 38 µm, experiences a drastic drop: it goes from a peak of ≈67% to <35% at 16 ppm. The cause is not a lack of hydrophobicity, but rather a kinetic "bottleneck" governed by the drainage of the liquid film. Having a larger specific surface area and lower inertia, these particles generate a longer, slower-draining film. Excess PAX forms surface multilayers, which raises the osmotic pressure within the film, thereby increasing its interfacial viscosity (Marangoni effect). Water takes too long to escape, the film does not break, and the particle rebounds or is swept away by the upward flow. Hence, a marginal overdosage results in fines alone, leading to losses of up to 40 points of recovery. In pyrite, the metallurgical response varies almost linearly with PAX dosage up to ~8 ppm, and only from 12 to 16 ppm do the coarse fractions (−150 + 90 and −90 + 75 µm) reach a plateau of 92%–95% recovery. This gradual behavior occurs because the surface is partially passivated by ferric oxides and sulfates, allowing the collector to be consumed first in displacing this layer and forming Fe-xanthate complexes that are less hydrophobic than the Cu-xanthate complexes of chalcopyrite. Consequently, the supersaturation that causes multilayering and re-dispersion in chalcopyrite fines is never generated. Furthermore, a large portion of the PAX remains chemically anchored, so that the concentration of free xanthate and K+ counterions in the liquid–gas film remains relatively low, and the interfacial viscosity barely increases. Film drainage does not slow down—not even for the finest fraction (−53 + 38 µm), which maintains a stable, albeit limited, recovery of around 40%. Thus, in pyrite, excess collector does not trigger the post-optimum drop: the mineral acts as a PAX sink until it becomes saturated, and fines recovery is controlled by the collision probability, not by drainage problems, making it clear that optimization should focus on increasing collector coverage (≥12 ppm) or on using nanocollectors capable of activating fines without drastically increasing reagent consumption.
It is important to highlight that the microflotation tests were conducted under nitrogen aeration in the Hallimond cell; however, the pulp conditioning was previously carried out in an open beaker for 15 min. During this period, pyrite can undergo spontaneous surface oxidation upon contact with dissolved oxygen, forming a thin film of Fe3+ oxyhydroxides and ferric sulfates. This film remains stable and retains its integrity even after the pulp is transferred to the Hallimond cell under a nitrogen atmosphere. This explains the different behavior observed between chalcopyrite and pyrite. In pyrite, the oxidized layer acts as a partial barrier, modulating collector adsorption and preventing the over-saturation effect that causes the collapse in fine chalcopyrite. In contrast, chalcopyrite, being more prone to direct interaction with PAX and less likely to form stable films within the same exposure time, exhibits the rebound effect caused by the surface tension gradient. Consequently, the oxidized film on pyrite originates during the air-conditioning stage, which is consistent with the experimental observations.
Figure 8a demonstrates that the PS-DVB-CTAB-PAX nanocollector, without free PAX, completely disrupts the size hierarchy observed with conventional xanthate on chalcopyrite: the fines (−53 + 38 µm) are the first to exceed 90% and reach a plateau close to 98% at just 300 ppm, while the −150 + 90 µm fraction requires twice the dose to match this recovery. This confirms that the nanoparticles act as mobile “hook-up” sites; their positive charge, imparted by CTAB, neutralizes the slightly negative surface of chalcopyrite in SIW and generates hydrophobic microclusters that increase the impact cross section of low-inertia particles, raising the collision frequency and eliminating the kinetic limitation to film drainage that affected pure PAX. When the same nanocollector is fed along with traces of free PAX (Figure 8b), the recovery increases even more rapidly: at ~100 ppm, chalcopyrite of all sizes already approaches 95%. Molecular xanthate first colonizes the high-energy Cu–S sites, lowering the local surface tension and facilitating the formation of a continuous hybrid monolayer by the partially PAX-coated nanoparticles. This synergy prevents supersaturation, allows the total collector dose to be reduced by one-third, and homogenizes the response between coarse and fine fractions.
