Physicochemical Conditions of Formation for Bismuth Mineralization Hosted in a Magmatic-Hydrothermal Breccia Complex: An Example from the Argentine Andes
Abstract
:1. Introduction
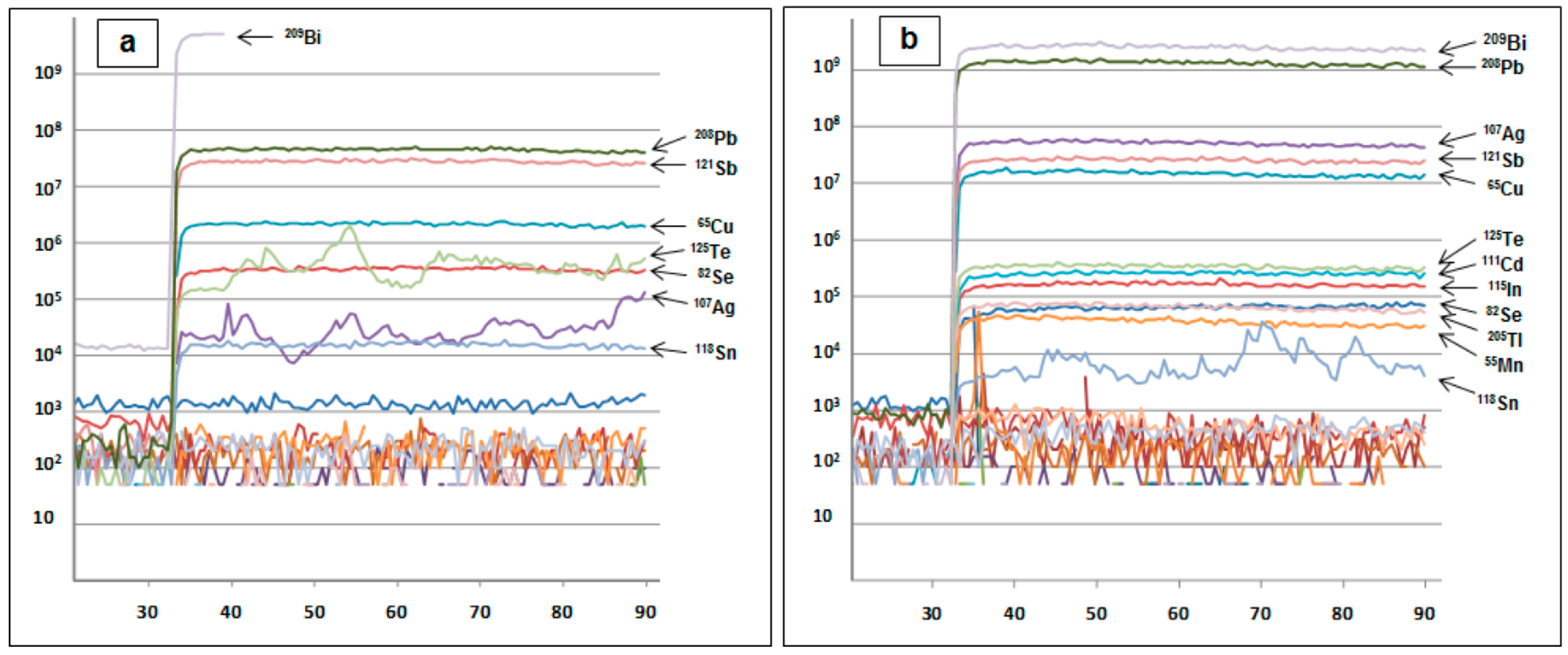
2. Methods
3. Results
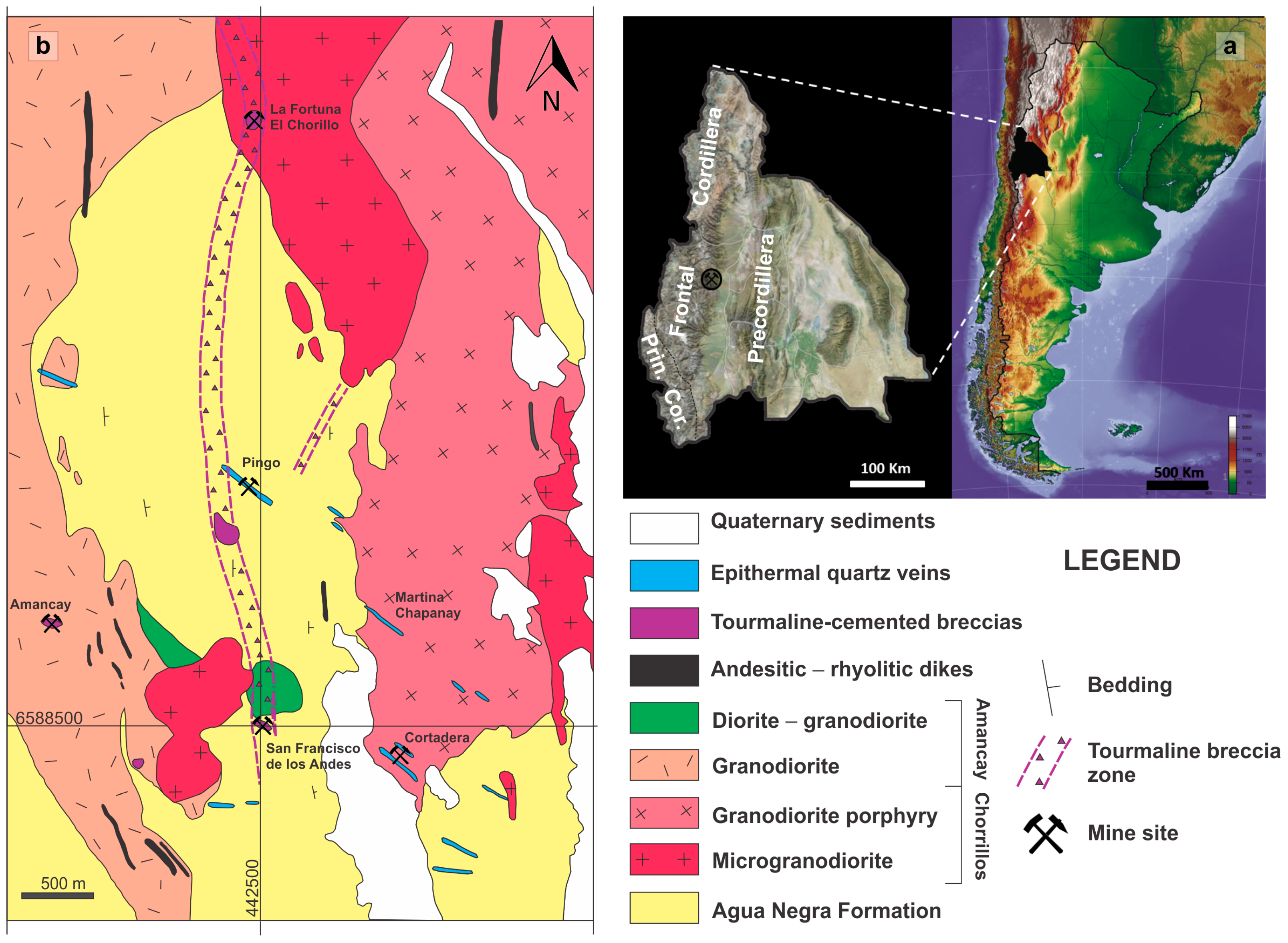
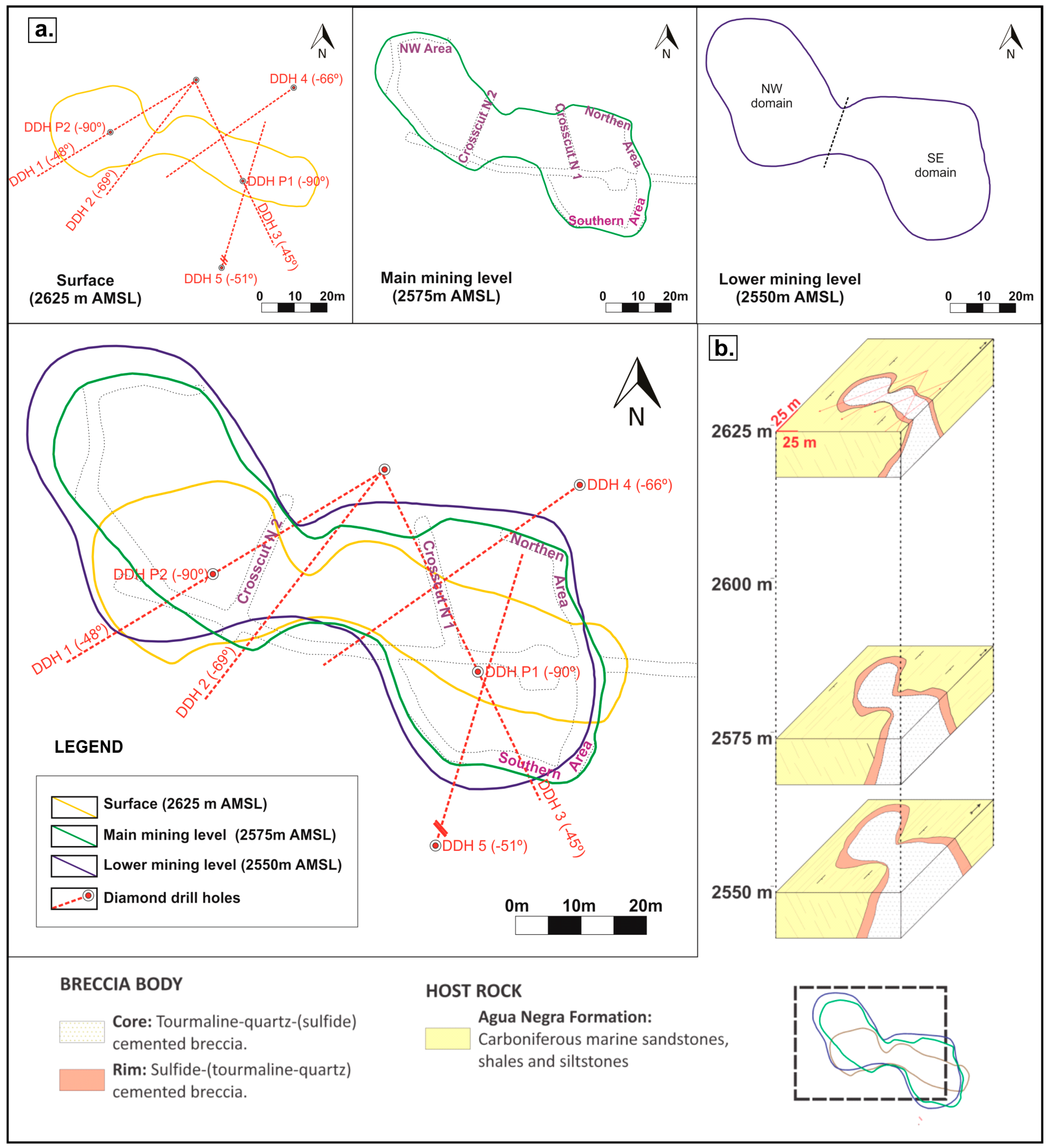
3.1. Morphology and Spatial Dimensions of the Breccia Complex
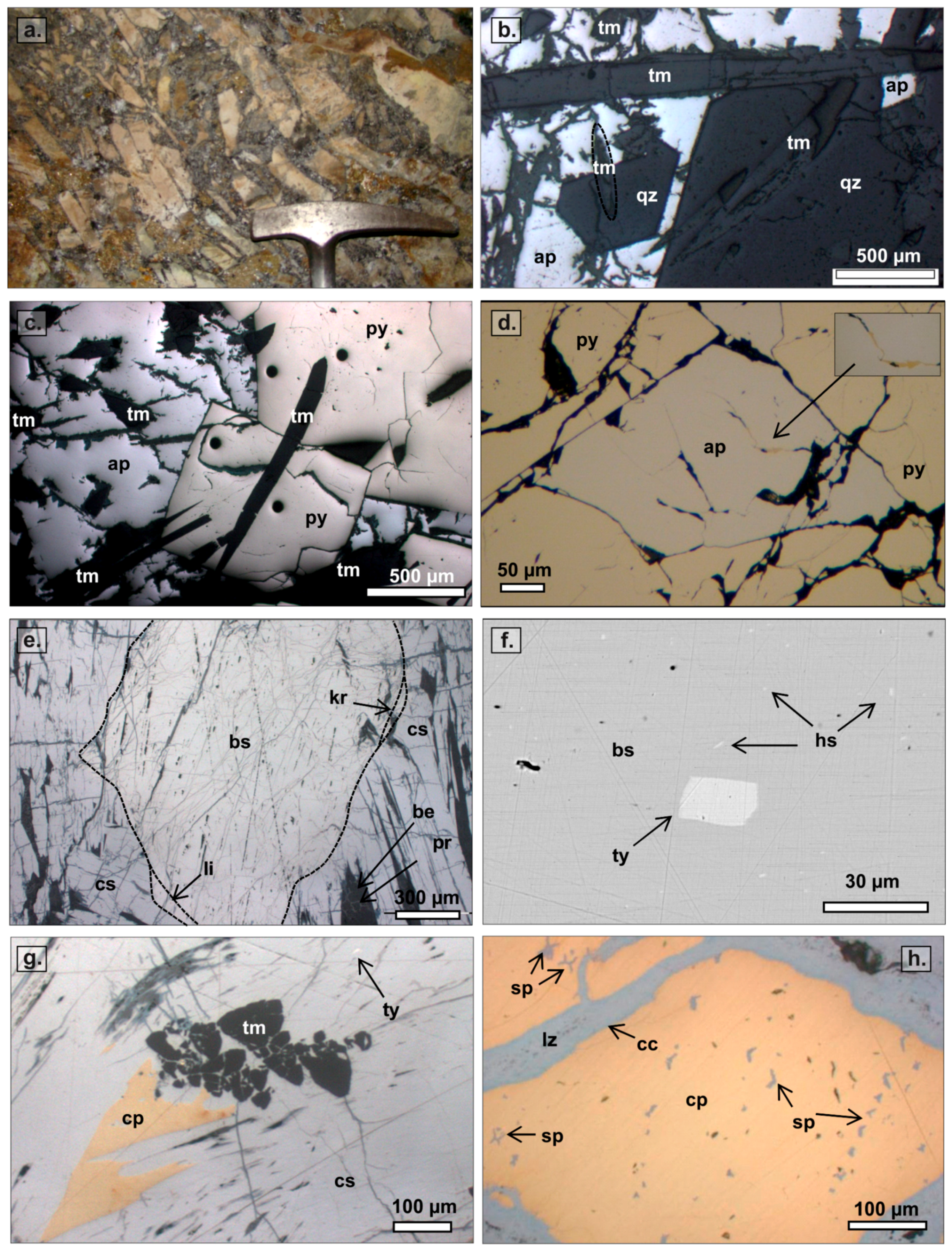
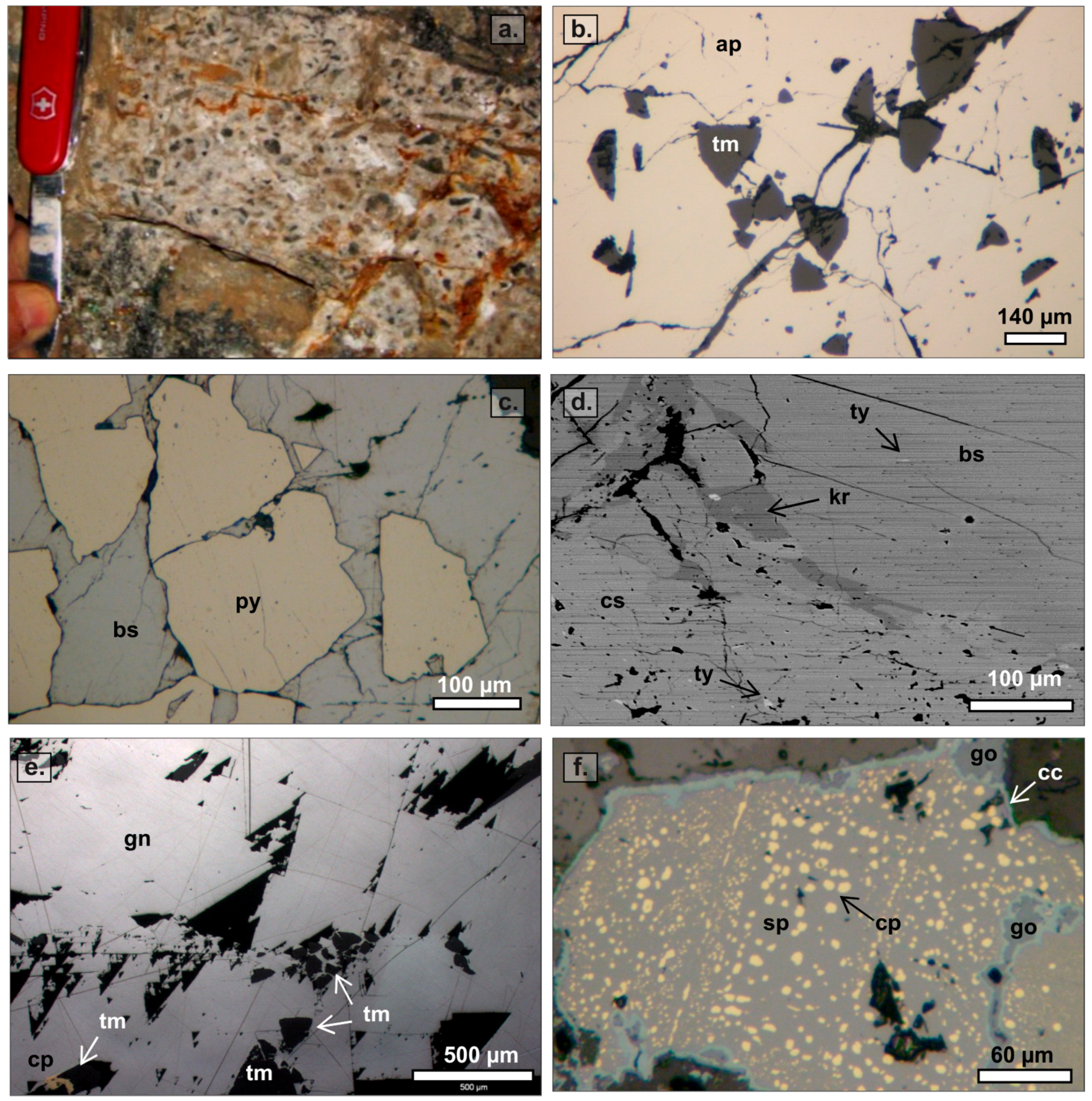
