Abstract
The extraction of manganese from a semi-oxidized manganese ore was investigated with sucrose as the reducing agent in dilute sulfuric acid medium. The kinetics of leaching manganese from the complex ore containing MnCO3 and MnO2 was also investigated. The effects of sucrose and sulfuric acid concentrations, leaching temperature and reaction time on the total Mn (TMn), MnO2 and MnCO3 leaching were investigated. Results showed that MnCO3 could more easily react with hydrogen ions than MnO2 in ores, and MnO2 decomposition could be advantageous for MnCO3 leaching. The leaching efficiencies of 91.8% for total Mn, 91.4% for MnO2 and 96.9% for MnCO3 were obtained under the following optimized conditions: 0.035 mol/L sucrose concentration, 5 mol/L sulfuric acid concentration, 60 min of reaction time and 363.2 K of leaching temperature. In addition, it was found that the leaching process of semi-oxidized manganese ore follows the shrinking core model and the leaching rate was controlled by chemical reaction and diffusion. The apparent activation energy of the total manganese, MnO2, and MnCO3 leaching were 40.83, 40.59, and 53.33 kJ·mol−1, respectively.
1. Introduction
Manganese and its products are widely used in steel, ferromanganese, non-ferrous alloys, dietary additives, fertilizers, paints, electronic components and other chemicals [1]. China is the largest producer and consumer of manganese products in the world, annually consuming more than 14 million tons of manganese ores [2]. However, only 6.4% of manganese ores were rich-grade, which had been depleted [3], and the import of rich-grade manganese ores has rapidly increased from 7.57 million tons in 2008 to 13 million tons in 2015. Therefore, the efficient and economic extraction of manganese from low-grade manganese ores in China is of practical significance.
As a low-grade manganese ore, semi-oxidized manganese ore is a rhodochrosite (MnCO3) that has been partly oxidized to manganese dioxide (MnO2). The mineral compositions found in ore are rhodochrosite (MnCO3), manganese oxide ore (MnO2) or mischcrystal structure of MnCO3 and MnO2. Previous studies mainly focus on the leaching of a single component of manganese ore. When the ores mainly contain MnCO3, manganese can be obtained directly through acid leaching. When the ores contain MnO2, the ores can be treated through reduction roasting followed by acid leaching [4,5,6,7] or directly through reductive leaching in dilute acid medium with different reducing agents, which includes waste tea [8], corncob [9], cornstalk [10], molasses [11], sawdust [12], glucose [13], oxalic acid [14], H2O2 [15,16], SO2 [17] and sphalerite [18]. Bioleaching technology has also been used to liberate manganese from ores [19]. However, there is no report on the simultaneous leaching of the two forms of manganese from semi-oxidized manganese ore. The relationship between the mineralogical structure and the leaching rate of the remaining semi-oxidized manganese ore is also poorly understood. The kinetics of simultaneously extracting various valence states of manganese from the ore also has not been studied.
Sucrose is a high-efficiency, environmentally friendly and renewable reductant in the leaching of manganese oxide ores [13]. Meanwhile, sucrose is the hydrolyzed product of biomass reductant in acid medium. As the structure and chemical components of sucrose are simpler than other biomass reductants, the use of sucrose can provide fundamental information which is pertinent to other biomass reductants. It has been used as a reducing agent to leach manganese dioxide in sulfuric acid solution [20]. Fractional factorial experiments have been used to study the significant effects and interactions among these factors. The chemical reaction that occurs during manganese leaching from manganese dioxide with sucrose as reductant can be described by the following reaction [20,21]. In addition, a shrinking core model and related statistical analysis of acid leaching manganiferous ores by sucrose were also reported [21].
The present study aimed to extract manganese from semi-oxidized manganese ores with sucrose as the reducing agent in sulfuric acid medium. The influences of different relevant factors, such as temperature and sucrose, and sulfuric acid concentrations, on the leaching rate of manganese (including total manganese, MnO2 and MnCO3) were investigated. Moreover, the kinetic model of the leaching process was investigated.
2. Materials and Methods
2.1. Materials
The sample of semi-oxidized manganese ore was obtained from the Daxin manganese mine (ore reserve, 131 million tons), Guangxi, China. The chemical composition of the manganese ore was characterized with X-ray fluorescence spectroscopy (Philips PW2404, Almeto, Holland) and an inductively coupled plasma spectrophotometer (Optima 5300 DV, PerkinElmer, Waltham, MA, USA). The results are presented in Table 1. The mineralogical structure was characterized through X-ray diffractometer (XRD, Rigaku model D/max-2500, Osaka, Japan). The XRD pattern is shown in Figure 1a. The chemical composition and XRD pattern indicated that the manganese (25.61%) in minerals was mainly composed of manganese dioxide (MnO2, 23.44%), manganous carbonate (MnCO3, 20.95%) and a few other valence phases. A large amount of gangue (SiO2) was also observed. Before use, all samples were crushed, ground, sieved and dried to provide desiccative raw material with a particle size of under 0.147 mm.

