Abstract
Illite is one of the main components in coal slime water and it sometimes makes the water extremely difficult to clarify. In this study, the aggregation mechanism of coal and illite particles was investigated using the extended Derjaguin-Landau-Verwey-Overbeek (XDLVO) theory and settling experiments of slime water containing coal and illite. The results show that electrostatic energy plays a dominant role and manifests repulsive force in the long-range (>4 nm). However, the leading role becomes a hydrophobic force determined by the polar surface interaction energy in the short-range (<4 nm). A coagulant (Ca2+) can lower the surface electric potential to make all particles easier to coagulate, while the surface hydrophobicity of coal and illite determines whether the particles aggregate. Cationic polyacrylamide (CPAM) can promote the sedimentation of particles flocs effectively and makes the supernatant clearer due to attractive electrostatic forces while anionic polyacrylamide (APAM) flocculates particles through the bridge mechanism. Although the hydration shell on the hydrophilic surface of illite appears to be harmful for either coagulation or flocculation, illite can accelerate sedimentation when it is attached to the coal due to its higher density.
1. Introduction
Coal slime water (slurry) is the waste-water produced during the wet processing of coal preparations. In the slurry, particles of rock, clay and coal are too small to float or sink [1,2,3]. It was reported that clay minerals [4], such as montmorillonite, kaolinite and illite [5,6,7,8], have a detrimental effect on coal slime dewatering and cleaning [9,10,11]. However, the coal slime water must be clarified and recycled in order to save water resources and reduce environmental pollution [12,13,14]. Hence, it is vital to understand how clays affect the aggregation and sedimentation of coal particles in order to make the clarification process more efficient.
It was reported in reference [7] that for coal-kaolinite suspensions, the addition of Ca2+ decreases the repulsive electrostatic force between the kaolinite particles, thus allowing the attractive van der Waals force to become the dominant interaction force. This allows the coal particles to aggregate with each other easily, as well as with kaolinite particles. This was verified by Gui et al. [15] using atomic force microscopy. Their study showed that the interaction forces between coal and kaolinite particles changed from being weakly repulsive to strongly attractive and increased monotonously, resulting in the occurrence of the kaolinite-coating phenomenon. As aggregation does not occur between kaolinite particles, kaolinite-coating may cause coal particles difficulty in settling and aggregating. In coal-montmorillonite suspensions, a clay platelet network is formed by montmorillonite particles in a “house of cards” model, and coal particles captured by the network then settle together with montmorillonite particles [8]. These works indicate that clays affect the coagulation and sedimentation of coal slime water depending on the added agents.
Illite content in slime water can be up to 20% of all clay particles and it seriously affects the sedimentation of the slime water [11]. However, while most researchers have investigated the impact of kaolinite or montmorillonite on coal slime water sedimentation, there are only a few studies that examine the impact of illite. Therefore, studying the influence of illite on slime water treatment is an important guiding significance in practical works. In this study, the coagulation of coal and illites particles at different dosages of CaCl2 (as the coagulant) was studied according to the extended Derjaguin-Landau-Verwey-Overbeek (XDLVO) theory and experiments. Additionally, flocculation-sedimentation experiments were conducted to understand the flocculation mechanism of particles by using different kinds of polyacrylamide.
2. Materials and Methods
2.1. Materials and Instruments
Coal samples were provided by the Xuehu Coal Preparation Plant (Yongcheng, China) and coal slime water was prepared by using high purity minerals, the methodology of which is provided in Section 2.2. In the experiment, calcium chloride was used as the cationic coagulant; cationic polyacrylamide (CPAM; molecular weight 10,000,000; charge density 40%; with an effective pH range of 3–8); and anionic polyacrylamide (Xinbang Environmental Protection Technology Co., Ltd., Xinxiang, China) (APAM; molecular weight greater than 10,000,000; charge density 25%; and an effective pH range of 7–12) were used as flocculants. Illite was provided by an online supplier, and its purity and mean diameter were 91.30% and 18 μm, respectively.
The particle size, zeta potentials, and contact angles with liquid medium were analyzed by a LS 100Q laser particle size analyzer (Beckman Coulter, Inc., Brea, CA, USA); a ZetaPALS high-resolution zeta potential analyzer (Brookhaven Instruments Corporation, Holtsville, NY, USA); and a optical contact angle measuring instrument DSA100 (Kruss GmbH, Hamburg, Germany), respectively. A X-ray Diffraction D8 Advance (Bruker AXS GmbH, Madison, WI, USA) was used to determine the purity of the illite. Supernatant turbidities were determined using a WGZ-1A turbidity meter (Shanghai Xinrui Instrument Co., Ltd., Shanghai, China).