3.2. Paragenetic Sequence
3.2.1. Hydrothermal Cement in the SE Domain
3.2.2. Hydrothermal Cement in the NW Domain
3.3. Bismuth Mineral Species and Related Phases
4. Discussion
4.1. Physicochemical Conditions during Bi Ore Deposition
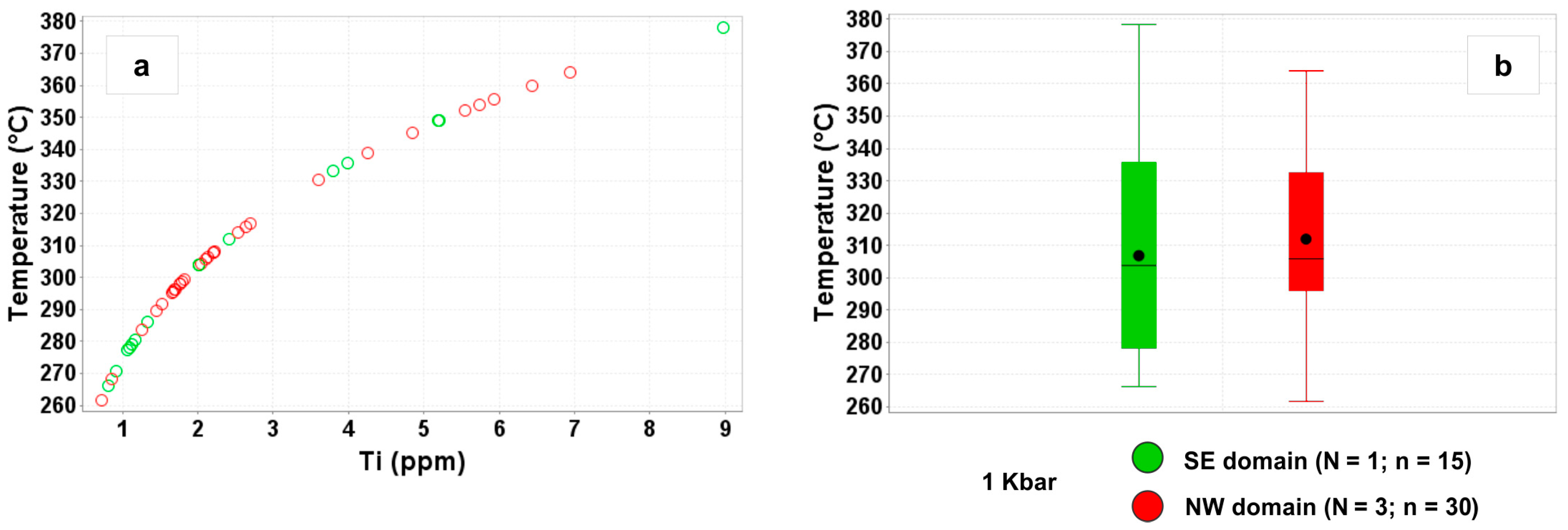
4.1.1. Temperature and Pressure
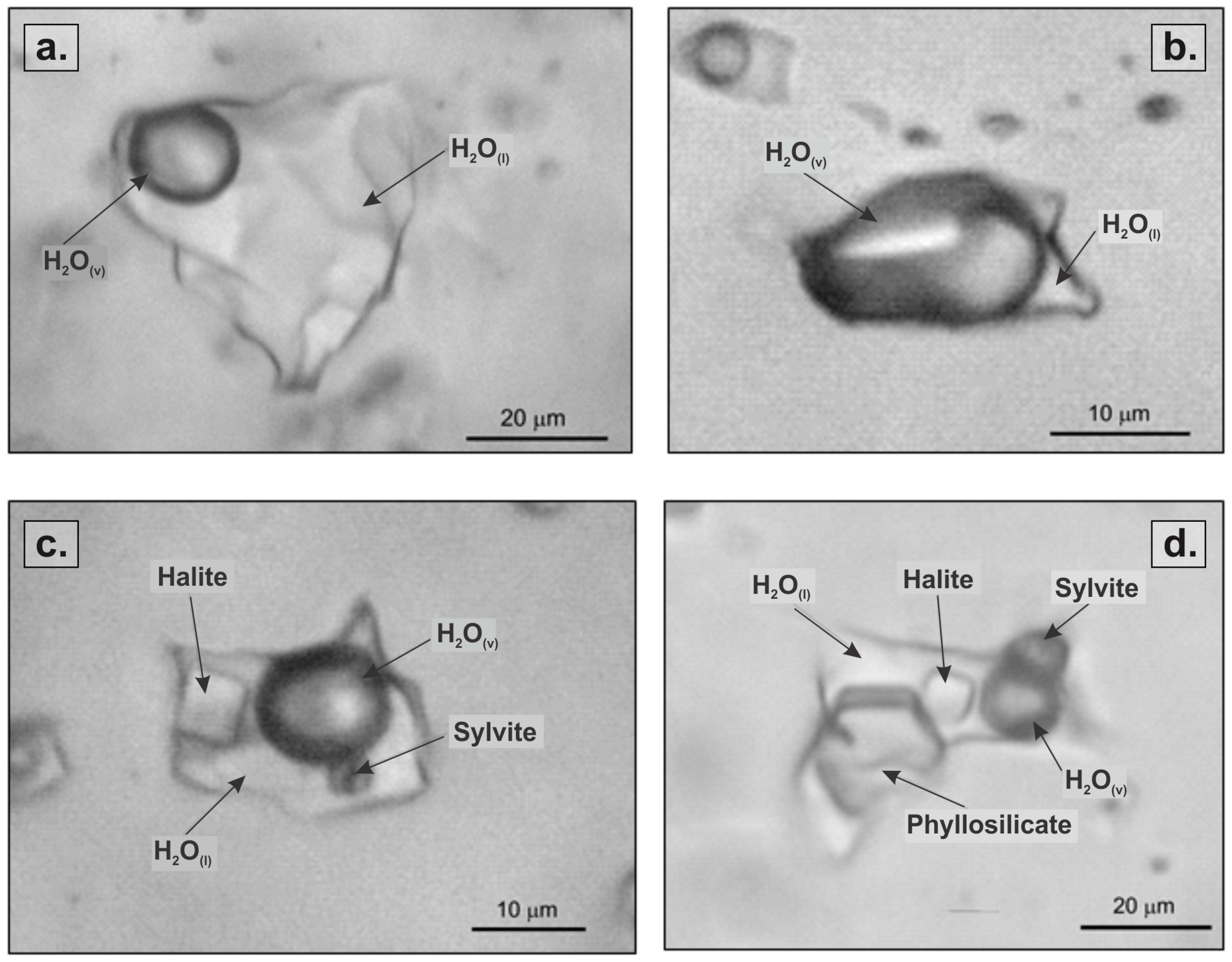
4.1.2. Phase Separation: Vapor-Rich Phase and Dense Brine Phase
4.1.3. Acidity/Alkalinity