Table 1.
Bulk chemical composition of semi-oxidized manganese ore sample (mass fraction, %).
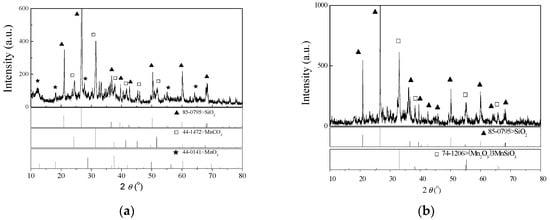
Figure 1.
X-ray diffractometer (XRD) pattern of semi-oxidized manganese ore (a) and its leaching residue (b).
2.2. Experiment Procedure
The leaching experiments were conducted in a 250 mL three-neck flask in a precision constant temperature water bath with an infinitely variable agitator. The reactor had three entrances, consisting of temperature measurement, stirrer and condenser. In a typical experiment, 10 g of samples were leached in 1–5 mol/L sulfuric acid solution and 0.015–0.045 mol/L sucrose with a stirring speed of 500 r/min at 333.2–363.2 K for 30–120 min, thereby keeping the solid-to-liquid ratio at 250 g/L. The samples of the leach solution were withdrawn at different times during the leach period for the total Mn (TMn). The leaching residues were washed with distilled water and dried for chemical analysis. All of the experiments were repeated in triplicate. The parameter errors were estimated by 95% confidence limits (Cls) (±1.96δ) [13]. The data points in Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6 represent the average values of the triplicate experiments and the error bars represent the 95% Cls.
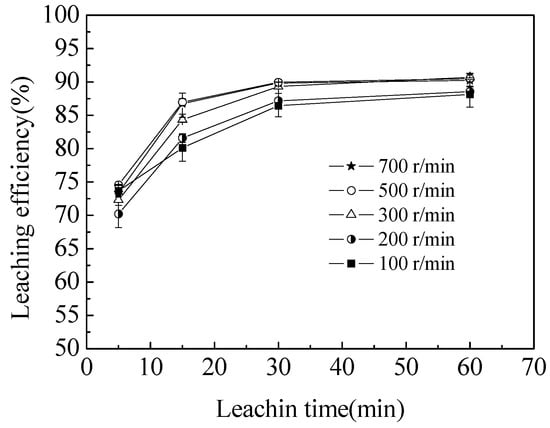
Figure 2.
Leaching rate of semi-oxidized manganese ore at different stirring speeds. The data points represent the average values from the triplicate experiments and the error bars represent the 95% confidence limits (Cls).
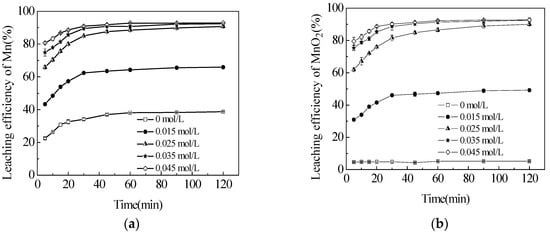
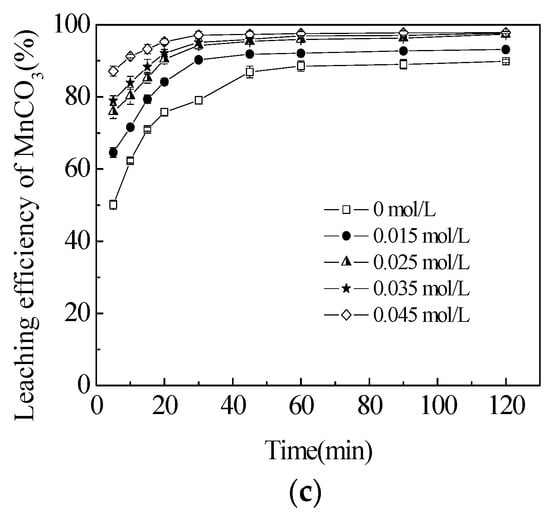
Figure 3.
Effect of sucrose concentration on the leaching efficiencies of total Mn (TMn) (a); MnO2 (b); and MnCO3 (c) in semi-oxidized manganese ore. The data points represent the average values from the triplicate experiments and the error bars represent the 95% Cls.
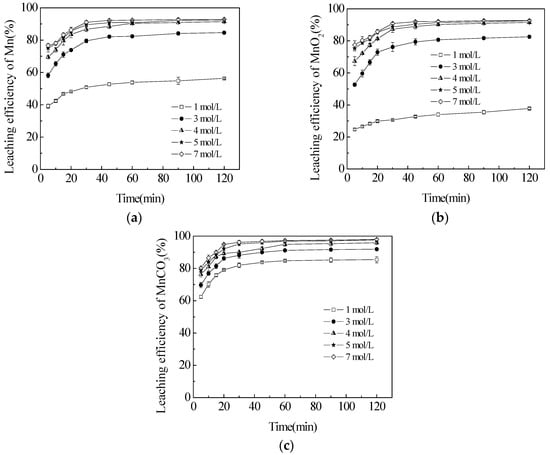
Figure 4.
