Abstract
Because of the acute toxicity of Cr(VI)-bearing substances, the pollution problem caused by chromite process residue has become a worldwide concern. In the view of relevant studies, the technologies based on the alkali treatment cannot fundamentally resolve the pollution problem, because the oxidation of Cr(III) to Cr(VI) is unavoidable during chromite decomposition. In contrast, the oxidation of Cr(III) to Cr(VI) can be controlled by the sulfuric acid treatment of chromite, and the Cr(VI) pollution can be eliminated from the original source of production. Many research studies focusing on the resolutions of the key obstacles hindering the development of the sulfuric acid treatment process have been carried out, and significant progress has been achieved. In this study, a clean hydrometallurgical process without the generation of hexavalent chromium is demonstrated. First, the chromite was decomposed and leached by sulfuric acid solution in the presence of an oxidant. Then, iron was hydrothermally removed from the acid solution as the precipitate of jarosite. Finally, chromium salts were obtained by adjusting the basicity of the solution, separation and drying. With the aim of realizing industrialization, future research emphasis on the development of the sulfuric acid treatment process is proposed in this study.
1. Introduction
Chromium salts play an important role in the national economy and are widely used in metallurgy, chemical industry, military, textile, and machinery. Among the chromium salts, the consumption of basic chromium sulfate and chromium oxides accounts for more than half of the total consumption, in addition to chromium acetate, chromium chloride, and chromium salts of organic acids. In recent years, the chromium salt industry in China has made significant progress in terms of production scale, equipment level, product quality, and environmental protection awareness. Since 2000, the chromium salt production capacity of China has surpassed the U.S., ranked the first in the world, with an average annual growth rate of 10%. In 2005, China’s total production of chromium salt reached 259 thousand tons [1], which further increased to 363 thousand tons by 2011, accounting for 37.3% of the world’s chromium salt production [2]. With the rapid development of the chromium salt industry, the pollution problem caused by the decomposition of chromite, the main raw material for chromium salt preparation, has become more and more serious. The residual chromium-containing materials after the extraction are either left on site or used as landfill. Some chromate compounds found in the leftover residues are highly soluble and migrate via the surface and ground water, threatening human health and the environment. The highly soluble chromates pass through concrete walls in many buildings and threaten the occupants, resulting in the evacuation and closure of buildings [3]. In China, the annual emission of 300,000 tons of highly toxic chromium residue and large amounts of chromium-containing dust has seriously worsened the surrounding environment [4]. A shocking incident was reported previously, where 1800 water wells were scrapped because of the chromium residue pollution in Northeast China. In addition, chromium salt factories in Shanghai, Qingdao, Yixing, and other domestic cities were forced to shut down because of the pollution problem [5].
At present, the pollution caused by the chromium salt industry has become a worldwide environmental issue that has repeatedly arisen in the U.S., Japan, and other developed countries. For example, the American AlliedSignal Company was forced to shut down and the chromium salt factory in Germany had to be moved from Bayer to South Africa because of serious pollution [6,7]. Therefore, the development of a clean treatment process for chromite has become a significant concern.
2. Chromium Salt Preparation Technology
Chromium-bearing ores are found in many forms, but the economically extractable form is mineral chromite, which is inert and insoluble in water in its spinel form. Chromium exists in various oxidation states, but the common and stable chemical states are trivalent and hexavalent chromium [8]. For chromium salt preparation, the water-insoluble Cr(III) (chromite) is converted into the water-soluble Cr(VI) in alkaline solution in the presence of an oxidant, followed by the separation of the insoluble gangue from the leachate containing Cr(VI) by physical separation. As a result, the majority of chromium salt preparation technologies are based on the alkali oxidizing treatment of chromite because of the low oxidation potential of Cr(III) to Cr(VI) in alkaline environment, following the reactions shown in Equations (1)–(3) [9]. The soluble chromate compounds leach during the extraction process, and Cr(III) salts can be prepared by the reduction of sodium dichromate (Na2CrO4) [10,11,12]. However, the residual chromate slowly dissolves and is left behind in the ore residue, comprising chemical compounds such as calcium chromate (CaCrO4) with a solubility of 0.00071 mol·kg−1 in water at 20–30 °C and iron oxide [13,14]. As the acute oral lethal dose of Cr(VI) compound is 115 g, and the water containing >0.1 mg·L−1 of Cr(VI) is poisonous to the human body [15], the U.S. Environmental Protection Agency (EPA) classifies Cr(VI) as a Group A Human Carcinogen [8]. Hence, the alkali oxidizing treatment of chromite seriously threatens the environment and human health.