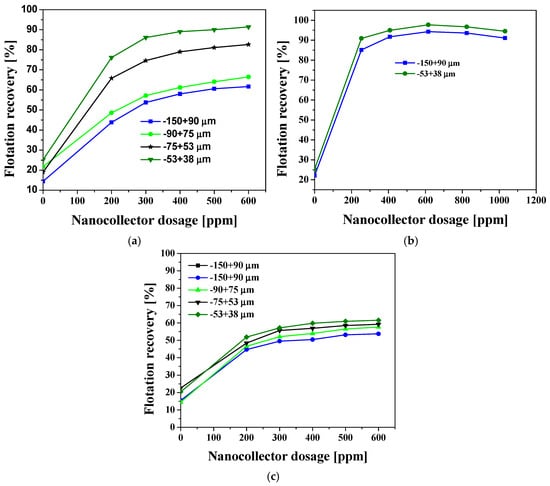
Figure 8.
Flotation recovery of chalcopyrite and pyrite in SIW at Natural pH: (a) chalcopyrite recovery with PS-DVB-CTAB-PAX, (b) chalcopyrite recovery with a mixture of PS-DVB-CTAB-PAX and PAX, and (c) pyrite recovery with PS-DVB-CTAB-PAX.
In Figure 8, it can be observed that, in the absence of reagent, the recovery of the fine fraction (−53 + 38 µm) is higher than that of the coarse fraction (−150 + 90 µm). This behavior is attributed to the fact that fine chalcopyrite particles possess a larger specific surface area, which increases the number of reactive sites and facilitates interaction with bubbles, even without the presence of a collector. Additionally, their partially oxidized or heterogeneous surfaces can generate hydrophobic patches that promote natural floatability, while their low inertia enhances attachment to bubbles under these conditions. In contrast, coarser particles rely more on collector-induced hydrophobicity to achieve efficient flotation, as their natural recovery is limited by surface heterogeneity and a lower probability of effective bubble contact. These factors explain the higher natural recovery observed for the fine fraction compared with the coarse fraction under identical conditions without a reagent.
In contrast, the curves in Figure 8c reveal that, on pyrite, the same nanocollector only allows recovery to be scaled up to 60%–70%, with the reverse order: the fine particles obtain a small additional benefit, but the overall gain is modest. The reason is twofold: the pyrite surface, being more oxidized and acid-base-free and less prone to electrostatic interactions with CTAB, adsorbs fewer nanoparticles, leaving hydrophilic Fe(OH)3 patches exposed; furthermore, the ability of PS-DVB-CTAB-PAX to release PAX by competitive exchange is limited, so that Fe-xanthate complexes are rarely generated. In the absence of supersaturation, the response is primarily controlled by the slow chemisorption of the polymer-anchored collector and by particle–bubble collisions, which explains the premature plateau. Overall, Figure 8 demonstrates that the nanocollector confers inherent Cu/Fe selectivity, as it maximizes chalcopyrite flotation, especially of difficult-to-flotation fines, drastically reduces xanthate consumption when used in a mixture, and simultaneously limits pyrite recovery, resulting in a net improvement in industrial Cu–Fe separation under high-salinity conditions.
The effectiveness of the separation was defined by Cu/Fe selectivity rather than absolute recovery values. Although pyrite exhibited moderate recoveries, the Total Organic Carbon (TOC) results show that the nanocollector displayed preferential adsorption of 96.6% on chalcopyrite compared to 86.4% on pyrite, which limited the floatability of the latter to below 70%. This differential behavior explains the improved separation of chalcopyrite and pyrite.
The reported recoveries correspond to metallurgical values calculated from the masses of concentrate and tailings. For chalcopyrite and pyrite, the feed grade was determined by the high mineral purity (>91% and >94%, respectively, confirmed by XRD). This ensures that the reported values accurately reflect metallurgical performance.
3.4. Adsorption Experiments
Figure 9 shows the adsorption of the PS-DVB-CTAB-PAX nanocollector on the two minerals tested. Adsorption was 96.6% on chalcopyrite and 86.4% on pyrite, a difference of 10.2 % in favor of the valuable mineral, justifying the preferential nature of the collector on the chalcopyrite surface.