4.1.4. Redox Conditions
4.2. Fluid Evolution and Metal Transport: S and Te Fugacities
5. Conclusions
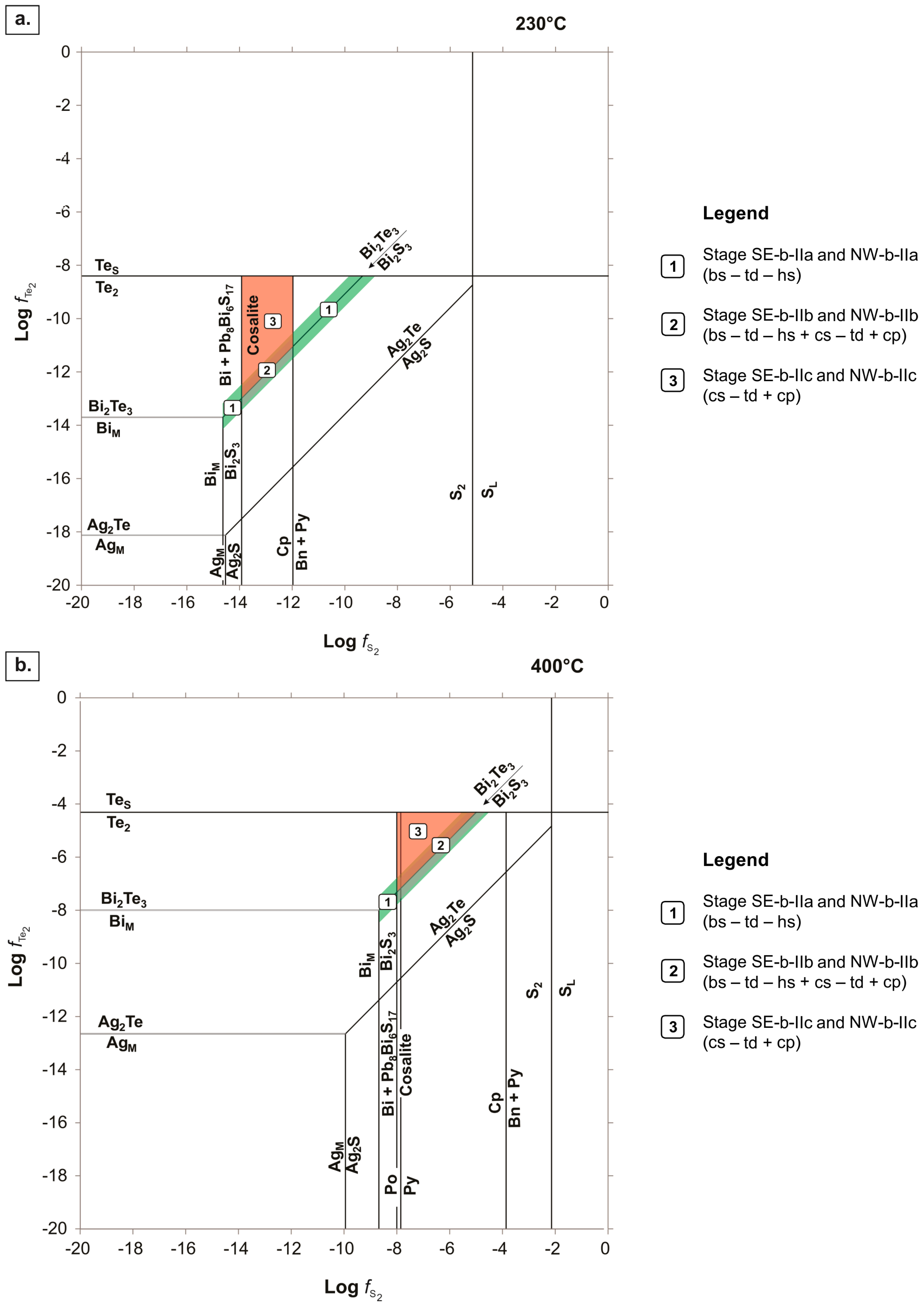
- Bismuthinite (with tetradymite–hessite inclusions): This mineral assemblage formed in equilibrium along the bismuthinite–tellurobismuthite monovariant line. This equilibrium line finishes where it meets the stability fields of BiM and TeS at opposite ends (Figure 10).
- Bismuthinite (with tetradymite–hessite inclusions) + cosalite (with tetradymite inclusions) + chalcopyrite: This mineral assemblage formed under much more restricted conditions than the previous assemblage (i.e., bismuthinite–tetradymite–hessite); along the bismuthinite–tellurobismuthite equilibrium line where it is constrained in the stability field where cosalite and chalcopyrite co-precipitate (at 230 °C), and where cosalite and Te2 coexist (at 400 °C; Figure 10).
- Cosalite (with tetradymite inclusions) + chalcopyrite: This mineral assemblage formed in the stability field constrained by the bismuthinite–tellurobismuthite monovariant line, and the equilibrium lines where cosalite, chalcopyrite, and Te2 are stable (at 230 °C; Figure 10). At 400 °C, the stability field for the cosalite–tetradymite–chalcopyrite assemblage was defined in the area limited by the equilibrium line that indicates the co-existence of tellurobismuthite, cosalite and Te2 (Figure 10).