The calcium-roasting treatment process, a typical alkaline process, of chromite is widely used in China [16]. The silicon, aluminum, and iron in chromite react with calcium oxide to form insoluble calcium silicate and calcium alumino-ferrite; therefore, the impurity in the Cr(VI)-rich leachate could be reduced [17]. In this process, a large amount of highly toxic emissions including Cr(VI)-bearing residue, gas, and dust is generated, posing a serious threat to the environment. Moreover, the discharge of toxic residue containing Cr(VI) is linked to a low recovery of chromium, usually in the range of 75%–81%. According to surveys, the toxic residue of industrial chromium salts is mainly linked to sodium dichromate production. The preparation of one ton of sodium dichromate can generate ~2.5 tons of residue, in which the Cr(VI) content is ≥0.4%, present in the carcinogenic calcium chromate phase [18,19].
The non-calcium roasting process has been gradually adapted to replace the calcium-roasting process in the developed chemical industries such as at the Occidental Chemical Company in the U.S. and the Elementis Chemical Company in the UK [20]. In this process, the calcium reagent is replaced by reactive recycling residue, thereby sharply decreasing the discharge of toxic residue, especially carcinogenic calcium chromate [21]. The recycling residue with a particle size of ≥75 μm is recovered from the leached residue after air oxidation, wet milling, and leaching in the non-calcium roasting process, comprising chromite, MgO, NaAlSiO4, Na4MgAl2Si3O12, 6NaAlSiO4·Na2CrO4, α-Fe2O3, Mg(Fe,Al)2O4, and others [22,23]. According to the various technical procedures, the non-calcium roasting process can be divided into different technologies such as no-padding granulation technology [24,25], wet pelletizing technology [26], tri-roasting and bi-leaching technology [27], bi-roasting and neutral leaching technology [28], preheating single-roasting technology [29], oxidizing roasting technology with carbon ferrochrome [30], and pre-roasting technology by controlling silicon and aluminum [31]. Wang et al. [4] found that in the non-calcium roasting process, the recovery of chromium could reach >90%. In this process, the amount of chromic residue can be reduced to one-third of that in the calcium-roasting process, and the Cr(VI) content in the residue drops to one-tenth. In addition, almost no carcinogenic calcium chromate is found in the residue from the non-calcium roasting process [23]. However, the basic chemical reactions of the non-calcium roasting process are roughly the same as the calcium-roasting process, hence Cr(VI) compounds are still generated [32,33]. In summary, the non-calcium roasting process cannot completely solve the problem of Cr(VI) pollution, even though the amount of toxic residue is significantly reduced [34].
The liquid phase oxidation technology for chromite in molten (or sub-molten) salt was proposed by the Institute of Process Engineering, at the Chinese Academy of Sciences, and later became a burgeoning treatment process for chromium salt preparation [10,35]. In this process, the chromite suspended in the molten (or sub-molten) alkaline medium is decomposed by oxidation, and then the chromium salt is prepared by separation, filtration, and purification steps. In relation to the liquid phase oxidation process of chromite in molten (or sub-molten) salt, a significant number of thermodynamic and kinetic studies have been conducted, indicating that this process possesses a strong reaction tendency [36,37,38,39,40]. Zhang et al., in collaboration with the Zhenxin chemical company in the Henan province of China, built a demonstration pilot-scale process with an annual output of 8000 tons of potassium chromate to produce high-quality chromium salts [5]. The reaction temperature and energy consumption were both effectively decreased compared to those in the traditional technologies.
