Abstract
Granulated blast furnace slag (GGBFS) is a potential resource of rare earth elements (REEs), and due to the complex mineralogy, extraction by conventional hydrometallurgical process makes it an acid-consuming method. Bioleaching is thus investigated using a chemo-organotrophic bacterium Gluconobacter oxydans (DSMZ 46616) for REE extraction from GGBFS containing 157 ppm Ce, 90 ppm La, 71 ppm Nd and 40 ppm Er, hosted in a Ca-Al-Si matrix. The gluconic acid generation by G. oxydans was assessed for its role in REE extraction from GGBFS. With 5% (w/v) GGBFS using a mixture of a non-adapted and a GGBFS-adapted culture, a maximum solubilization of 67% and 88% Nd was observed after 12 and 40 days of incubation, respectively. The total amount of gluconic acid excreted by the bacteria increased with leaching duration, which contributed to a rise in metal extraction. Scanning electron microscope-energy dispersive analysis (SEM-EDAX) analysis of the solid residue showed bacterial cells in corrosion pits, and thereby assisting in metal solubilization.
1. Introduction
Rare earth elements (REEs) are widely used for traditional sectors, including metallurgy, petroleum and textiles industry, and agriculture. A broad and rapidly expanding range of applications rely upon the chemical, catalytic, electrical, magnetic, and optical REE properties, especially in many high-tech applications such as hybrid cars, wind turbines, compact fluorescent lights, flat-screen televisions, mobile phones, disc drives, and defense technologies. Furthermore, their applications in clean energy technologies and defense systems have brought global attention to REE [1]. Given the growing economic and persistent strategic importance of these sectors, there is no doubt that continuous access to REE resources is strategically important, both in developing and developed countries. However, the distribution of REE all over the world is uneven. The countries with a deficit of land-based resources (mines) are also looking at alternative/secondary sources to obtain the coveted group of metals [2].
During the iron production process, large amounts of blast furnace slag (BFS) are generated, which is estimated to be 175–225 million tons per year worldwide [3]. Blast furnace slag has several potential applications in other industries, such as in the concrete/cement industry and wastewater treatment industry for phosphate and dye removal [4]. The concrete industry aims to replace Portland cement as much as possible with other cementitious materials, such as fly ash, ground granulated blast furnace slag (GGBFS), limestone fines, and silica fume. Some of these materials, such as fly ash or GGBFS, are wastes from industrial processes [5]. Several possible processes for the recycling and recovery of metals from blast furnace slag are under investigation. Base metal recovery from GGBFS has been widely examined [6]. The associated rare earth elements such as La, Ce, Nd, Pr, Y, Er, and Dy in GGBFS [7,8] provide an additional advantage for the aim of circular economy (the residue after extraction can be used for cement making [9,10].
Limited research has been carried out globally on the use of biohydrometallurgy in the extraction of REEs from such industrial wastes [11,12,13,14]. When considering the usage of Gluconobacter (G.) oxydans in bioleaching of REEs, Antonick et al. (2019) performed an extraction of REE from phosphogypsum using four different types of lixiviants such as sulfuric acid, phosphoric acid, commercial gluconic acid, and bio-lixiviant (mostly composed of gluconic acid produced by G. oxydans), where bio-lixiviant leached more amount of Y than sulfuric, phosphoric and commercial gluconic acids with 91.2% leaching recovery [12]. Likewise, Abhilash et al. (2021) used G. oxydans to extract REEs from red mud and achieved 83%–94% Sc in 18–20 d [11]. Using acid leaching, Abhilash et al. (2017) attempted the recovery of REEs from air-cooled blast furnace slag by sulfuric acid leaching [7], resulting in an extraction of 92% La, 36% Ce, 35% Nd, and 52% Er at 1%–5% pulp density (PD). Owing to the characteristics of the material for its amenability towards REE extraction and good recoveries at room temperature by microbial intervention, bioleaching can be attempted to evaluate the efficacy of REE extraction, thereby reducing the use of acids [13,14]. The disruption of phosphate-specific transport system genes in G. oxydans enhances REE bioleaching by up to 18% [15]. This work thus focuses on using the gluconic acid-producing chemo-organotrophic bacterium, G. oxydans to extract REEs from GGBFS, whilst varying the parameters in the presence of non-adapted and adapted cultures. The binding of bacteria on slag surface resulting in REE complexation-cum-dissolution is also highlighted.