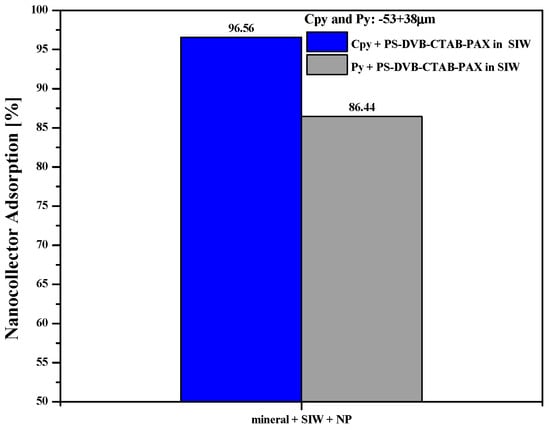
Figure 9.
Nanocollector adsorption on chalcopyrite and pyrite surfaces measured by total organic carbon.
From a physicochemical perspective, the greater adsorption on chalcopyrite was attributed to stronger electrostatic interactions between the slightly negative surface of the copper sulfide and the cationic CTAB groups anchored to the copolymer. Once deposited, the nanoparticles developed localized superhydrophobicity that favored the formation of stable microagglomerates. On pyrite, the higher proportion of oxidized ferric species generated a less attractive surface field and led to lower preferential adsorption, despite sharing the same ionic strength and pH. This behavior reflects that surface chemistry—and not just net charge—governs the nanocollector’s affinity.
Differential adsorption supports the Cu/Fe selectivity observed in the flotation tests. By forming a denser hydrophobic layer on the chalcopyrite, PS-DVB-CTAB-PAX increased the probability of bubble-particle collision and adhesion, thereby boosting fines recovery without the need for overdosing thiol collectors. At the same time, the lower coverage on pyrite limited its floatability, improving overall separation and reducing concentrate dilution.
3.5. Contact Angle Measurements
Figure 10 presents the contact angles measured on chalcopyrite and pyrite under three experimental conditions. For chalcopyrite, the baseline value in deionized water was 56.4°, indicating a moderately hydrophobic surface. After the addition of potassium amyl xanthate (PAX), the contact angle increased to 60.2°, confirming the collector’s ability to induce surface hydrophobicity. Treatment with the PS-DVB-CTAB-PAX nanocollector further increased the angle to 86.5°, demonstrating a significant enhancement in hydrophobicity and placing the system within the range of highly non-wetting surfaces.
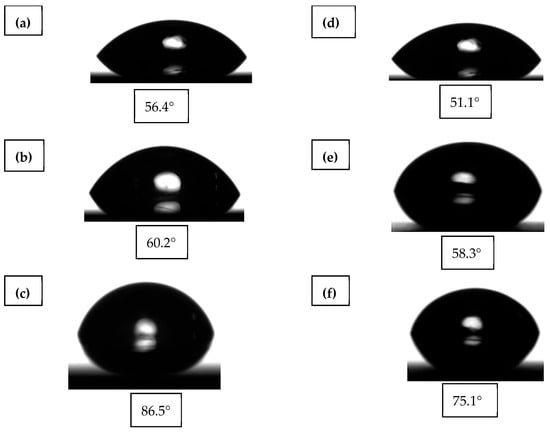
Figure 10.
Average contact angle of chalcopyrite after treatment with: (a) DI Water without collector; (b) PAX (4.05 mg L−1) and (c) PS-DVB-CTAB-PAX. Average contact angle of Pyrite after treatment with: (d) DI Water without collector; (e) PAX (4.05 mg L−1) and (f) PS-DVB-CTAB-PAX.