Effect of H2SO4 concentration on the leaching efficiencies of TMn (a); MnO2 (b); and MnCO3 (c) in semi-oxidized manganese ore. The data points represent the average values from the triplicate experiments and the error bars represent the 95% Cls.
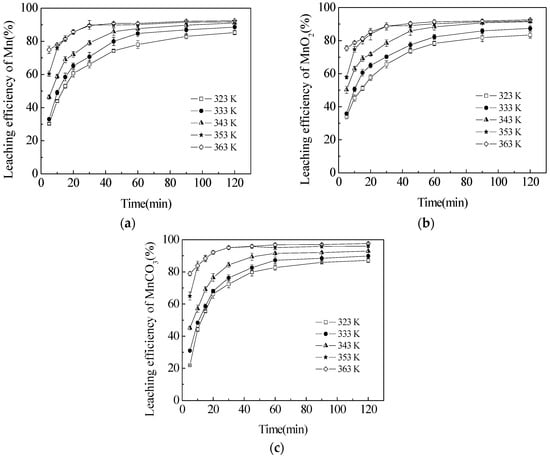
Figure 5.
Effect of the reaction temperature on the leaching efficiencies of TMn (a); MnO2 (b); and MnCO3 (c) in semi-oxidized manganese ore. The data points represent the average values from the triplicate experiments and the error bars represent the 95% Cls.
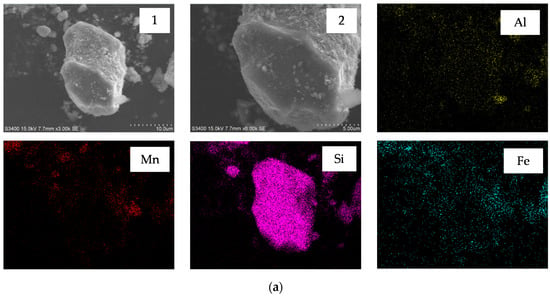
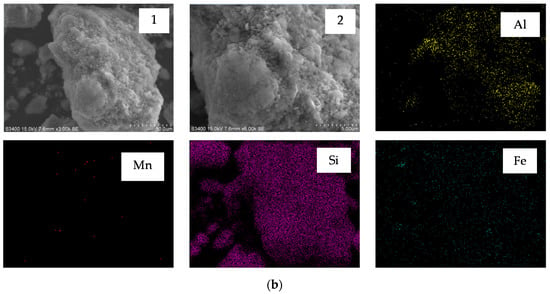
Figure 6.
Scanning electron microscope (SEM) images and energy dispersive X-ray spectrometry (EDS) element mapping of (a) semi-oxidized manganese ore; and (b) leaching residue.
2.3. Analytical Methods
The Mn contents of ores, leaching solution or leaching residues were detected via ammonium ferrous sulfate titration method [4]. Then, 0.2 g of the sample was diluted in 40 mL (1 wt %) perchloric acid at boiling water for 30 min and then filtrated and heated to measure the content of MnCO3 in mineral and residue. After the white smoke emerged from the filtrate, 2 mL (37 wt %) hydrochloric acid was added and diluted with water to 50 mL, and the Mn content was detected via titration method.
The content of MnO2 in mineral and residue was measured via iodometry. Approximately 0.2 g of the sample was weighed and placed into a 250 mL iodine flask. Then, 50 mL distilled water and 4 g of KI were added. After the solid of potassium iodide dissolved completely, 5 mL of diluted HCl solution (17 wt %) was added. Then, the bottle was sealed with a cork and stored in darkness for 30 min. In this period, the bottle was shaken violently at times to dissolve the sample completely. Finally, normality sodium thiosulfate standard solution was applied to titrate the obtained solution with 3 mL of starch solution (5 wt %) as an indicator. Then, the MnO2 content of the sample was calculated with the following formula:
where V is the volume of sodium thiosulfate solution consumed during the titration, mL; C is the normality sodium thiosulfate standard solution, 0.1 mol/L; 0.04347 is the amount of substance of MnO2, g/millimol of thiosulfate; and m is the weight of sample.
The leaching efficiencies of TMn, MnCO3 and MnO2 were determined with the following equation:
where w is the percentage of leaching efficiency; (TMn/MnCO3/MnO2)0 (g) is the total manganese or MnCO3 or MnO2 content before leaching; and (TMn/MnCO3/MnO2)i (g) is the total manganese or MnCO3 or MnO2 value after leaching.