2. Materials and Methods
2.1. Blast Furnace Slag
The material used in this study was granulated blast furnace slag (GBFS) which has been collected from TATA STEEL, Jamshedpur. The slag was crushed, ground, and passed through a sieve of 150 mesh size to generate GGBFS. A representative sample was prepared by the coning and quartering method for analysis of its major and critical metals using inductively coupled plasma optical emission spectroscopy (ICP-OES) after dissolution in aqua regia. The morphology and mineralogy of the ground sample was evaluated by SEM, electron probe microanalysis (EPMA), and X-ray diffraction (XRD).
2.2. Growth Characteristics of Gluconobacter oxydans and Adaptation
Gluconobacter (G.) oxydans DSM 46616 was cultivated at 35 °C in DSMZ medium 105 (glucose: 100 g/L; yeast extract: 10 g/L; calcium carbonate: 20 g/L) as advised by the German Collection of Microorganisms and Cell Cultures GmbH (DSMZ, Braunschweig, Germany). G. oxydans oxidizes glucose (carbon source) to gluconic acid, as demonstrated in our previous study on REE bioleaching from red mud [10]. Liquid cultures were supplemented with GGBFS for adaptation with increasing amounts of GGBFS to a final content of 10% (w/v). Cell density, redox potential (Eh), and pH were determined as described previously [10] and used to evaluate the adaptation efficiency.
2.3. Bioleaching Experiments
Bacterial cells as inoculum (10% v/v) were added to 100 mL medium containing 5% (w/v) sterilized GGBFS in a 250 mL Erlenmeyer flask and incubated at 35 °C and 120 rpm, unless stated otherwise. The experiments were conducted in two different modes, i.e., using non-adapted cells and a GGBFS adapted culture at 20% (v/v) inoculum unless specifically described. For adaptation, 100 mL of medium was prepared for G. oxydans in a 250 mL Erlenmeyer flask and sterilized in an autoclave. The sterilized GGBFS sample was added into the media using 5% and 10% pulp densities, and 10% (v/v) bacterial culture was inoculated in the respective flasks, with regular monitoring of redox potential (Eh in mV vs. Ag/AgCl) and pH. Liquid samples were withdrawn at regular intervals to analyze bacterial cell numbers, pH value, redox potential, and concentrations of metals. The cell number was determined in a counting chamber (Petroff Hauser) under a phase-contrast microscope. Control experiments were performed by using distilled water and fresh leaching medium, with addition of mercuric chloride for sterility. Parameters like glucose concentration (10%–40%), pulp density (5%–30% w/v), type of inoculum (adapted vs. non-adapted), and leaching time (0.5–10 h) were varied to observe their effect on the REE extraction. All experiments were run in triplicates with <±1.2% variation. At the end of the leaching period, the leaching residue was filtered, followed by washing with water, and dried overnight at 40 °C. Liquid samples for metal analysis were stabilized by adding a pre-determined volume of leach liquor in 0.65% nitric acid. The recovery of REE and other metals was analyzed using ICP-OES and inductively coupled plasma mass spectroscopy (ICP-MS). The leaching residues were characterized by EPMA and SEM examinations.
3. Results
3.1. GGBFS Characterization
As depicted in Table 1, a high concentration of CaO, Al2O3, and SiO2 was observed in GGBFS via ICP-OES analysis of dissolved samples. Nearly 157 ppm Ce, 90 ppm La, 71 ppm Nd, and 40 ppm Er were detected in GGBFS with ~84% rich matrix of CaO, SiO2, and Al2O3. EPMA mapping analysis (Figure 1) reported a presence of the targeted REEs. It also revealed that concentrations of calcium silicate and aluminosilicates were spatially distributed across the entire sample. However, the desired rare earth elements were found to be accumulated in calcium-rich spots, as shown in the mapping of the elements (Figure 1).