2.2. Experimental Methods
2.2.1. Preparation of Coal Slime Water
After drying, grinding, screening and float-and-sinking in heavy-fluid (1.40 kg/L) consisting of carbon tetrachloride and benzene, a high purity fine coal was obtained. This was then dried at 60 °C for 3 h. The ash content of the coal sample was 3.74% and its average particle size was 36 μm.
In order to understand the aggregation mechanisms of coal-coal, illite-illite and coal-illite particles, the coal sample and illite were mixed with deionized water (conductivity 93.1 μs/cm) to prepare three kinds of coal slime water: (1) a slurry containing coal only; (2) a slurry containing illite only; and (3) a slurry containing both coal and illite at a mass ratio of 2:1. All concentrations of were set to 60 g/L and were used after soaking for half an hour.
2.2.2. Theoretical Calculation of Particle Aggregation
The XDLVO theory suggests that the aggregation or dispersion of particles is determined by the total action potential energy VT. From a thermodynamic standpoint, the attraction or adhesion between two interacting surfaces occurs when VT is negative; repulsion occurs when VT is positive. The VT is given by Van Oss [16] as:
where VE is the electrostatic double layer repulsive energy; VW is the van der Waals attractive energy; and VH is the polar surface interaction energy (hydrophobic energy). The calculation formulas of energies are shown in Table 1.
VT = VE + VW + VH

Table 1.
XDLVO theoretical calculation formulas.
2.2.3. Coagulation and Flocculation-Sedimentation of Coal Slime Water
Dosages of 0.1 and 10 mM CaCl2 solution were added respectively to every 100 mL sample of each kind of slime water prepared in Section 2.2.1. After mixing thoroughly, they were allowed to settle for 2 h, and the clarification layer height recorded every 10 min. Finally, the turbidity of supernatant 0.5 cm from the free surface was determined.
Similarly, the same dosages of CaCl2 solution were added to every 100 mL of slime water. In addition, the CPAM solution was added with a dosage of 4.5 mg/L. After mixing, it settled for 10 min with the clarification layer height recorded every 30 s before finally measuring the supernatant turbidity was measured. These experiments were then conducted again, with the APAM substituting the CPAM.
3. Results and Discussion
3.1. Interaction between Particles
The contact angles of three kinds of liquid (water, formaldehyde and glycerol) on solid samples (coal and illite) are shown in Table 2. These were used to calculate the As and solid surface dispersion parameters (rSd, rS+, rS-) according to Equation (Ib) (where the liquid used was deionized water, and the Hamaker constant was 4.84 × 10−20 J [20]) and Equation (IIIb) listed in Table 1. The zeta potentials of the samples under different calcium ion concentrations are shown in Table 3, and were used to calculate VE. The liquid surface energy parameters used in Equation (IIIb) are given in Table 4; the h0 (= 0.158 nm) is the minimum separation distance due to the Born repulsion [21], which allows for the calculation of VW and VH. These calculations allow the relationship between the three potential energies (VE, VW and VH) and the particle distance to be obtained.

Table 2.
The contact angles of coal and illite in three liquid mediums at pH 8.2.

Table 3.
The zeta potential of samples under different calcium concentration at pH 8.2.

Table 4.
The surface energy parameters of liquids (mJ/m) [8].
As shown in Figure 1, the van der Waals attractive energy (VW) between all particles is always negative, which is beneficial for the coagulation of particles. However, when the particle distance is greater than 4 nm, it has little influence on collision and coagulation as its absolute value is much lower than both that of the electrostatic energy (VE) and the polar surface interaction energy (VH). This was also shown in the works of Nabweteme [22].

Figure 1.
Predicted potential energy between particles with different calcium ion concentrations (coagulant) by XDLVO: (a) coal-coal; (b) illite-illite and (c) coal-illite. The absence of “Ca2+” in the legend means that no coagulant was added.
The VH depends strongly on surface properties of the particles. It was shown that the VH between coal particles displayed hydrophobic attraction energy and is favorable for coagulation; while the VH between the coal-illite and illite-illite particles manifest repulsive interaction energies. Furthermore, the VH increased sharply at distances shorter than 60 nm and plays a leading role in the determination of the aggregation state of the particles. These results were similar to the particle interaction force of coal slurry containing montmorillonite [8].