The content of MnSiO3 in mineral and residue was measured as follows. Approximately 0.2 g of the sample was incinerated at 500 °C for 2 h. Then, the incinerated sample was placed into a 100 mL conical flask, followed by the addition of 25 mL HCl (37 wt %) and SnCl2 solution (10 wt %). After filtration, the residue was dissolved with 15 mL HCl (37 wt %), 5 mL HNO3 (69 wt %) and 2 mL HClO4 (70 wt %) in a PTFE (polytetrafluoroethylene) beaker. Then, it was filtrated and diluted with water to 50 mL, and the Mn content was detected through titration method. The relative error of the measured Mn contents, MnCO3 contents, MnO2 contents, and MnSiO3 contents had high accuracy, and the precision was less than ±0.1%. The recoveries were in the range of 97.9% to 102.1%.
2.4. Ore Characterization
The surface morphologies were examined with field emission scanning electron microscope (SEM Hitachi Limited model, S-3400N, Tokyo, Japan) equipped with energy dispersive X-ray spectrometry (EDS; IXRF Systems, Inc., Model550i, Austin, TX, USA).
3. Results and Discussion
3.1. Effect of Agitation Speed
The effect of agitation speed on the leaching rate of total manganese was investigated in the range of 100–700 r·min−1 under the conditions of the initial sulfuric acid concentration of 5 mol/L, 0.035 mol/L sucrose and temperature of 363.2 K. As shown in Figure 2, the extraction of total manganese increased when the agitation speed increased from 100 to 500 r·min−1 because of the enhanced diffusion of liquid reactants [22]. However, a slight decrease was observed when the agitation speed increased from 500 to 700 r·min−1, and this decrease was ascribed to the violent agitation that caused the particles to adhere onto the inner wall of the three-neck flask. This phenomenon reduced the leaching efficiency. The experimental research verified that the effect of external diffusion could be easily ignored when the agitation speed was over 500 r·min−1 [23]. Therefore, the agitation speed was kept constant at 500 r·min−1 in the following tests.
3.2. Effect of Sucrose Concentration on the Leaching Efficiencies of TMn, MnCO3 and MnO2
The manganese dioxide in semi-oxidized manganese ore could not be directly leached with sulfuric acid without reductant. The effects of sucrose concentration on the leaching efficiencies of TMn, MnCO3 and MnO2 were investigated in the range of 0.015 to 0.045 mol/L at the initial sulfuric acid concentration of 5 mol/L and temperature of 363.2 K to improve the leaching efficiency of manganese. The results are presented in Figure 3. Without adding sucrose, the leaching efficiency of TMn had a sharp increase in the first 45 min of the reaction (Figure 3b). Then, the leaching efficiency of TMn increased slightly from 36.5% to 38.2%. The efficiency of MnCO3 was reciprocated by a similar sharp increase in TMn (Figure 3a), thereby suggesting that MnCO3 could be easily extracted by sulfuric acid without reductants [24]. Although approximately 5% MnO2 was decomposed, the possibility was that the Mn2+ released from MnO2 would be apparently based on the small quantities of ferrous or biomass in ores that could be used as reductants [25]. The leaching efficiency of TMn increased dramatically with the increase of sucrose concentration from 0.015 mol/L to 0.035 mol/L. However, this increase was not remarkable in the later concentration of 0.035–0.045 mol/L with a slight augmentation in the leaching rate from 92.4% to 92.9%. Nearly 8% of TMn also remained in the leaching residues that included 7.3% MnO2 and 2.2% MnCO3. These observations demonstrated that the two manganese phases could not be recovered completely. Table 1 shows that 37.7% of silica existed in the semi-oxidized manganese ore. This finding implied that gangue encapsulation might be the main reason that restricts manganese from being leached. The leaching efficiency of MnCO3 rose slightly within a narrow range from 93.1% to 97.4% when the sucrose concentration reached 0.025 mol/L. This slight increase was the result of the exposure of MnCO3, which was encapsulated in MnO2, in the leaching process and then by its reaction with sulfuric acid. Then, the increase in the sucrose concentration had no significant effect on MnCO3 leaching efficiency. However, even if the phases of MnO2 and MnCO3 could be recovered completely under the aforementioned conditions, the approximately 2% TMn that remained in the ores could not be leached. This observation confirmed that some other manganese phases could not be dissolved easily in an acid solution with a reductant. Therefore, the optimal sucrose concentration should be 0.035 mol/L to leach manganese from the semi-oxidized manganese ore as completely as possible, and to decrease the sucrose concentration. This optimal sucrose concentration corresponded to the TMn leaching efficiency of 91.8%, including 91.4% for MnO2 and 96.9% for MnCO3 dissolved in 60 min. This sucrose concentration was used in all subsequent experiments.