Xu et al. [41] decomposed chromite in NaOH solution in the presence of oxidizing gases including O2, air, O3 or their mixtures, and then prepared sodium chromate by pressure leaching. In this process, sodium dichromate and chromium anhydride are produced from sodium chromate by the electrolytic method. Wang et al. [42] utilized an electrochemical field to enhance the oxidative decomposition of chromite in a KOH sub-molten salt medium, affording the maximum extraction of chromium as 99%. In the aforementioned processes, the consumption of electricity is high, even though the discharge of toxic residue is limited. Hemmings et al. adapted a hydrothermal process with vacuum evaporation to treat chromite and successfully prepared chromic anhydride and sodium bisulfate [2]. Notably, none of the above-mentioned processes has achieved commercial-scale production.
As stated above, although many studies have been carried out to improve the chromium salt production process, none of these breaks away from the basic principle of oxidizing Cr(III) to Cr(VI) for chromite decomposition. As a result, the pollution problem caused by Cr(VI)-bearing substances in the chromium salt industry has not yet been resolved. Cr(III) is insoluble in alkaline solution; however, it is soluble in sulfuric acid solution. The oxidation from Cr(III) to Cr(VI) is therefore avoided in the sulfuric acid treatment process, which is cleaner and more environmentally friendly compared to the alkaline treatment process. Nevertheless, two obstacles hinder the development of the sulfuric acid treatment process: (i) the chromium in the chromite is difficult to leach because of the high stability of the chromite spinel in sulfuric acid solution at room temperature and atmospheric pressure; (ii) iron, magnesium, and aluminum ions can be released into the leachate concomitantly as chromium is leached, and the removal of impurities is especially difficult for the separation of Fe3+ and Cr3+. With increasing requirements for environmental protection, the development of a sulfuric acid treatment process has attracted significant attention, and the resolution of the two obstacles has become a worldwide focus of research.
3. Leaching Process of Chromite in Sulfuric Acid Solution
The term “chromite” refers to chromium-containing spinels, with a composition of AO·B2O3, where the divalent cation A can be Fe2+ or Mg2+, and the trivalent cation B can be Fe3+, Cr3+, or Al3+ [43]. Chromite spinel (FeCr2O4) is the main mineral phase of the chromite ore [44]. As shown in Figure 1, chromite spinel belongs to the cubic system, in which the 32 oxygen atoms stack in the central plane of the large cubic cell, forming 64 tetrahedral voids and 32 octahedral voids [45]. For the classic chromite spinel, among these voids, eight tetrahedral sites are occupied by Fe(II) and 16 octahedral sites are occupied by Cr(III). Chromite spinel has a stable and compact spinel lattice structure that is barely soluble in sulfuric acid solution at room temperature and atmospheric pressure. For the chromite ore in nature, a part of the Fe(II) can be replaced by Mg(II) and a part of the Cr(III) can be replaced by Al(III) or Fe(III). Thus, (Mg,Fe)(Cr,Al,Fe)2O4 as the chromium-bearing phase is isomorphic, in which Mg(II) and Fe(II) occupy the tetrahedral sites of the lattice, whereas Cr(III), Al(III), and Fe(III) occupy the octahedral sites [46,47].