The VE is related to the solution medium. The cationic coagulant can reduce the absolute values of the particle surface potentials by compressing the electric double layer, which is beneficial to the aggregation of the particles. The VE has a greater effect on the long range, and it displayed a dominant influence when the particle spacing was greater than 2 nm.
With regard to the interaction energies between coal particles, it is known that the zeta potential decreases when the calcium ion concentration is 10 mM and the VT between the coal particles is negative, which results from the VE and VH when the distance is greater than 200 nm. The VE increases with the reduction of particle spacing, which causes the VT to change from negative to positive and the appearance of a maximum energy barrier at a distance of 30 nm. However, the VT becomes negative again quickly due to the rapid increase of the VH, which is beneficial to coagulation. Thus, coal particles may aggregate stably if they can overcome the energy barrier and become close to each other by providing enough momentum. When the calcium ion concentration is 0.1 mM, the tendency of the VT is similar and the dominant force is also VE, whereas the energy barrier is greater than that of 10 mM.
In the present work, due to the hydrophilic surfaces (which mainly react at short-range) and the electrostatic negativity (which mainly work at long-range) of the illite particle surfaces, the VT values of coal-illite and illite-illite are always shown as repulsive forces, which is not conducive to aggregation and makes it difficult to settle slime water mixed with illite. The repulsive forces between the hydrophilic surface of illite can be attributed to the hydration layer, where hydration shells near hydrophilic surfaces immersed in water have been reported [23,24]. When two hydrophilic surfaces are close to each other, the shell tends to protect itself from being broken, so repulsive forces appear. These hydrophilic repulsive forces can be much stronger than DLVO forces in the short-range [25].
It should be emphasized that these calculation results achieved using the XDLVO theory cannot absolutely determine whether particles coagulate. Particle agglomeration is also related to the particle hydraulic effect, which means that particles may adhere to each other only if the kinetic energy provided by fluid flow is large enough to overcome the repulsive energy.
3.2. Coagulation Sedimentation of Particles
All the experiments were conducted at a neutral pH. The results show that the pH wavered around 8.2 very slightly and there were no obvious changes when coagulants or/and flocculants were added.
The change rate of the clarification layer height represents the sedimentation velocity of the particles. As shown in Figure 2, the change of the calcium ion concentration had no remarkable effect on the sedimentation rate of all the groups; however, it is interesting that the sedimentation rate of the slime water containing illite and coal was superior to that of the others. This indicates that illite particles (2600–2900 kg/m3) may adhere to coal (less than 1400 kg/m3) and accelerate the sedimentation rate by improving the average density of aggregation. Although this seems unlikely due to the strong repulsive forces between coal and illite (as shown by the calculation results in Figure 1c), similar phenomena were observed in the coal-kaolinite (calculated force attractive) and coal-montmorillonite (calculated force repulsive) systems [8]. This is possibly due to the adhesion of the coal surface and clay platelet edges as Marek [26] reported, where clay platelets attach to hydrophobic (but not hydrophilic) surfaces by their edges as a result of the presence of nano-bubbles existing on the edges of the clay crystals. The deviation between the calculations and experiments was caused by using the general contact angles of illite on liquids. However, as illites infrequently attach to each other due to the repulsion forces between the hydrophilic flat and the low frequency of collision from edge to edge, they settled down more slowly (Figure 2). Coal particles tended to settle more quickly than illite as they can adhere to each other due to hydrophobic attractive forces. When sedimentation experiments on the three kinds of slurry in the absence of calcium were carried out, no clarification layer could be observed. When comparing these results to Figure 2, it demonstrates that a coagulant can improve particle aggregation by decreasing the electrostatic repulsion.
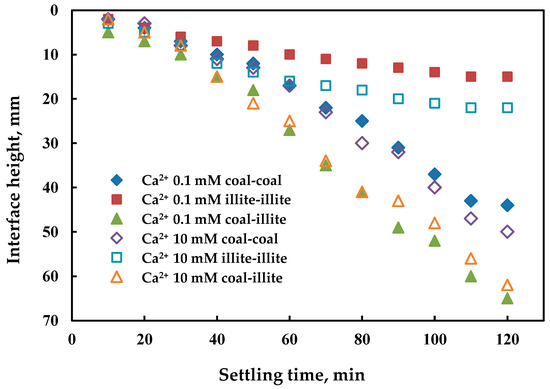
Figure 2.