Table 1.
ICP-OES analysis of GGBFS after dissolution in aqua regia.
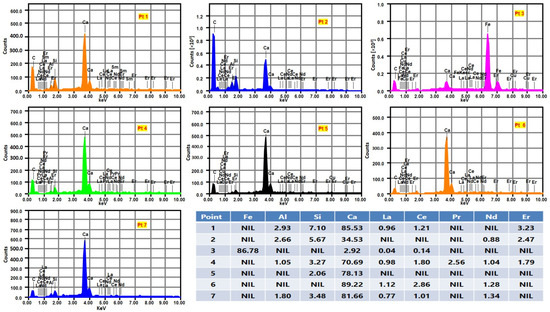
Figure 1.
Mapping of elements and relative distribution of REEs in GGBFS.
3.2. Growth Kinetics and Gluconic Acid Production by G. oxydans
Growth kinetics was determined by cell counting every hour over 5 h. The initial cell count was 7.2 × 106 cells/mL and reached a maximum cell count of 1.9 × 108 cells/mL after 5 h, with a generation time of 3.49 h. The ability of G. oxydans to produce gluconic acid was monitored using the D-gluconic acid estimation kit [10]. A fully grown non-adapted culture of G. oxydans produced 63 g/L gluconic acid in 10 d (Figure 2a).
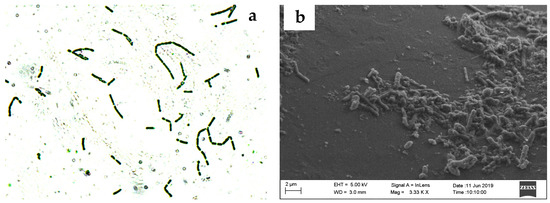
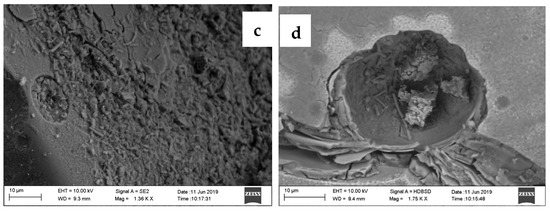
Figure 2.
Microscopic observation of G. oxydans (a) sub-cultured in medium and viewed in light microscopy (1000×); (b) G. oxydans cells adapted on GGBFS surface viewed in SEM; (c) Attachment of adapted G. oxydans on GGBFS; and (d) pitting on the surface of GGBFS.
3.3. Adaptation of G. oxydans on 5%–10% (w/v) GGBFS
In the case of adaptation of 5% PD slag with G. oxydans, the cell count increased from 1 × 108 cells/mL to 1 × 109 cells/mL on day 6 at 5% PD (Figure 3). Figure 4a shows the trend of pH and redox potential in the presence of 5% PD from day 1 to day 6, where the pH decreased from 6.8 to 5.3 within 6 days, while Eh decreased from 165 mV to 40 mV.
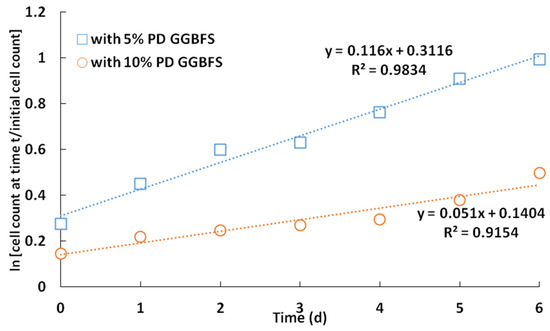
Figure 3.
Growth of G. oxydans in the presence of 5% and 10% PD GGBFS.
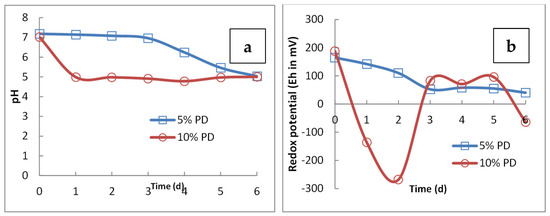
Figure 4.