- High fS2 and fTe2 conditions prevailed during stages SE-b-II and NW-b-II Bi-ore deposition; hydrothermal fluids must have had high aBi and aAg to stabilize Bi-tellurosulfides, sulfosalts, and sulfides, as well as Ag-tellurides and Ag-rich, Bi-sulfosalts.
- Bismuth and Ag telluride/tellurosulfide result from intermittent contributions of magmatic Te2(g) to hydrothermal mineralizing fluids released from the deep-seated crystalizing Tocota Pluton, implying a genetic link between the breccia complex and the underlying magmatic system. Magmatic volatile-rich vapor plumes probably drove fragmentation and buoyantly ascended though the breccia column.
- Abundant galena and sphalerite coupled with the absence of altaite (PbTe) and Pb or Zn-bearing sulfosalts in the NW domain imply lower Te2 and S2 fugacities throughout stage NW-b-III; a drastic drop in fS2 and particularly fTe2 is consistent with intermittent incorporation of Te via magmatic Te2(g)-rich plumes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Llambías, E.J.; Malvicini, L. The geology and genesis of the Bi-Cu mineralized breccia-pipe, San Francisco de los Andes, San Juan, Argentina. Econ. Geol. 1969, 64, 271–286. [Google Scholar] [CrossRef]
- Testa, F.J. Geology, Alteration, Mineralization and Geochemistry of Tourmaline Breccia Complexes in the Andes: Rio Blanco-Los Bronces, Chile and San Francisco de Los Andes, Argentina. Ph.D. Thesis, University of Tasmania, Hobart, Australia, 2017. [Google Scholar]
- Cardó, R.; Segal, S.; Korzeniewski, L.I.; Palacio, M.B.; Chernicoff, C. Estudio Metalogenético de brechas hidrotermales portadoras de mineralización de Bi-Au-Cu en el ámbito de Cordillera Frontal, provincia de San Juan. In Serie Contribuciones Técnicas, Recursos Minerales N° 31; Servicio Geológico Minero Argentino; SEGEMAR: Buenos Aires, Argentina, 2008; pp. 2–28. (In Spanish) [Google Scholar]
- Testa, F.J.; Villanueva, C.; Cooke, D.R.; Zhang, L. Lithological and Hydrothermal Alteration Mapping of Epithermal, Porphyry and Tourmaline Breccia Districts in the Argentine Andes Using ASTER Imagery. Remote Sens. 2018, 10, 203. [Google Scholar] [CrossRef]
- Testa, F.; Cooke, D.; Zhang, L.; Mas, G. Bismoclite (BiOCl) in the San Francisco de los Andes Bi–Cu–Au Deposit, Argentina. First Occurrence of a Bismuth Oxychloride in a Magmatic–Hydrothermal Breccia Pipe and Its Usefulness as an Indicator Phase in Mineral Exploration. Minerals 2016, 6, 62. [Google Scholar] [CrossRef]
- Angelelli, V. Yacimientos Metalíferos de la República Argentina; Comisión de Investigaciones Científicas de la Provincia de Buenos Aires: La Plata, Argentina, 1984. (In Spanish) [Google Scholar]
- Lencinas, A.N. Informe sobre Mina San Francisco de los Andes; Compañía Minera Aguilar S.A.: San Juan, Argentina, 1990. (In Spanish) [Google Scholar]
- Bedlivy, D.; Llambías, E.J. Arseniatos de Cu, de Fe, y de Pb de San Francisco de los Andes, Provincia de San Juan, República Argentina. Rev. la Asoc. Geológica Argentina 1969, 24, 29–40. (In Spanish) [Google Scholar]
- Malvicini, L. Luzonita plumbifera de San Francisco de los Andes provincia de San Juan, República Argentina. Rev. la Asoc. Geológica Argentina 1969, 24, 127–131. (In Spanish) [Google Scholar]
- Bedlivy, D.; Llambías, E.J.; Astarloa, J. Rooseveltit von San Francisco de los Andes und Cerro Negro de la Aguadita, San Juan, Argentinien. Tschermaks Miner. Petrogr. Mitt. 1972, 17, 65–75. [Google Scholar] [CrossRef]
- Bedlivy, D.; Mereiter, K. Preisingerite, Bi3O(OH)(AsO4)2, a new species from San Juan Province, Argentina: Its description and crystal structure. Am. Mineral. 1982, 67, 833–840. [Google Scholar]
- Garrels, R.M.; Christ, C.L. Solutions, Minerals, and Equilibria; Harper & Row: New York, NY, USA, 1965. [Google Scholar]
- Meyer, C.; Hemley, J.J. Wall rock alteration. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Holt, Rinehart and Winston, Inc.: New York, NY, USA, 1967; pp. 166–232. [Google Scholar]