The sedimentation rate of particles with different calcium ion concentrations, where the interface height is the distance from the free surface to the interface between the supernatant and suspension.
Supernatant turbidity after sedimentation is shown in Figure 3. It illustrates that the supernatant turbidity decreased to different degrees with the increase in coagulation (CaCl2), which demonstrates that a coagulant can increase the adhesion probability by decreasing the electrostatic repulsive forces of the particles. For the three different water samples, the order of turbidity, illite-illite > coal-illite > coal-coal, was exactly consistent with the theoretical results calculated by the XDLVO theory. This result proves that the VH plays a leading role in the coal-illite system. Although illite may be attached to coal by its edge, it could not be captured fully and it made the supernatant more turbid than the pure coal slurry.
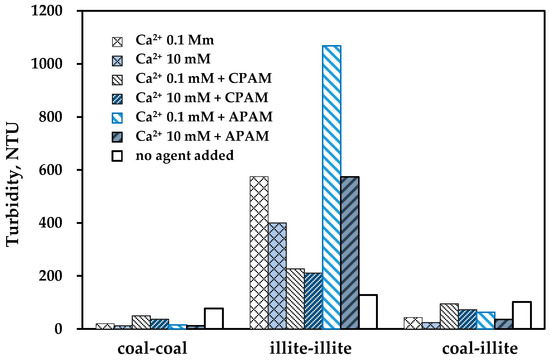
Figure 3.
The supernatant turbidity of coal slime water after sedimentation using different agents; the dosage of PAM was 4.5 mg/L, if available.
3.3. Flocculation Sedimentation of Particles
The curves presented in Figure 4a demonstrate that the sedimentation velocity of all particles increased significantly and sedimentation times were shortened from more than 120 min (Figure 2) to 10 min after adding the CPAM, while APAM presents little influence (Figure 4b). This may be primarily attributed to electrostatic forces. As the particles carry negative charge over the studies, minerals can be attracted to the cationic group of CPAM and repulsed by the negative charge group of APAM. As the PAMs are macromolecules, large flocs were formed when flocculation was conducted, which means that the sedimentation velocity can be greatly improved. Furthermore, the shortened sedimentation time could be responsible for the relatively higher supernatant turbidity when adding CPAM, both in the coal-coal and coal-illite samples shown in Figure 3. Figure 4a also shows the sedimentation velocity order of illite-illite > coal-illite > coal-coal, which can be attributed to the different densities of the minerals we mentioned in Section 3.2.
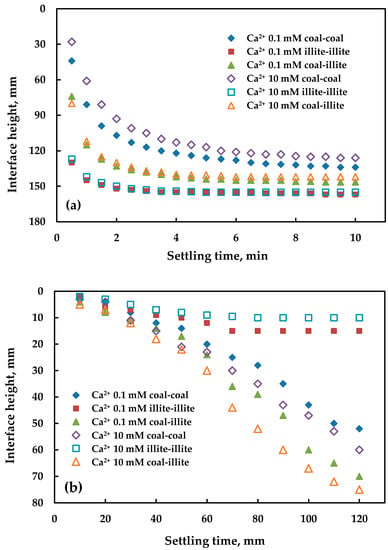
Figure 4.
The sedimentation rate of particles when both calcium and PAM were added: (a) cationic PAM; (b) anionic PAM. The dosage of the two kinds of PAM was 4.5 mg/L.
The reported results that APAM is effective when used in combination with metal ions [10,27] did not agree with the results shown in Figure 4, where additional sedimentation experiments were carried out in conditions with added calcium (10 mM) and APAM (1.0, 1.5, 2.0 mg/L). In the pure coal and mixed coal-illite samples, the optimal supernatant turbidities (4.6 NTU for coal-coal and 9.8 NTU for coal-illite) were obtained when 1.5 mg/L APAM was added, with the turbidity increasing as more APAM was added. This demonstrates that calcium ions acted as bridges between particles and the anionic groups of flocculant when a suitable dosage of APAM was used; excessive APAM may increase repulsive electrostatic forces, which may be adverse to flocculation [26], as Figure 4b shows. Of interest is the fact that no obvious sedimentation was observed in the pure illite samples in all of the additional experiments. It appears that the hydration shell on illite can stop the bridge mechanism of the illite-calcium-anionic group of APAM.