In the case of pyrite, lower contact angles were obtained under the same conditions, with 51.1° in deionized water, 58.3° after PAX treatment, and 75.1° after exposure to the nanocollector. These values reflect its more hydrophilic nature and the presence of an oxidized surface layer that limits adsorption compared to chalcopyrite. The high contact angles achieved with the nanocollector are attributed to the preferential adsorption of cationic nanoparticles on high-energy Cu–S sites of chalcopyrite, which is favored by electrostatic interactions with the CTAB groups. Once adsorbed, the polymer particles generate localized superhydrophobic domains, reducing solid–liquid interfacial free energy and increasing nanoscale roughness. Consequently, these effects enhance bubble–particle collision and adhesion efficiency, especially for fine particles, reducing the need for excessive thiol collector dosages and reinforcing the Cu/Fe selectivity demonstrated in previous sections.
The contact angle measurements provide preliminary evidence of the enhanced hydrophobic behavior induced by the PS-DVB-CTAB-PAX nanocollector compared to conventional reagents. The progressive increase in the contact angle from 56.4° in distilled water to 60.2° with PAX, and finally to 86.5° with the nanocollector, clearly indicates that surface modification by the polymeric nanoparticles significantly improves chalcopyrite hydrophobicity. In contrast, pyrite displayed smaller variations (from 51.1° to 75.1°), consistent with its partially oxidized surface and lower adsorption capacity. These differences in contact angle values suggest that the nanocollector not only enhances surface hydrophobicity but also contributes to the selective separation of chalcopyrite over pyrite, supporting the trends later confirmed by TOC and flotation results.
3.6. Morphological Analysis of Nanoparticles by SEM-EDX and TEM
Two of the main parameters evaluated in the characterization of nanoparticles (NPs) are size and shape.
Figure 11 presents the characterization of PS-DVB-CTAB-PAX nanoparticles obtained through electron microscopy. The SEM images (Figure 11a) show a spherical morphology with a smooth and uniform surface, while the TEM images (Figure 11b) reveal a tendency toward agglomeration into clusters, yet maintaining a well-defined spherical shape and homogeneous particle size.
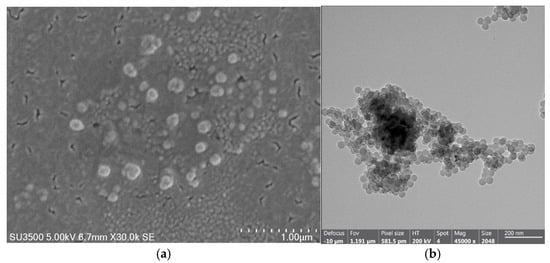
Figure 11.
(a) SEM-EDX image of PS-DVB-CTAB-PAX nanoparticles and (b) TEM image of PS-DVB-CTAB-PAX nanoparticles.
3.7. Particle Size Distribution of the Nanocollectors
Figure 12 shows the particle size distribution of the PS-DVB-CTAB-PAX nanocollectors. Graph (a) corresponds to the frequency histogram, while graph (b) presents the probability plot (Q-Q plot) comparing the empirical distribution with a theoretical normal distribution.
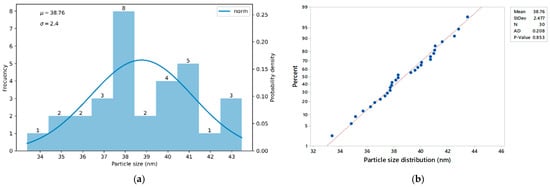
Figure 12.
Particle size distribution: (a) and (b) PS-DVB-CTAB-PAX nanocollector.
The histogram (a), based on the analysis of 30 PS-DVB-CTAB-PAX nanoparticles, reveals a moderately narrow distribution with particle sizes ranging from 33.37 nm to 43.48 nm. Most particles cluster around the mean value of 38.8 nm with a standard deviation of 2.4 nm, indicating low dispersion and suggesting a consistent synthesis process. Moreover, the Q-Q plot (b) confirms a good fit to normality (p-value = 0.853).
3.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
Figure 13 compares the spectra of polystyrene (PS), the PS-DVB-CTAB-PAX copolymer, and the PAX reagent. The PS spectrum exhibits characteristic signals in the regions of 3000–3100 cm−1, attributed to C–H stretching vibrations of the aromatic ring, and 1500–1600 cm−1, corresponding to C=C stretching vibrations of the same ring. When compared with the PS-DVB-CTAB-PAX copolymer spectrum, notable differences are observed, particularly in the 1100–1300 cm−1 region, where new bands appear, indicating the incorporation of functional groups derived from copolymerization.