Variation of (a) pH and (b) redox potential (Eh in mV vs. Ag/AgCl) with time due to activity of G. oxydans in the presence of 5% and 10% PD GGBFS.
In the presence of 10% PD GGBFS, the pH decreased to 5 in 1 day, however, the final pH on the 6th day was 5.2. A characteristic variation in redox potential, likely due to a delayed lag phase of cell growth, was seen. The redox potential increased from the lowest value of −335 mV to −80 mV (Figure 4).
G. oxydans was getting adapted proficiently at a low pulp density of GGBFS and growth occurred at low pH and reduced redox potential. In the case of 10% PD, even though the cell number was lower as compared to 5% PD, the value of Eh was increasing towards more oxidative conditions. Cell attachment to the slag surface was observed (Figure 5).
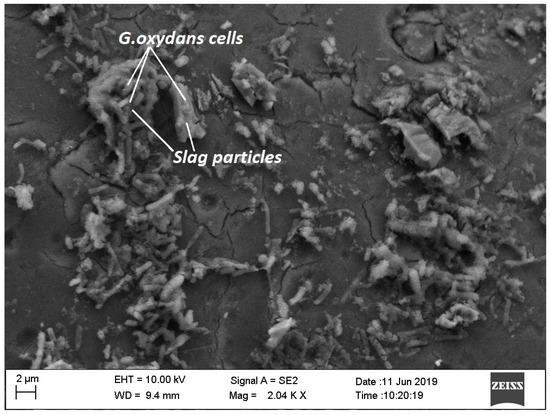
Figure 5.
Attachment of G. oxydans on GGBFS slag particles during adaptation at 5% PD.
3.4. Bioleaching Studies for Extraction of REE from GGBFS
As shown in Figure 6, the use of non-adapted and adapted cells of G. oxydans influenced the recovery of REE from GGBFS. With non-adapted G. oxydans, 13% La, 19.4% Ce, 18.6% Nd, and 11.2% Er using 5% GGBFS, and 11.4% La, 18.7% Ce, 16.2% Nd, and 10.6% Er using 10% GGBFS were measured after 12 d, respectively. The effect of using an adapted culture of G. oxydans improved the bioleaching performance with the slurry of 5% GGBFS, thereby increasing the REE extraction to 43.4% La, 58.1% Ce, 67.4% Nd, and 37.3% Er in 12 d. A slight decrement in REE recovery was seen in the case of 10% PD (Table 2), which could be attributed to the substantial effect of higher solids that impede the secretion of gluconic acid (Table 3). In control sets, owing to no bio-lixiviant, <0.2% total REEs recovery was observed, which could be attributed to the sulfur content in GGBFS. The rise in pulp density beyond 10% (w/v) decreased the REE extraction using non-adapted and adapted cultures of G. oxydans, however, it only improved the Al dissolution in solution under the same conditions. Bioleaching resulted in 39.06 ppm La, 91.26 ppm Ce, 47.86 ppm Nd, and 14.9 ppm Er in leach liquor.
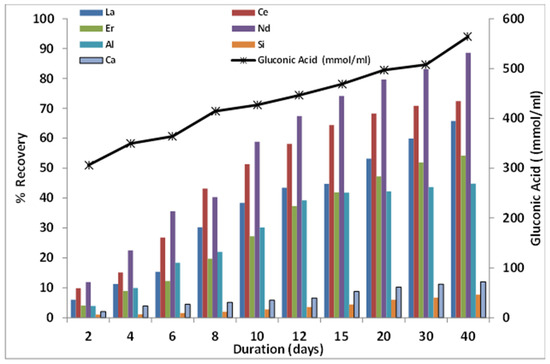
Figure 6.
Variation of REE recovery from 5% (w/v) GGBFS in the presence of varying gluconic acid concentrations using an adapted culture of G. oxydans.

Table 2.
Variation of REE recovery from GGBFS in the presence of adapted and non-adapted cultures of G. oxydans in 12 days.

Table 3.