4. Conclusions
Particle surface hydrophobicity plays a predominant role in sedimentation. Coal particles are likely to settle due to hydrophobicity, while illite particles are difficult to coagulate due to hydrophilicity. However, illite particles may adhere to coal particles on their hydrophobic edges, and their higher density is conducive to improving the settling velocity of coal slurry. The repulsive electrostatic forces play an important role when the particle distance is greater than 4 nm, which is detrimental to particle aggregation. Increasing the concentration of cations can increase the probability of particle adhesion as it reduces the negative charge on the particle surface. CPAM can attract particles with negative charges and form big flocs to accelerate sedimentation, while adsorption bridging is the main mechanism for promoting particle flocculation with APAM. Although the hydration shells on the hydrophilic surfaces of illite appear to be harmful for either coagulation or flocculation, illite can accelerate sedimentation when attached to coal particles due to its higher density.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 51604273, 51304194, 51304195) and the Jiangsu Natural Science Foundation financially, BK20130181; it is also part of a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author Contributions
Zhe Lin, Panting Li and Yali Kuang conceived and designed the experiments; Panting Li and Dou Hou performed the experiments; Zhe Lin and Panting Li analyzed all the data; Zhe Lin, Panting Li and Guanghui Wang wrote the paper. All authors read and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| A | Hamaker constant, A132 represents the effective Hamaker constant of the interaction between substance 1 and 2 in medium 3, A11 is the Hamaker constant for substance 1 in vacuum, so as A22. |
| c | Concentration for amount of substance of ions, mol/L |
| e | elementary charge, 1.602 × 10−19 C |
| H | surface distance between particles |
| h0 | relaxation length |
| K | Boltzmann constant, 1.38 × 10−23 J/K |
| NA | Avogadro’s number, 6.022 × 1023 |
| R | particle radius |
| r+ | surface energy electron acceptor component |
| r− | surface energy electron donor component |
| T | absolute temperature |
| VH0 | interfacial polar interaction energy constant |
| Z | ionic valence in solution |
| θ | contact angle |
| rd | surface energy dispersive component |
| εα | absolute dielectric constant of dispersion medium, F/m, the absolute dielectric constant of water is 7.172 × 10−10 F/m. [28] |
| κ−1 | length of Debye, m |
| ψ | surface potential. It was replaced by zeta potential in this study |
| ψ | surface potential. It was replaced by zeta potential in this study |
| Subscripts | |
| 1,2 | particles can be coal or illite in the present work |
| 3 | disperse medium (water here) |
| L | Liquid |
| S | Solid |
References
- Xu, H.X.; Cheng, G. A new method to evaluate the performance of slime water treatment. Energy Source 2016, 38, 777–782. [Google Scholar] [CrossRef]
- Gong, G.Q.; Zhang, Y.J.; Zheng, H.L. Experimental study on the transmittance of the supernatant of refractory slime water. J. Chem. Eng. Jpn. 2016, 49, 417–424. [Google Scholar] [CrossRef]
- Xie, W.; Cao, G.; Ren, X. Effect of flotation promoter on the rate of coal slime flotation. J. Min. Sci. 2014, 50, 601–607. [Google Scholar] [CrossRef]
- Tian, S.D.; Zhuo, Y.Q.; Zhan, Z.H.; Shu, X.Q.; Kang, Z.Z. Distribution of clay minerals in light coal fractions and the thermal reaction products of these clay minerals during combustion in a drop tube furnace. Energies 2016, 9, 428–442. [Google Scholar] [CrossRef]
- Liang, L.X.; Xiong, J.; Liu, X.J.; Luo, D.X. An investigation into the thermodynamic characteristics of methane adsorption on different clay minerals. J. Nat. Gas Sci. Eng. 2016, 33, 1–10. [Google Scholar] [CrossRef]
- Li, G.; Guo, S.H.; Hu, J.X. The influence of clay minerals and surfactants on hydrocarbon removal during the washing of petroleum-contaminated soil. Chem. Eng. J. 2016, 286, 191–197. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Liu, J.T.; Wang, Y.T. Effects of water hardness on the dispersion of fine coal and kaolinite in coal slurry. J. China Coal Soc. 2008, 9, 1058–1062. [Google Scholar]