3.3. Effect of Sulphuric Acid Concentration on the Leaching Efficiencies of TMn, MnCO3 and MnO2
The influence of sulfuric acid concentration on the dissolution of semi-oxidized manganese ore was examined with temperature of 363.2 K, 0.035 mol/L sucrose. The result is shown in Figure 4. When the H2SO4 concentration was 1 mol/L, the leaching efficiencies of TMn and MnO2 were stabilized at 56.3% and 37.8%, respectively, while 81.4% MnCO3 was dissolved rapidly within 30 min. This phenomenon revealed that MnCO3 and MnO2 generated a competition relation to hydrogen ions, and a certain increase in sulfuric acid concentration was an accelerator to leach semi-oxidized manganese ore in which the MnCO3 phase could obtain hydrogen ions more easily than the MnO2 phase. Therefore, the decomposition rate of MnCO3 was faster than that of the latter at low acid concentrations. As the acid concentration increased to 3 mol/L, the leaching efficiency of TMn increased evidently and reached 79.6% within 30 min. However, this increase was not remarkable in the later period from 30 to 120 min with a slight growth in the leaching efficiency from 79.6% to 84.6%. This observation indicated that the leaching activity of semi-oxidized manganese ore in hydrometallurgy process was relatively low compared to manganese dioxide, which was reported by Veglio [20]. The further leaching of MnCO3 also verified that a part of MnCO3 was truly encapsulated in MnO2. Meanwhile, the leaching efficiency of MnO2 could reach 82.5% in 120 min. This finding indicated that increasing the acid concentration could relieve the competitive relations between MnCO3 and MnO2 for hydrogen ions. The leaching efficiencies of TMn and MnO2 also increased obviously with the increase of sulfuric acid concentration from 1 to 5 mol/L. However, they did not apparently move up after 5 mol/L with a spot of augmentation from 92.4% to 92.9% and 92.5% to 92.6%. The leaching efficiency of MnCO3 also changed from 97.8% to 97.9%. Consequently, the extra consumption of sulfuric acid should be avoided, and the sulfuric acid concentration of 5 mol/L was adequate for the next leaching process.
3.4. Effect of Temperature on the Leaching Efficiencies of TMn, MnCO3 and MnO2
The influence of the reaction temperature on the leaching efficiency of Mn was examined in the range of 323.2 to 363.2 K. As shown in Figure 5, the temperature had a significant effect on the leaching efficiencies of TMn, MnO2 and MnCO3. The leaching efficiencies of TMn, MnO2 and MnCO3 increased from 85.3%, 83.4% and 87.1% to 92.4%, 92.6% and 97.7%, respectively, by increasing the temperature from 323.2 to 363.2 K. In consideration of the equipment and leaching cost, the most favorable temperature was chosen as 363.2 K.
3.5. Characterization of Semi-Oxidized Manganese Ore and Leaching Residue
The mineralogical forms of semi-oxidized manganese ore samples before and after leaching treatment were characterized with XRD and SEM with EDS element mapping, which are depicted in Figure 1, Figure 6 and Figure 7, respectively.
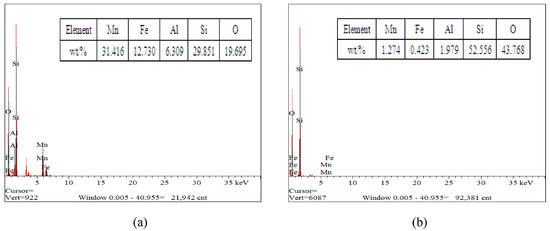
Figure 7.
EDS of raw manganese ore or residues under different conditions: (a) semi-oxidized manganese ore; and (b) leaching residue.
Figure 6 (a1,a2) shows that the particle of ore had smooth surface morphology, whereas those etched with H2SO4/sucrose had rough surface and many micropores in the surface after leaching (Figure 6 (b1,b2)). The EDS element mapping (seen in Figure 7) also demonstrated that Mn, Al and Fe elements had obviously different distributions in original ore and leaching residue. Figure 6b shows that the distribution of Mn, Al and Fe was lower than that of the element in ore, particularly Mn. This observation indicated that these elements dissolved after leaching in acid medium with reductant [12]. The dissolution of partial Fe agreed with the result of Section 3.1, that approximately 5% MnO2 was decomposed without a reducing agent. Nevertheless, the leaching experiments also showed that approximately 8% of TMn could not be leached with high acid consumption and excessive reductant. The comparison of Figure 6a,b indicated that the particles of ore had not appeared to fracture after leaching, which might have resulted in incomplete leaching.
The XRD patterns showed that the gangue was overwhelmingly the most common mineral component in the ore sample and leaching residue. This finding was in accordance with EDS element mapping in Figure 7. Compared with Figure 1a, Figure 1b also represents observable minor peaks of 3Mn2O3·MnSiO3 and an absence of phases of MnO2 and MnCO3. However, their mineral contents were excessively low, such that the fraction of 3Mn2O3·MnSiO3, MnO2 and MnCO3 in the leaching residue could not be reliably determined from the XRD pattern. Hence, the chemical characteristics of the residue sample are analyzed in Table 2. The content of insoluble manganese in residue (leached from 10 g of ore) was approximately 0.20 g, which accounted for approximately 7.8% of TMn. The contents of MnO2, MnCO3 and MnSiO3 were 0.11, 0.03 and 0.04 g, which accounted for 56.78%, 13.07% and 18.09%, respectively, which were still contained in the leaching residue as the amount of gangue. The schematic of various manganese leaching from semi-oxidized manganese ore was shown in Figure 8.