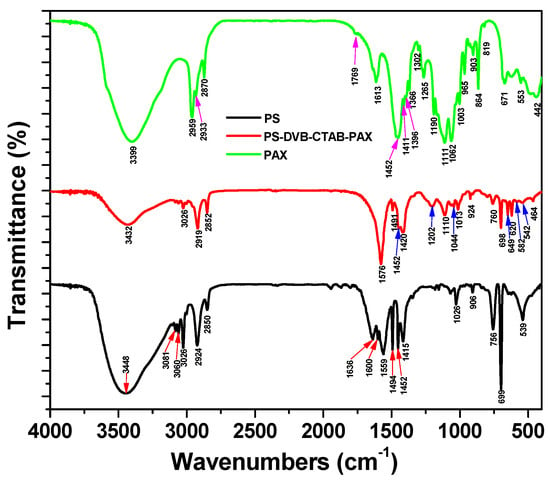
Figure 13.
IR spectra of PS, PS-DVB-CTAB-PAX copolymer, and PAX.
The PAX reagent spectrum is characterized by signals in the 3000–2800 cm−1 range, associated with –C–H groups (CH3 at 2959 cm−1, CH2 at 2933 cm−1, and CH3 at 2870 cm−1) [,]. Additionally, a band at 1769 cm−1 corresponds to the C=O bond of a ketone or aldehyde, characteristic of PAX due to the use of n-amyl alcohol in its synthesis. The signals between 1452 and 1003 cm−1, together with the intense band at 1062 cm−1, are assigned to C=S bond vibrations (carbon disulfide) [,].
It is noteworthy that the signals at 1452 cm−1 and 1411 cm−1 observed in the PAX spectrum coincide with those at 1452 cm−1 and 1420 cm−1 in the PS-DVB-CTAB-PAX copolymer, confirming the presence of the C=S group in the copolymeric structure [,]. Likewise, the band at 2933 cm−1, associated with the –C–H (CH2) group, suggests the effective integration of PAX into the copolymer [,].
FTIR spectra confirmed the incorporation of PAX functional groups into the PS-DVB-CTAB-PAX structure, as evidenced by the characteristic C=S (1062 cm−1) and CH2 (2933 cm−1) bands. These results, combined with SEM and TEM analyses, demonstrate the hybrid chemical–electrostatic adsorption mechanism of the nanocollector on chalcopyrite.
4. Conclusions
The results show that the PS-DVB-CTAB-PAX nanocollector represents a significant advance in reagent engineering for copper sulfide flotation. At the metallurgical level, the reagent increased the overall recovery of chalcopyrite to 98% in the −53 + 38 µm fraction, significantly outperforming potassium amyl xanthate (PAX), whose maximum recovery of fine particles failed to reach 70%.
The results show that the PS-DVB-CTAB-PAX nanocollector offers significant advantages over the conventional PAX collector due to its high specific surface area, which provides multiple simultaneous binding sites (multivalent effect), its ability to be functionalized with chemical groups that allow tuning selectivity toward specific minerals, and its flexible polymeric structure capable of generating superhydrophobic microdomains that enhance bubble–particle adhesion. In this context, the present study serves as proof of concept, demonstrating the feasibility of the PS-DVB-CTAB-PAX nanocollector under simulated industrial water conditions. This establishes the foundation for future research focused on multicomponent systems and validates its performance at the pilot scale.
The combination of the PS-DVB-CTAB-PAX nanocollector with PAX yielded an apparent synergistic effect, increasing recovery to values of >98%, while simultaneously maintaining recoveries above 95% for coarse particles, without the typical decrease associated with thiol collector supersaturation. The synergism is explained by (i) the preferential adsorption of the nanocollector on free surface sites, which seals hydrophilic voids left by PAX, and (ii) the localized superhydrophobicity induced by the nanoparticles, which reduces the interfacial free energy and inhibits re-detachment during drainage of the liquid film.