- Zhang, M.Q.; Liu, Q.; Liu, J.T. Extended DLVO theory applied to coal slime-water suspensions. J. Cent. South Univ. 2012, 19, 3558–3563. [Google Scholar] [CrossRef]
- Mcfarlane, A.J.; Bremmell, K.E.; Addai, M. Optimising the dewatering behaviour of clay tailings through interfacial chemistry, orthokinetic flocculation and controlled shear. Powder Technol. 2005, 160, 27–34. [Google Scholar] [CrossRef]
- Sabah, E.; Erkan, Z.E. Interaction mechanism of flocculants with coal waste slurry. Fuel 2006, 85, 350–359. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, C.; Shen, Z.Y.; Qi, X. The properties and sedimentation characteristics of extremely sliming coal slime water. J. China Coal Soc. 2010, 35, 312–315. [Google Scholar]
- Barraza, J.; Guerrero, J.; Pineres, J. Flotation of a refuse tailing fine coal slurry. Fuel Process. Technol. 2013, 106, 498–500. [Google Scholar] [CrossRef]
- Zhang, J.S.; Chen, N.; Fu, G.; Yan, M.W.; Kim, Y.C. The safety attitudes of senior managers in the Chinese coal industry. Int. J. Environ. Res. Public Health 2016, 13, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, J.F.; Bai, X.; Song, B.; Wang, R.D.; Zhou, T.H.; Jia, J.L.; Pu, H.X. Leaching behavior and potential environmental effects of trace elements in coal gangue of an open-cast coal mine area, Inner Mongolia, China. Minerals 2016, 6, 50–68. [Google Scholar] [CrossRef]
- Gui, X.H.; Xing, Y.W.; Rong, G.Q.; Cao, Y.J.; Liu, J.T. Interaction forces between coal and kaolinite particles measured by atomic force microscopy. Powder Technol. 2016, 301, 349–355. [Google Scholar] [CrossRef]
- Van Oss, C.J. Acid-base interfacial interactions in aqueous media. Colloids Surf. A 1993, 78, 1–49. [Google Scholar] [CrossRef]
- Brant, J.A.; Childress, A.E. Assessing short-range membrane-colloid interactions using surface energetics. J. Membr. Sci. 2002, 203, 257–273. [Google Scholar] [CrossRef]
- Brant, J.A.; Childress, A.E. Membrane–colloid interactions: Comparison of extended DLVO predictions with AFM force measurements. Environ. Eng. Sci. 2002, 19, 413–427. [Google Scholar] [CrossRef]
- Churaev, N.V.; Derjaguin, B.V. Inclusion of structural forces in the theory of stability of colloids and films. J. Colloid Interface Sci. 1984, 2, 542–553. [Google Scholar] [CrossRef]
- Duran, J.D.G.; Ramos-Tejada, M.M.; Arroyo, F.J.; Gonzalez-Caballero, F. Rheological and electrokinetic properties of sodium montmorillonite suspensions: I. Rheological properties and interparticle energy of interaction. J. Colloid Interface Sci. 2000, 229, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hoek, E.M.V.; Agarwal, G.K. Extended DLVO interactions between spherical particles and rough surfaces. J. Colloid Interface Sci. 2006, 298, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Nabweteme, R.; Yoo, M.; Kwon, H.S.; Kim, Y.J.; Hwang, G.; Lee, C.H.; Ahn, I.S. Application of the extended DLVO approach to mechanistically study the algal flocculation. J. Ind. Eng. Chem. 2015, 30, 289–294. [Google Scholar] [CrossRef]
- Peng, C.; Song, S.; Fort, T. Study of hydration layers near a hydrophilic surface in water through AFM imaging. Surf. Interface Anal. 2006, 38, 975–980. [Google Scholar] [CrossRef]
- Pashley, R.M.; Israelachvili, J.N. Molecular layering of water in thin films between mica surfaces and its relation to hydration forces. J. Colloid Interface Sci. 1984, 2, 511–523. [Google Scholar] [CrossRef]
- Yoon, R.H.; Vivek, S. Effects of short-chain alcohols and pyridine on the hydration forces between silica surfaces. J. Colloid Interface Sci. 1998, 204, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Żbik, M.; Horn, R.G. Hydrophobic attraction may contribute to aqueous flocculation of clays. Colloids Surf. A Physicochem. Eng. Asp. 2003, 222, 323–328. [Google Scholar] [CrossRef]
- Duong, C.; Choung, J.; Xu, Z.; Szymanski, J. A novel process for recovering clean coal and water from coal tailings. Miner. Eng. 2000, 13, 173–181. [Google Scholar] [CrossRef]
- Harvey, P.A.; Nguyen, A.V.; Evans, G.M. Influence of electrical double-layer interaction on coal flotation. J. Colloid Interface Sci. 2004, 25, 337–343. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).