Table 2.
Content of insoluble manganese phase in residues.
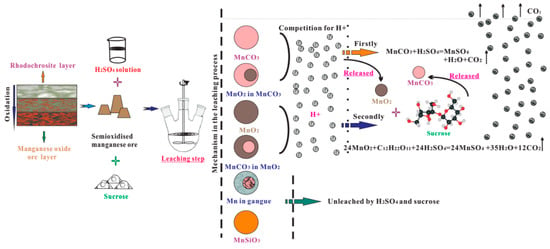
Figure 8.
Schematic of the manganese leaching from semi-oxidized manganese ore by sucrose in H2SO4 medium.
3.6. Kinetic Analysis
As mentioned above, the gangue was the main mineral component in the leaching residue and the initial ore surface appeared smooth but became evidently rough after leaching, with numerous pits. The morphology of reacted particles supported the appropriateness of using the shrinking core model (SCM) to describe the kinetics of leaching in semi-oxidized manganese ore. Generally speaking, the classical models could be concluded to three equations as follows [23,26,27]: surface chemical reaction controlled kinetic model (Equation (4)), internal diffusion controlled kinetic model (Equation (5)) and mixed-controlled kinetic model (Equation (6)).
where x is the fraction reacted of Mn and t is the leaching time (min); kd, k is the apparent reaction rate constant (min−1), respectively.
In the range of 5–30 min, the leaching efficiency values yield sensible rate measurements, so it was convenient for studying the leaching mechanism through kinetic data and may be exploited for industrial application. This article mainly studied the dynamic behavior when the leaching tests started in 5 min. The experimental data (Figure 5) were correlated with various kinetic models (Equations (4)–(6)) for solid–liquid reactions to obtain the kinetic equation and apparent activation energy. The following diffusion-controlled kinetic equation (Equation (6)) was found to fit the data best with a correlation coefficient greater than 0.97. The results are shown in Figure 9.
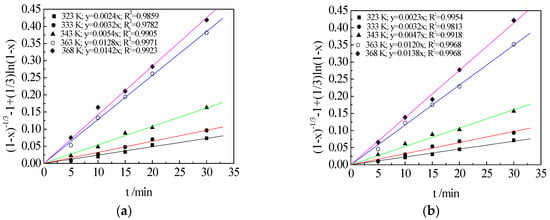
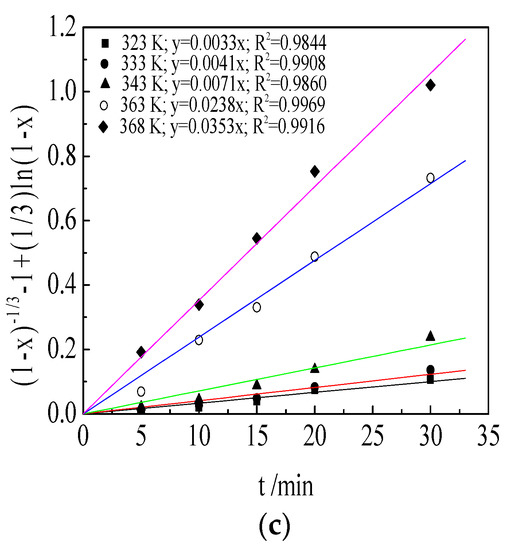
Figure 9.
Plots of (1 − x)1/3 − 1 + (1/3)ln(1 − x) versus time at different temperatures of leaching TMn (a); MnO2 (b); and MnCO3 (c) in semi-oxidized manganese ore.
As shown in Figure 9, the kinetic data of TMn, MnCO3 and MnO2 under different reaction temperatures were calculated using Equation (6). The leaching process followed the shrinking core model, and the leaching rate was controlled by chemical reaction and diffusion.
The apparent activation energy (Ea) was determined based on the following Arrhenius equation:
where k is a reaction rate constant, and k0 is the frequency factor. According to the diffusion constants at different temperatures of leaching process, the plots of lnk versus temperature were investigated. The results are indicated in Figure 10.
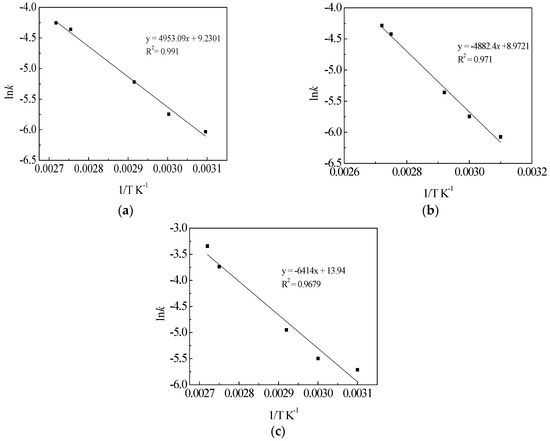
Figure 10.