The total organic carbon, contact angle, and metallurgical recovery results consistently validate the adsorption mechanism of the PS-DVB-CTAB-PAX nanocollector on chalcopyrite and pyrite. Total organic carbon analysis confirmed preferential adsorption of 96.6% on chalcopyrite versus 86.4% on pyrite, correlating with the higher local density of Cu–S sites and the electrostatic affinity of the CTAB groups that promote the formation of a continuous nanometric monolayer. This coating generated localized superhydrophobicity, as evidenced by an increase in contact angle from 56.4° (for water) to 86.5° with the nanocollector. In contrast, pyrite exhibited lower hydrophobicity (75.1°) and reduced adsorption, consistent with its oxidized surface and limited buoyancy (<70%). These physicochemical differences directly translated into flotation behavior, where chalcopyrite achieved recoveries close to 98%, confirming strong Cu/Fe selectivity. From a phenomenological perspective, the nanoparticles acted as adhesion nuclei, enhancing the effective bubble–particle interaction area and mitigating kinetic limitations in fine particles, thereby explaining the superior recovery and selectivity observed experimentally.
The results confirm a direct relationship between the surface adsorption of the nanocollector and the metallurgical recovery of chalcopyrite. The Total Organic Carbon analysis revealed a progressive increase in adsorption with dosage, reaching 96.6% on chalcopyrite, which was reflected in the flotation tests through a proportional rise in recovery. Consistently, the contact angle values increased from 56.4° (in water) to 86.5° with the nanocollector, indicating greater hydrophobicity associated with broader surface coverage. Overall, these findings experimentally support the proposed mechanism, where a higher nanocollector dosage leads to greater adsorption and, consequently, higher recovery of the valuable mineral.
From a practical standpoint, the results obtained with the PS-DVB-CTAB-PAX nanocollector suggest a high potential for application in industrial flotation circuits, particularly in scenarios where maximizing fine particle recovery and reducing the consumption of traditional collectors are required. The observed synergy with PAX and the selectivity toward pyrite indicate that this type of nanocollector could help improve metallurgical efficiency and process sustainability by reducing dependence on conventional reagents. These findings support the feasibility of advancing toward pilot-scale testing in larger systems to evaluate their performance under more complex and representative operational conditions of the mining industry.
Author Contributions
Conceptualization, E.D.M. and R.I.J.; methodology, E.D.M. and Y.L.B.; validation, P.R., L.A.C. and R.I.J.; formal analysis, L.A.C. and R.I.J.; investigation, E.D.M.; data curation, E.D.M. and Y.L.B.; writing—original draft preparation, E.D.M. and R.I.J.; writing—review and editing, P.R. and L.A.C.; supervision, R.I.J. and L.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Centro CRHIAM Project ANID/Fondap/1523A0001, AFB23001 ANID project Agencia Nacional de Investigación y Desarrollo (ANID), FONDECYT Regular N◦ 1211606. Y.B. acknowledges FONDECYT postdoctoral Nº 3240106.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
Luis Cisternas and Ricardo I. Jeldres thank the AFB23001 ANID project. Ricardo I. Jeldres thanks Centro CRHIAM Project ANID/Fondap/1523A0001 and Fondecyt 1211606. Pedro A. Robles thanks the Pontificia Universidad Católica de Valparaíso. Enoque Mathe gratefully acknowledges the financial support provided by Fondecyt Project 1211606 and the Minera Centinela Scholarship. He also extends his appreciation to the Doctoral Program in Mineral Process Engineering at the University of Antofagasta for the infrastructure and institutional support received.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bilal, M.; Park, I.; Hornn, V.; Ito, M.; Hassan, F.U.; Jeon, S.; Hiroyoshi, N. The Challenges and Prospects of Recovering Fine Copper Sulfides from Tailings Using Different Flotation Techniques: A Review. Minerals 2022, 12, 586. [Google Scholar] [CrossRef]
- Suppes, R.; Heuss-aßbichler, S. Resource potential of mine wastes: A conventional and sustainable perspective on a case study tailings mining project. J. Clean. Prod. 2021, 297, 126446. [Google Scholar] [CrossRef]
- Ober, J.A. Mineral Commodity Summaries 2018; USGS: Reston, VA, USA, 2018; ISBN 9781411341999.