Variation in gluconic acid concentration (mM) in the presence of different cultures of G. oxydans.
4. Discussion
To assess the role of gluconic acid in extraction of REEs from GGBFS, the leaching period was prolonged over 40 d. It was observed that prolonged duration of leaching led to further decrement of pH from 5.2 on the 6th day to 4.42 on the 12th day and further to 2.86 on the 40th day. As shown in Table 3, the rise in gluconic acid concentration from 12 d (446 mM) to 40 d (564 mM) improved the extraction of REEs, especially Nd, from 67.4% to 88.6%, with a very meager effect on co-extraction of Ca, Al, and Si using 5% (w/v) GGBFS (Figure 6). The evidence of cells attached to GGBFS even after 40 d of leaching (Figure 2c) and the surface corrosion (Figure 2d) likely led to the rise in metal extraction.
The rise in REEs dissolution is linked with the improved secretion of gluconic acid. Gluconic acid forms complexes with lanthanides Gluconic acid, CO2H(CHOH)4CH2OH, is a poly-hydroxylated carboxylic acid with potentially labile protons that can be displaced during metal complexation at low pH. The Ln2Gl complexes formed are anticipated with low log β values due to the different number of H+ ions, favoring easy displacement [16].
5. Conclusions
Ground granulated blast furnace slag (GGBFS) was assessed as a potential resource of REE (La, Ce, Er, Nd). The bioleaching of REE from GGBFS using G. oxydans (DSMZ 46616) was tested in laboratory shake flask experiments with a non-adapted and an adapted culture with two different pulp densities. The recovery of REEs using the non-adapted G. oxydans was 13% La, 19.4% Ce, 18.6% Nd, and 11.2% Er using 5% GGBFS, and 11.37% La, 18.74% Ce, 16.2% Nd, and 10.6% Er using 10% GGBFS, respectively, in 12 d. Using the adapted cultures of G. oxydans, the extraction improved to 42.% La, 56.2% Ce, 65% Nd, and 34.1% Er in 12 d at 10% pulp density. Adaptation of G. oxydans to GGBFS improved the ability of the bacteria to bind to the aluminosilicates matrix and ensure pitting to solubilize the REEs by secretion of gluconic acid. The rise in gluconic acid concentration in 12 d (446 mM) until the 40th day (564 mM) improved the extraction of REEs, especially Nd, from 67.4% to 88.62%, with the very meager dissolution of Ca, Al, and Si.
This method could be useful for leaching of REEs from the huge accumulation of GBFS beds, more like a heap bioleaching phenomena, due to the extended duration of leaching, but stands meritorious due to very minimal gangue dissolution. The work is further investigated for improvement in selective extraction of REEs and base metals (Zn, Pb, etc.), with no dissolution of gangue (Al, Ci, Ca, and Mg), ensuring the holistic utilization of leach residue.
Author Contributions
Conceptualization, A.; methodology, A., S.H. and P.M.; validation, A. and A.S.; formal analysis, A.; investigation, A. and A.S.; resources, A.G., S.H. and S.S.; data curation, A. and A.S.; writing—original draft preparation, A.; writing—review and editing, A.S., S.H., P.M. and A.; visualization, A.; supervision, A.S.; project administration, A.S. and S.S.; funding acquisition, A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded as a part of the CSIR-NML and TATA STEEL Collaboration (SSP-1176). Raman Research Fellowship awarded to Dr. Abhilash by the Council of Scientific and Industrial Research to work at BGR, Hannover, Germany, is acknowledged for the preliminary investigations.
Data Availability Statement
Restrictions apply to the availability of these data.