- Robie, R.A.; Waldbaum, D.R. Thermodynamic properties of minerals and related substances at 298.15 K (25.0 °C) and one atmosphere (1.013 bars) pressure and at higher temperatures. In US Geological Survey Bulletin 1259; USGS: Washington, DC, USA, 1968; p. 256. [Google Scholar]
- Craig, J.R.; Barton, P.B. Thermochemical approximations for sulfosalts. Econ. Geol. 1973, 68, 493–506. [Google Scholar] [CrossRef]
- Robie, R.; Hemingway, B.; Fisher, J. Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 pascals) pressure and at higher temperatures. In US Geological Survey Bulletin 1452; USGS: Washington, DC, USA, 1978; p. 456. [Google Scholar]
- Barton, P.B.J.; Skinner, B. Sulfide mineral stabilities. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Wiley Intersci.: New York, NY, USA, 1979; pp. 278–403. [Google Scholar]
- Afifi, M.; Kelly, W.C.; Essene, E.J. Phase relations among tellurides, sulfides, and oxides: I. Thermochemical data and calculated equilibria. Econ. Geol. 1988, 83, 377–394. [Google Scholar] [CrossRef]
- Afifi, A.M.; Kelly, W.C.; Essene, E.J. Phase relations among tellurides, sulfides, and oxides: II. Applications to telluride-bearing ore deposits. Econ. Geol. 1988, 83, 395–404. [Google Scholar] [CrossRef]
- Robie, R.A.; Hemingway, B.S. Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 pascals) pressure and at higher temperatures. In US Geological Survey Bulletin 2131; USGS: Washington, DC, USA, 1995; p. 461. [Google Scholar]
- Krauskopf, K.B.; Bird, D.K. Introduction to Geochemistry, 3rd ed.; McGraw-Hill: New York, NY, USA, 1995. [Google Scholar]
- Sillitoe, R.H. Ore-related breccias in volcanoplutonic arcs. Econ. Geol. 1985, 80, 1467–1514. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Barton, P.B.; Bethke, P.M. Chalcopyrite disease in sphalerite: Pathology and epidemiology. Am. Mineral. 1987, 72, 451–467. [Google Scholar]
- Cooke, D.R.; McPhail, D.C.; Bloom, M.S. Epithermal gold mineralization Ancupan Banguio district Philippine: Geology, mineralization, alteration and the thermochemical environmenot of ore deposition. Econ. Geol. 1996, 91, 243–272. [Google Scholar] [CrossRef]
- Thomas, J.B.; Watson, E.B.; Spear, F.S.; Shemella, P.T.; Nayak, S.K.; Lanzirotti, A. TitaniQ under pressure: The effect of pressure and temperature on the solubility of Ti in quartz. Contrib. Mineral. Petrol. 2010, 160, 743–759. [Google Scholar] [CrossRef]
- Wark, D.A.; Watson, E.B. The TitaniQ: A Titanium-in-quartz geothermometer. Contrib. Mineral. Petrol. 2006, 152, 743–754. [Google Scholar] [CrossRef]
- Frikken, P.H.; Cooke, D.R.; Walshe, J.L.; Archibald, D.; Skarmeta, J.; Serrano, L.; Vargas, R. Mineralogical and isotopic zonation in the Sur-Sur tourmaline breccia, Río Blanco-Los Bronces Cu-Mo deposit, Chile: Implications for ore genesis. Econ. Geol. 2005, 100, 935–961. [Google Scholar] [CrossRef]
- Bodnar, R. Revised equation and table for determining the freezing point depression of H2O-NaCl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Lecumberri-Sanchez, P.; Steele-MacInnis, M.; Bodnar, R. A numerical model to estimate trapping conditions of fluid inclusions that homogenize by halite disappearance. Geochim. Cosmochim. Acta 2012, 92, 14–22. [Google Scholar] [CrossRef]
- Skewes, M.A.; Holmgren, C.; Stern, C.R. The Donoso copper-rich, tourmaline-bearing breccia pipe in central Chile: Petrologic, fluid inclusion and stable isotope evidence for an origin from magmatic fluids. Miner. Depos. 2003, 38, 2–21. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusions. Mineral. Soc. Am. 1984, 12, 644. [Google Scholar]
- Corbett, G.J.; Leach, T.M. Southwest Pacific Rim Gold–Copper Systems: Structure, Alteration, and Mineralization; Special Publication 6; Society of Economic Geologists: Littleton, CO, USA, 1998; p. 238. [Google Scholar]
- Burnham, C.W.; Ohmoto, H. Late stage processes of felsic magmatism. Soc. Min. Geol. Japan 1980, 8, 1–11. [Google Scholar]