- Kapur, A.; Graedel, T.E. Copper Mines Above. Environ. Sci. Technol. 2006, 40, 3135–3141. [Google Scholar] [CrossRef] [PubMed]
- Legawiec, K.J.; Polowczyk, I. Evolution of ideas towards the implementation of nanoparticles as flotation reagents. Physicochem. Probl. Miner. Process. 2020, 56, 280–289. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalisation and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef]
- Hajati, A.; Shafaei, Z.; Noaparast, M.; Farrokhpay, S.; Aslani, S. Investigating the effects of particle size and dosage of talc nanoparticles as a novel solid collector in quartz flotation. Int. J. Min. Geo-Eng. 2019, 53, 1–6. [Google Scholar] [CrossRef]
- Yang, S.; Pelton, R. Nanoparticle Flotation Collectors II: The Role of Nanoparticle Hydrophobicity. Langmuir 2011, 27, 11409–11415. [Google Scholar] [CrossRef]
- Sigauke, T.; Johnson, O.T. A Review of Polystyrene Nanoparticles as Selective Collectors in the Copper Froth Flotation Process. Trans. Indian Inst. Met. 2025, 78, 128. [Google Scholar] [CrossRef]
- Yang, S.; Pelton, R.; Raegen, A.; Montgomery, M.; Dalnoki-Veress, K. Nanoparticle flotation collectors: Mechanisms behind a new technology. Langmuir 2011, 27, 10438–10446. [Google Scholar] [CrossRef]
- Yang, S.; Bi, B.; Razavizadeh, M.; Pelton, R.; Bruin, G. Nanoparticle Flotation Collectors The Influence of Particle Softness. ACS Appl. Mater. Interfaces 2013, 5, 4836–4842. [Google Scholar] [CrossRef]
- Dong, X.; Marway, H.S.; Cranston, E.D.; Pelton, R.H. Relating Nanoparticle Shape and Adhesiveness to Performance as Flotation Collectors. Ind. Eng. Chem. Res. 2016, 55, 9633–9638. [Google Scholar] [CrossRef]
- Yang, S.; Pelton, R.; Abarca, C.; Dai, Z.; Montgomery, M.; Xu, M.; Bos, J.A. Towards nanoparticle flotation collectors for pentlandite separation. Int. J. Miner. Process. 2013, 123, 137–144. [Google Scholar] [CrossRef]
- Estrada, D.; Murga, R.; Rubilar, O.; Amalraj, J.; Gutierrez, L. On the Use of Styrene-Based Nanoparticles to Mitigate the Effect of Montmorillonite in Copper Sulfide Recovery by Flotation. Polymers 2024, 16, 1682. [Google Scholar] [CrossRef]
- He, G.C.; Ding, J.; Huang, C.H.; Kang, Q. Synthesis of nanoparticle emulsion collector HNP and its application in microfine chalcopyrite flotation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 292, 012029. [Google Scholar] [CrossRef]
- Abarca, C.; Ali, M.M.; Pelton, R.H. Choosing mineral flotation collectors from large nanoparticle libraries. J. Colloid Interface Sci. 2018, 516, 423–430. [Google Scholar] [CrossRef]
- Murga, R.; Rodriguez, C.; Amalraj, J.; Vega-Garcia, D.; Gutierrez, L.; Uribe, L. Use of Polystyrene Nanoparticles as Collectors in the Flotation of Chalcopyrite. Polymers 2022, 14, 5259. [Google Scholar] [CrossRef]
- An, M.; Liao, Y.; Gui, X.; Zhao, Y.; He, Y.; Liu, Z.; Lai, Q. An investigation of coal flotation using nanoparticles as a collector. Int. J. Coal Prep. Util. 2020, 40, 679–690. [Google Scholar] [CrossRef]
- Choi, J.; Seo, J.; Kim, S.B.; Kim, W. Flotation behavior of malachite using hydrophobic talc nanoparticles as collectors. Minerals 2020, 10, 756. [Google Scholar] [CrossRef]