Plots of lnk versus reciprocal temperature of leaching TMn (a); MnO2 (b); and MnCO3 (c) in semi-oxidized manganese ore.
As can be seen in Figure 10, the data of lnk versus 1/T were plotted for different temperatures, and the leaching equation of TMn was obtained: lnk = −4910.8/T + 9.1093. The regression equation was found to have an R2 of 0.991. Thus, the apparent activation energy was determined to be 40.83 kJ·mol−1. Likewise, the leaching equations for MnO2 and MnCO3 were lnk = −4882.4/T + 8.9721 and lnk = −6414/T + 13.94 with R2 of 0.9710 and 0.9679, and the apparent activation energies were 40.59 and 53.33 kJ·mol−1, respectively. The apparent activation energy of TMn, MnO2 and MnCO3 indicated that the leaching processes of semi-oxidized manganese ore were controlled by diffusion through the insoluble layer of the associated minerals [23,27]. The value clearly confirms that this process was controlled by chemical reaction and diffusion, simultaneously.
4. Conclusions
The reductive leaching process for semi-oxidized manganese ore with sucrose as the reducing agent in sulfuric acid medium was reported in this paper. The leaching rules of different valence states of manganese leaching were discussed. The research could be summarized as per below.
The total manganese leaching efficiency could reach 91.8% when the sucrose concentration and sulfuric acid hugely exceeded the stoichiometric concentration (based on the actual Mn(II) and Mn(IV) content of the ore) for 60 min at 363.2 K. The leaching efficiencies of MnO2 and MnCO3 in the ore were 91.4% and 96.9%, respectively.
The leaching process showed that the leaching rate of MnCO3 was faster than that of MnO2 in the ore, given that MnCO3 could easily access H+. Approximately 5% MnO2 was decomposed without a reducing agent due to the dissolution of partial Fe in ores. The decomposition of MnO2 could also expose the MnCO3 that was encapsulated in it, and MnCO3 could be aided to participate in the reaction.
The shrinking core model can be used to describe the leaching process, and the leaching rate was controlled by chemical reaction and diffusion. The apparent activation energy of total manganese, MnO2, and MnCO3 leaching were 40.83, 53.33, 40.59 kJ·mol−1, respectively.
Acknowledgments
This work was financially supported by the NSFC (No. 51164002, 51664002), the Province Science and Technology Key Projects of Guangxi (No. 1598015-4, 2016AA01115) and the Innovation Project of Guangxi Graduate Education (YCBZ2014014).
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used Yuhong Wang and Shenglong Jin conceived and designed the experiments; Yuhong Wang and Shenglong Jin performed the experiments; Yuhong Wang and Yan Lv analyzed the data; Shenglong Jin and Yan Lv contributed reagents/materials/analysis tools; Yuhong Wang, Yanjuan Zhang and Haifeng Su wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, J.; Dreisinger, D.; Glück, T. Manganese electrodeposition—A literature review. Hydrometallurgy 2014, 141, 105–116. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, G.; Yan, H.; Zhao, Y.; Li, T.; Feng, X. Reduction of low-grade manganese dioxide ore pellets by biomass wheat stalk. Acta Metall. Sin. Engl. Lett. 2013, 26, 167–172. [Google Scholar] [CrossRef]
- Xie, C.; Xu, L.; Peng, T.; Chen, K.; Zhao, J. Leaching process and kinetics of manganese in low-grade manganese ore. Chin. J. Geochem. 2013, 32, 222–226. [Google Scholar] [CrossRef]
- Zhang, Y.; You, Z.; Li, G.; Jiang, T. Manganese extraction by sulfur-based reduction roasting-acid leaching from low-grade manganese oxide ores. Hydrometallurgy 2013, 133, 126–132. [Google Scholar] [CrossRef]
- Cai, Z.; Feng, Y.; Li, H.; Du, Z.; Liu, X. Co-recovery of manganese from low-grade pyrolusite and vanadium from stone coal using fluidized roasting coupling technology. Hydrometallurgy 2013, 131–132, 40–45. [Google Scholar] [CrossRef]
- Yang, K.D.; Ye, X.J.; Su, J. Response surface optimization of process parameters for reduction roasting of low-grade pyrolusite by bagasse. Trans. Nonferrous Met. Soc. China 2013, 23, 548–555. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, G.; Cheng, Z. Thermal analysis and kinetic modeling of manganese oxide ore reduction using biomass straw as reductant. Hydrometallurgy 2010, 105, 96–102. [Google Scholar] [CrossRef]