- Guilbert, J.M.; Park, C.F. The Geology of Ore Deposits; Freeman: New York, NY, USA, 1985. [Google Scholar]
- Sverjensky, D.A.; Hemley, J.J.; D’Angelo, W.M. Thermodynamic assessment of hydrothermal alkali feldspar-mica-aluminosilicate equilibria. Geochim. Cosmochim. Acta 1991, 55, 989–1004. [Google Scholar] [CrossRef]
- Inoue, A. Formation of clay minerals in hydrothermal environments. In Origin and Mineralogy of Clays; Velde, B., Ed.; Springer: Berlin, Germany, 1995; pp. 268–330. [Google Scholar]
- Pirajno, F. Hydrothermal Processes and Mineral Systems; Springer Science & Business Media: Berlin, Germany, 2009. [Google Scholar]
- Voicu, G.; Bardoux, M.; Jébrak, M. Tellurides from the Paleoproterozoic Omai gold deposit, Guiana shield. Can. Mineral. 1999, 37, 559–573. [Google Scholar]
- Cook, N.J.; Ciobanu, C.L. Bismuth tellurides and sulphosalts from the Larga hydrothermal system, Metaliferi Mts, Romania: Paragenesis and genetic significance. Mineral. Mag. 2004, 68, 301–321. [Google Scholar] [CrossRef]
- Voudouris, P. A comparative mineralogical study of Te-rich magmatic-hydrothermal systems in northeastern Greece. Mineral. Petrol. 2006, 87, 241–275. [Google Scholar] [CrossRef]
- Cooke, D.R.; McPhail, D.C. Epithermal Au-Ag-Te mineralization, Acupan, Baguio district, Philippines: Numerical simulations of mineral deposition. Econ. Geol. 2001, 96, 109–131. [Google Scholar]
- Cook, N.J. What makes a gold-telluride deposit? In GSA Denver Annual Meeting, Session No. 72, Abstracts with Programs, v. 39; Geological Society of America: Washington, DC, USA, 2007; p. 196. [Google Scholar]
- Jensen, E.P.; Barton, M.D. Gold deposits related to alkaline magmatism. Rev. Econ. Geol. 2000, 13, 279–314. [Google Scholar]

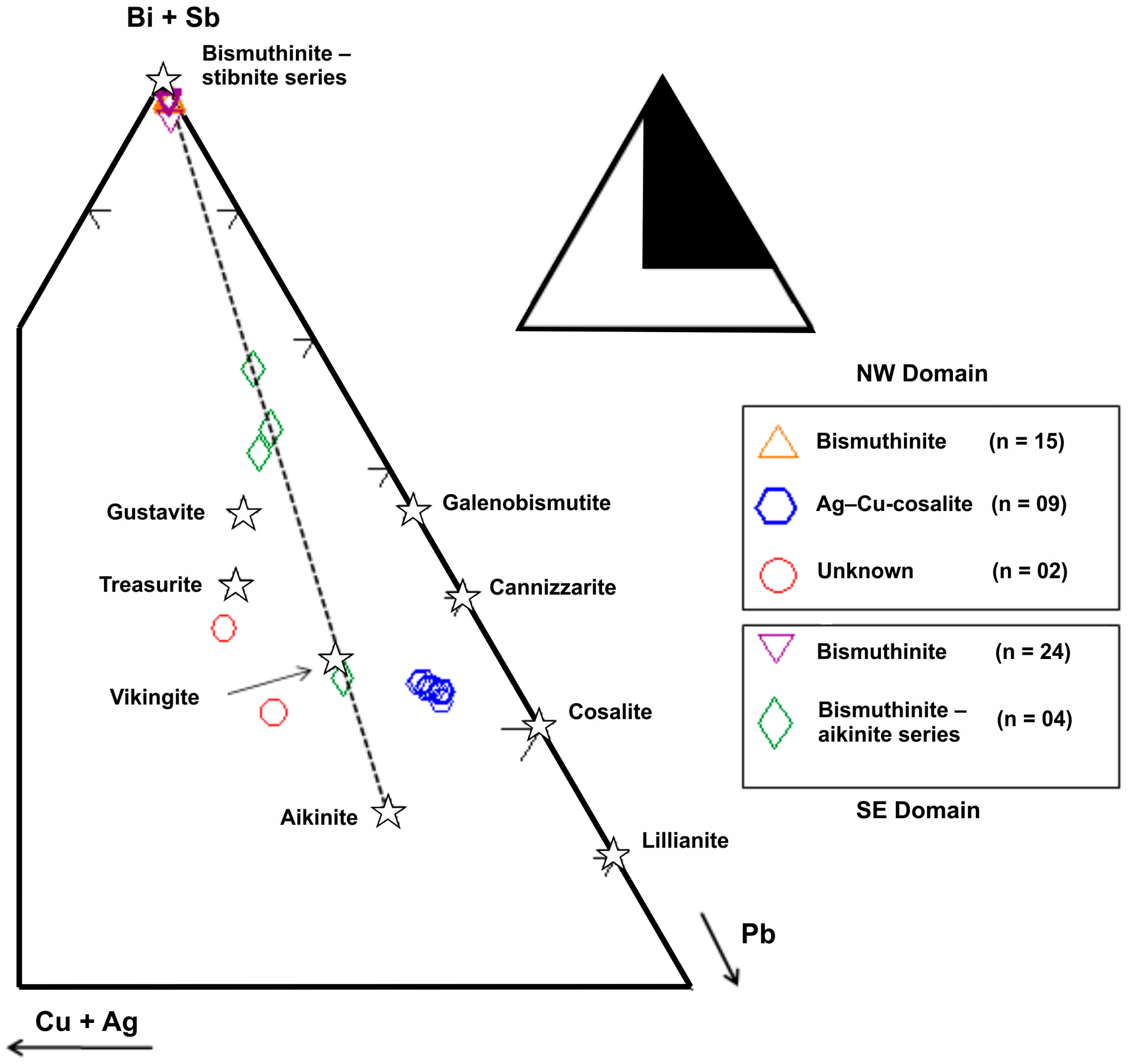
| NW Domain | SE Domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4b-II Bismuthinite | 4b-II Cosalite | Unknown | Unknown | 3b-II Bismuthinite | 3b-II Tetradymite | 3b-II Bismuthinite–Aikinite Series n = 4 | ||||
| Gladite–Salzburgite Member | Paarite–Krupkaite Member | Friedrichite | ||||||||
| n = 15 | n = 9 | n = 1 | n = 1 | n = 24 | n = 7 | n = 1 | n = 1 | n = 1 | n = 1 | |
| Bi | 79.31 | 43.33 | 47.56 | 40.49 | 79.46 | 60.81 | 63.04 | 59.63 | 58.52 | 43.62 |
| Sb | 0.93 | 1.13 | 0.55 | 1.68 | 0.55 | 0.27 | 0.53 | 0.50 | 0.14 | 1.08 |
| Pb | 0.99 | 34.74 | 20.95 | 26.09 | 0.84 | 0.03 | 13.98 | 16.88 | 17.09 | 28.98 |
| Cu | 0.34 | 2.09 | 6.71 | 7.53 | 0.31 | 0.08 | 4.27 | 5.20 | 6.53 | 9.14 |
| Ag | 0.01 | 2.61 | 7.58 | 6.56 | 0.02 | 0.17 | 0.00 | 0.00 | 0.13 | 0.10 |
| S | 18.34 | 16.08 | 16.74 | 16.62 | 17.91 | 5.02 | 17.44 | 17.52 | 17.32 | 16.78 |
| Se | 0.18 | 0.11 | 0.19 | 0.18 | 0.23 | 0.31 | 0.17 | 0.14 | 0.18 | 0.06 |
| Te | 0.06 | 0.14 | 0.18 | 0.21 | 0.30 | 34.18 | 0.04 | 0.07 | 0.07 | 0.05 |
| ∑ | 100.16 | 100.23 | 100.46 | 99.37 | 99.62 | 100.89 | 99.48 | 99.93 | 99.98 | 99.81 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testa, F.J.; Zhang, L.; Cooke, D.R. Physicochemical Conditions of Formation for Bismuth Mineralization Hosted in a Magmatic-Hydrothermal Breccia Complex: An Example from the Argentine Andes. Minerals 2018, 8, 486. https://doi.org/10.3390/min8110486
Testa FJ, Zhang L, Cooke DR. Physicochemical Conditions of Formation for Bismuth Mineralization Hosted in a Magmatic-Hydrothermal Breccia Complex: An Example from the Argentine Andes. Minerals. 2018; 8(11):486. https://doi.org/10.3390/min8110486
Chicago/Turabian StyleTesta, Francisco J., Lejun Zhang, and David R. Cooke. 2018. "Physicochemical Conditions of Formation for Bismuth Mineralization Hosted in a Magmatic-Hydrothermal Breccia Complex: An Example from the Argentine Andes" Minerals 8, no. 11: 486. https://doi.org/10.3390/min8110486
APA StyleTesta, F. J., Zhang, L., & Cooke, D. R. (2018). Physicochemical Conditions of Formation for Bismuth Mineralization Hosted in a Magmatic-Hydrothermal Breccia Complex: An Example from the Argentine Andes. Minerals, 8(11), 486. https://doi.org/10.3390/min8110486