- Hrůzová, K.; Matsakas, L.; Sand, A.; Rova, U.; Christakopoulos, P. Organosolv lignin hydrophobic micro- and nanoparticles as a low-carbon footprint biodegradable flotation collector in mineral flotation. Bioresour. Technol. 2020, 306, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Valdivieso, A.; Sánchez Lopez, A.A.; Song, S. On the cathodic reaction coupled with the oxidation of xanthates at the pyrite/aqueous solution interface. Int. J. Miner. Process 2005, 77, 154–164. [Google Scholar] [CrossRef]
- Nieto, S.; Piceros, E.; Toledo, P.G.; Robles, P. Compressive Yield Stress of Flocculated Kaolin Suspensions in Seawater. Polymers 2023, 15, 530. [Google Scholar] [CrossRef]
- Nieto, S.; Toro, N.; Robles, P.; Edelmira, G.; Gallegos, S.; Jeldres, R.I. Flocculation of Clay-Based Tailings: Differences of Kaolin and Sodium Montmorillonite in Salt Medium. Materials 2022, 15, 1156. [Google Scholar] [CrossRef]
- Nuruzatulifah, A.M.; Nizam, A.A.; Ain, N.M.N. Synthesis and Characterization of Polystyrene Nanoparticles with Covalently Attached Fluorescent Dye. Mater. Today Proc. 2016, 3, S112–S119. [Google Scholar] [CrossRef]
- Jung, M.H.; Yun, H.G.; Kim, S.; Kang, M.G. ZnO nanosphere fabrication using the functionalized polystyrene nanoparticles for dye-sensitized solar cells. Electrochim. Acta 2010, 55, 6563–6569. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, S.K.; Lee, J.Y.; Kim, J.; Lee, S. Synthesis of Polystyrene Nanoparticles with Monodisperse Size Distribution and Positive Surface Charge Using Metal Stearates. Macromol. Res. 2008, 16, 178–181. [Google Scholar] [CrossRef]
- Fornasiero, D.; Eijt, V.; Ralston, J. An electrokinetic study of pyrite oxidation. Colloid Surf. 1992, 62, 63–73. [Google Scholar] [CrossRef]
- Angelico, R.; Ceglie, A.; Ji-zheng, H.; Yu-rong, L.; Palumbo, G.; Colombo, C. Chemosphere Particle size, charge and colloidal stability of humic acids coprecipitated with Ferrihydrite. Chemosphere 2014, 99, 239–247. [Google Scholar] [CrossRef]
- Abodinar, A.; Smith, A.M.; Morris, G.A. Short communication A novel method to estimate the stiffness of carbohydrate polyelectrolyte polymers based on the ionic strength dependence of zeta potential. Carbohydr. Polym. 2014, 112, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.; Xiao, Q.; He, N.; Ren, Z.; Lartey, C.; Gerson, A.R. The Influence of Common Monovalent and Divalent Chlorides on Chalcopyrite Flotation. Minerals 2017, 7, 111. [Google Scholar] [CrossRef]
- Cabrera-german, D.; García-valenzuela, J.A.; Martínez-gil, M.; Suárez-campos, G. Applied Surface Science Assessing the chemical state of chemically deposited copper sulfide: A quantitative analysis of the X-ray photoelectron spectra of the amorphous-to-covellite transition phases. Appl. Surf. Sci. 2019, 481, 281–295. [Google Scholar] [CrossRef]
- Mayo, D.W.; Miller, F.A.; Hannah, R.W. Course Notes on the Interpretation of Infrared and Raman Spectra; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; ISBN 9786468600. [Google Scholar]
- Leja, L.H.L.G.W.P.J. Infrared spectra of xanthate compounds 11. Can. J. Chem. 1961, 39, 745–754. [Google Scholar]
- Marín, O.A.; Ordóñez, J.I.; Gálvez, E.D.; Cisternas, L.A. Pourbaix diagrams for copper ores processing with seawater. Physicochem. Probl. Miner. Process. 2020, 56, 625–640. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).