- Tang, Q.; Zhong, H.; Wang, S.; Li, J.Z.; Liu, G.Y. Reductive leaching of manganese oxide ores using waste tea as reductant in sulfuric acid solution. Trans. Nonferrous Met. Soc. China 2014, 24, 861–867. [Google Scholar] [CrossRef]
- Tian, X.K.; Wen, X.X.; Yang, C.; Liang, Y.J.; Pi, Z.B.; Wang, Y.X. Reductive leaching of manganese from low-grade manganese dioxide ores using corncob as reductant in sulfuric acid solution. Hydrometallurgy 2010, 100, 157–160. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhu, G.C.; Zhao, Y.L. Study in reduction-roast leaching manganese from low-grade manganese dioxide ores using cornstalk as reductant. Hydrometallurgy 2009, 96, 176–179. [Google Scholar] [CrossRef]
- Su, H.F.; Wen, Y.X.; Wang, F.; Sun, Y.Y.; Tong, Z.F. Reductive leaching of manganese from low-grade manganese ore in H2SO4 using cane molasses as reductant. Hydrometallurgy 2008, 93, 136–139. [Google Scholar] [CrossRef]
- Hariprasad, D.; Dash, B.; Ghosh, M.K.; Anand, S. Leaching of manganese ores using sawdust as a reductant. Miner. Eng. 2007, 20, 1293–1295. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Furlani, G.; Valentini, P.; Vegliò, F.; Toro, L. Leaching of low-grade manganese ores by using nitric acid and glucose: Optimization of the operating conditions. Hydrometallurgy 2004, 75, 157–167. [Google Scholar] [CrossRef]
- Sahoo, R.N.; Naik, P.K.; Das, S.C. Leaching of manganese from low-grade manganese ore using oxalic acid as reductant in sulphuric acid solution. Hydrometallurgy 2001, 62, 157–163. [Google Scholar] [CrossRef]
- El Hazek, M.N.; Lasheen, T.A.; Helal, A.S. Reductive leaching of manganese from low grade Sinai ore in HCl using H2O2 as reductant. Hydrometallurgy 2006, 84, 187–191. [Google Scholar] [CrossRef]
- Nayl, A.A.; Ismail, I.M.; Aly, H.F. Recovery of pure MnSO4·H2O by reductive leaching of manganese from pyrolusite ore by sulfuric acid and hydrogen peroxide. Int. J. Miner. Process. 2011, 100, 116–123. [Google Scholar] [CrossRef]
- Sun, W.Y.; Su, S.J.; Wang, Q.Y.; Ding, S.L. Lab-scale circulation process of electrolytic manganese production with low-grade pyrolusite leaching by SO2. Hydrometallurgy 2013, 133, 118–125. [Google Scholar] [CrossRef]
- Lan, Y.Z. Laboratory study: Simultaneous leaching silver-bearing low-grade manganese ore and sphalerite concentrate. Miner. Eng. 2004, 17, 1053–1056. [Google Scholar]
- Xin, B.P.; Li, T.; Li, X.; Dan, Z.G.; Xu, F.Y.; Duan, N.; Zhang, Y.T.; Zhang, H.Y. Reductive dissolution of manganese from manganese dioxide ore by autotrophic mixed culture under aerobic conditions. J. Clean. Prod. 2015, 92, 54–64. [Google Scholar] [CrossRef]
- Vegliò, F.; Toro, L. Fractional factorial experiments in the development of manganese dioxide leaching by sucrose in sulphuric acid solutions. Hydrometallurgy 1994, 36, 215–230. [Google Scholar] [CrossRef]
- Beolchini, F.; Peterangelpapini, M.; Toro, L.; Trifoni, M.; Vegliò, F. Acid leaching of manganiferous by sucrose: Kinetic modeling and related statistical analysis. Miner. Eng. 2001, 14, 175–184. [Google Scholar] [CrossRef]
- Su, H.; Wen, Y.; Wang, F.; Li, X.; Tomg, Z. Leaching of pyrolusite using molasses alcohol wastewater as a reductant. Miner. Eng. 2009, 22, 207–209. [Google Scholar] [CrossRef]
- Su, H.; Liu, H.; Wang, F.; Lü, X.; Wen, Y. Kinetics of reductive leaching of low-grade pyrolusite with molasses alcohol wastewater in H2SO4. Chin. J. Chem. Eng. 2010, 18, 730–735. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lin, Q.Q.; Li, L.F.; Fu, J.G.; Zhu, Z.S.; Wang, C.Q.; Qian, D. Study on hydrometallurgical process and kinetics of manganese extraction from low-grade manganese carbonate ores. Int. J. Min. Sci. Technol. 2014, 24, 567–571. [Google Scholar] [CrossRef]
- Das, S.C.; Sahoo, P.K.; Rao, P.K. Extraction of manganese from low-grade manganese ores by FeSO4 leaching. Hydrometallurgy 1994, 8, 35–47. [Google Scholar] [CrossRef]
- Wu, F.; Zhong, H.; Wang, S.; Lai, S. Kinetic of reductive leaching of manganese oxide ore using cellulose as reductant. J. Cent. South Univ. 2014, 21, 1763–1770. [Google Scholar] [CrossRef]
- Xue, J.; Zhong, H.; Wang, S.; Li, C.; Li, J.; Wu, F. Kinetics of reduction leaching of manganese dioxide ore with Phytolacca americana in sulfuric acid solution. J. Saudi Chem. Soc. 2014, 9, 1–6. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).