Acknowledgments
Special thanks to André Marx, Mathias Hilsberg, and Jens Stummeyer for analytical support and assistance at BGR; also, support from Jay Narayan Patel and Rohit, respectively, for analytical and EPMA support at CSIR-NML is deeply acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Y.; Zheng, B. What Happens after the Rare Earth Crisis: A Systematic Literature Review. Sustainability 2019, 11, 1288. [Google Scholar] [CrossRef] [Green Version]
- Abhilash; Akcil, A. Critical and Rare Earth Elements: Recovery from Secondary Resources, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Lidelöw, S. Leaching Behavior of Air-Cooled Blast-Furnace Slag under Intermittent and Continuous Wetting. Ph.D. Thesis, Universitetstryckeriet, Lulea, Sweden, 2011. [Google Scholar]
- Brand, A.S.; Fanijo, E.O. A Review of the Influence of Steel Furnace Slag Type on the Properties of Cementitious Composites. Appl. Sci. 2020, 10, 8210. [Google Scholar] [CrossRef]
- Shanmuganathan, N.; Akbar Basha, S.; Sheikibrahim, K.; Mohammed Fahad, A.S. Ground Granulated Blast Furnace Slag (GGBS OR GGBFS) and Fly Ash (FA) in Concrete—A Study Report. Int. J. Civ. Eng. 2018, 5, 13–17. [Google Scholar]
- Binnemans, K.; Tom Jones, P.; Fernández, A.M.; Torres, V.M. Hydrometallurgical Processes for the Recovery of Metals from Steel Industry By-Products: A Critical Review. J. Sustain. Metall. 2020, 6, 505–540. [Google Scholar] [CrossRef]
- Abhilash; Meshram, P.; Sarkar, S.; Venugopalan, T. Exploring blast furnace slag as a secondary resource for extraction of rare earth elements. Miner. Metall. Process. 2017, 34, 178–182. [Google Scholar] [CrossRef]
- Sinha, S.; Abhilash; Meshram, P.; Pandey, B.D. Metallurgical processes for the recovery and recycling of lanthanum from various resources—A review. Hydrometallurgy 2016, 160, 47–59. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Ehrig, K.; Addai-Mensah, J.; Skinner, W. Recovery of Rare Earth Elements Minerals from Iron-Oxide-Silicate-Rich Tailings: Research Review. Eng 2022, 3, 259–275. [Google Scholar] [CrossRef]
- Abhilash; Meshram, P. Recovery of Rare Earth Elements from Metallurgical Wastes. In Sustainable and Economic Waste Management—Resource Recovery Techniques, 1st ed.; Md Anawar, H., Strezov, V., Abhilash, Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 247–263. [Google Scholar]
- Abhilash; Hedrich, S.; Schippers, A. Distribution of scandium in red mud and extraction using Gluconobacter oxydans. Hydrometallurgy 2021, 202, 105621. [Google Scholar] [CrossRef]
- Antonick, P.J.; Hu, Z.; Fujita, Y.; Reed, D.W.; Das, G.; Wu, L.; Shivaramaiah, R.; Kim, P.; Eslamimanesh, A.; Lencka, M.M.; et al. Bio- and mineral acid leaching of rare earth elements from synthetic Phosphogypsum. J. Chem. Thermodyn. 2019, 132, 491–496. [Google Scholar] [CrossRef]
- Fathollahzadeh, H.; Becker, T.; Eksteen, J.J.; Kaksonen, A.H. Microbial contact enhances bioleaching of rare earth elements. Bioresour. Technol. Rep. 2018, 3, 102–108. [Google Scholar] [CrossRef]
- Schmitz, A.M.; Pian, B.; Medin, S.; Reid, M.C.; Wu, M.; Gazel, E.; Barstow, B. Generation of a Gluconobacter oxydans knockout collection for improved extraction of rare earth elements. Nat. Commun. 2021, 12, 6693. [Google Scholar] [CrossRef] [PubMed]
- Thompson, V.S.; Gupta, M.; Jin, H.; Vahidi, E.; Jindra, M.A.; Nguyen, V.; Fujita, Y.; Sutherland, J.W.; Jiao, Y.; Reed, D.W.; et al. Techno-economic and life cycle analysis for bioleaching rare-earth elements from waste materials. ACS Sustain. Chem. Eng. 2018, 6, 1602–1609. [Google Scholar] [CrossRef]
- Warwick, P.; Evans, N.; Vines, S. Studies on metal gluconic acid complexes. MRS Online Proc. Libr. 2006, 932, 959–966. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).