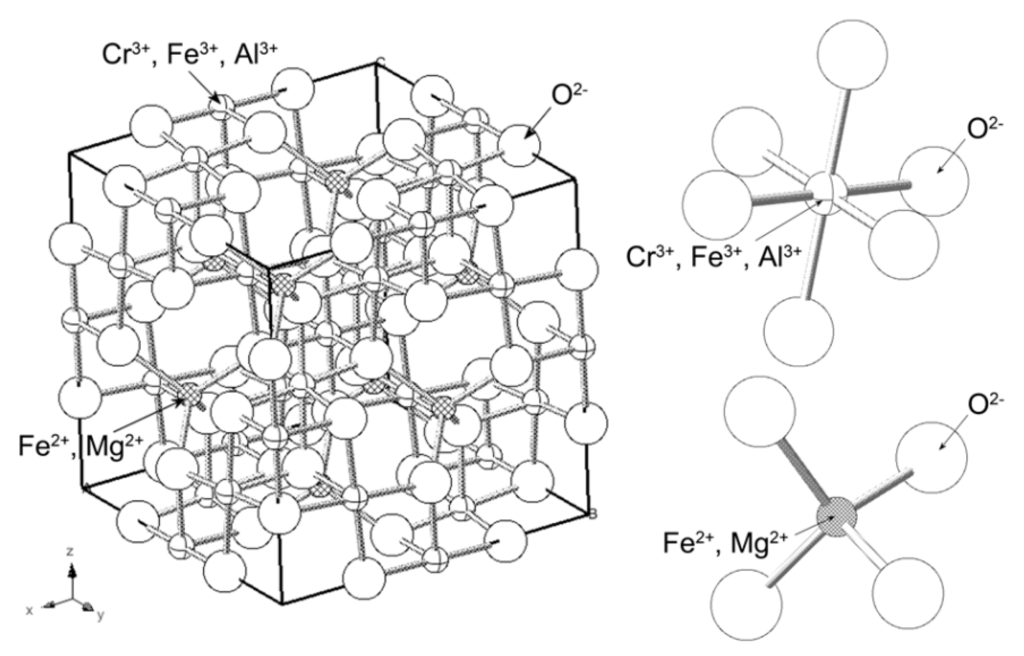
Figure 1.
Crystalline structure of chromite spinel [45].
With the aim to investigate the leaching thermodynamics of chromite in sulfuric acid solution, a potential (E)-pH diagram for the Fe-Cr-H2O system based on the principle of simultaneous equilibrium was developed using Factsage 6.4, as shown in Figure 2 [48]. In all the colored regions, chromium is soluble as either Cr3+ or Cr2O72−. However, to realize the dissolution of Cr(III) from chromite, only the potential and pH values in the green and the blue regions should be chosen based on the following reasons. In the red region, the chromium in Cr2O72− is Cr(VI), which is toxic. In the yellow region, although the chromium in Cr3+ is Cr(III), the valence of iron is same as that in the chromite spinel, and the structure of the spinel is difficult to disrupt, because no oxidation reaction occurs. Hence, only in the green and blue regions can chromium in the chromite spinel be dissolved effectively in the form of Cr3+ by the oxidation of Fe(II) to Fe(III). Clearly, an appropriate oxidation potential and low pH value are essential for the decomposition of chromite spinel and, in particular, the oxidation potential plays a key role in preventing the generation of Cr(VI).
Biermann et al. [49] investigated the decomposition mechanism of chromite by sulfuric acid. The initial step involves the attack of protons on the chromite lattice, bringing the metallic constituents into the solution in a similar ratio as that in the lattice. The second step is the precipitation of polynuclear products that can slow down the attack on the chromite. The extraction of chromium by leaching in sulfuric acid is limited without an oxidant. With the goal of improving the leaching behavior of chromite in sulfuric acid solution, Geveci et al. [50] used perchloric acid as the catalyst in the leaching process at atmospheric pressure, achieving 83% chromium extraction. Vardar et al. [51] determined that the apparent activation energy for the leaching of chromite in sulfuric acid at atmospheric pressure is 77 kJ·mol−1 in the presence of perchlorate. Shi et al. [52,53] used sodium bichromate as an oxidant in the leaching process at atmospheric pressure and 110–170 °C, achieving a chromium extraction of 82%. All of the above investigations indicate that no Cr(VI) was generated in the sulfuric acid leaching process. It is still worth noting that the extraction of chromium is not sufficiently high, probably because of the inappropriate choice of oxidant. Liu et al. [54,55,56] found that adding a certain amount of oxidant could notably improve the chemical potential of the sulfuric acid solution, thus significantly accelerating the chromite sulfuric acid leaching process, e.g., the extraction of chromium was 93% without the generation of Cr(VI).
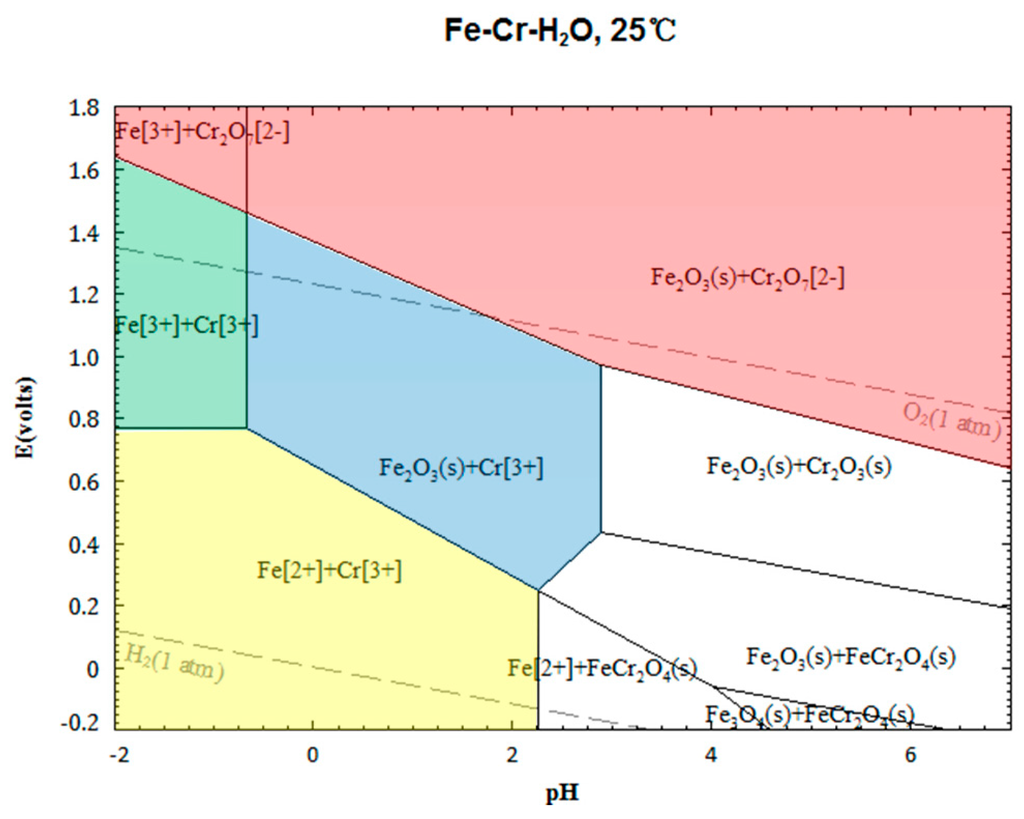
Figure 2.
E-pH diagram of the Cr-Fe-H2O system.
Zhao et al. [57,58] investigated the sulfuric acid leaching behavior of chromite in the presence of oxidant affording the optimal chromium extraction of 96.4%. The sulfuric acid leaching behavior of the chromite in Zhao et al.’s study can be illustrated as shown in Figure 3, where four types of spinel, denoted as I, II, III, and IV, represent different states at specific stages of the leaching process. Spinel I shows that the particle becomes smaller with the duration of the leaching. Three corrosion depths marked as Db(p), Db(g), and Dg illustrate that the phase boundary is the most prone to corrosion followed by the grain boundary, whereas the grain surface is relatively stable. Spinel particles fall off or react to completion, leaving holes in the silicon-rich phase (cf. Spinel II). The precipitation of sulfate may occur locally if the temperature is too high. Some of the inner spinel phase may react when the solution flushes the solid layer and is in direct contact with the spinel (cf. Spinel III); however, other deeper spinel phases cannot be leached over short times (cf. Spinel IV), as the silicon-rich phase acts as a barrier. The model developed by Zhao et al. is based on the experimental results of leaching chromite lump, and the sulfuric acid leaching behavior of the chromite with the optimized particle size should be further studied.
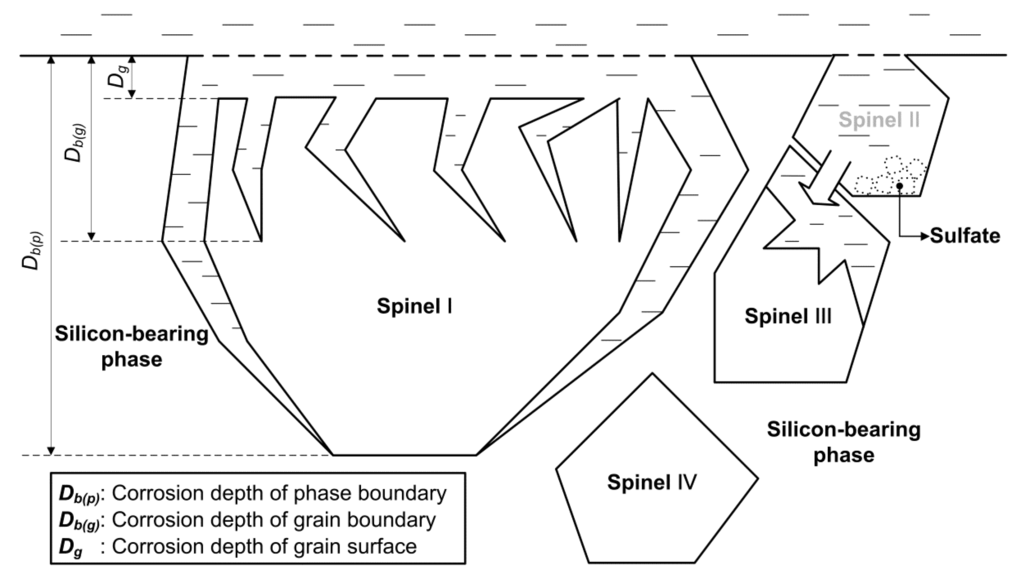
Figure 3.
Leaching mechanism of chromite with sulfuric acid [57].
Although by using an appropriate oxidant the extraction of chromium can exceed 90% under optimal conditions, further studies are required to explore the choice of oxidant and its amount, revealing the mechanisms governing chromite spinel decomposition at a microscopic level.
4. Separation of Cr3+ and Fe3+ in Sulfuric Acid Leaching Solution
The main components in the sulfuric acid leaching solution of chromite are the sulfates of chromium, ferrous, aluminum, and magnesium. The ionic radius of Cr3+ and Fe3+ (0.62 and 0.64 Å, respectively) are so close that good separation of Cr3+ and Fe3+ is difficult to achieve. In contrast, Al3+ and Mg2+ are relatively easier to remove from the solution. Chromium salt products have strict limits on the impurity content of iron. The Chinese chemical industry standard (HG/T 267895) requires the ferrous content to be <0.1% in industrial basic chromium sulfate, and the Russian standard (ΓOCT2912-79) limits FeO content to below 0.01% [59]. Hence, one of the most important reasons for the failure of the sulfuric acid leaching process at industrial scale is the difficulty of separating Cr3+ and Fe3+ [60]. A significant number of studies have been carried out, and substantial progress has been made in the development of separation technology for Cr3+ and Fe3+ in the sulfuric acid solution. Some of this research is described in more detail in the following section.
4.1. Separation by Solvent Extraction
4.1.1. Liquid-Liquid Extraction of Chromium
In 1977, Stauter et al. [61] successfully separated Cr6+ and Fe3+ in sulfuric acid leaching solution by using the liquid-liquid extraction method. As the separation of Cr3+ and Fe3+ is much more difficult, later investigation [53] indicated that Cr3+ could be extracted into the organic phase, and thus Cr3+ and Fe3+ could be separated in the presence of primary amine (R, R′–CH–NH2) as the extractant. After stripping by sulfuric acid, the separation coefficients of chromium and iron were >200.
4.1.2. Liquid-Liquid Extraction of Iron
Akash et al. [62] investigated the separation efficiency of Fe3+ from the Cr3+-bearing solution and reported Cyanex923 as an effective extractant. Shi et al. [63] extracted Fe3+ from the Cr3+-bearing solution using octadeyl dimethyl tertiary amine as the extractant. Ma et al. [64] extracted Fe3+ from the Cr3+-bearing solution using bis(2-ethylhexyl) phosphoric acid (D2EHPA) as the extractant. After the extraction, the ferrous form was stripped from the organic phase by hydrochloric acid.
The prominent advantage of the solvent extraction method is its economical basis, because the extractant can be recycled. However, the expectation for sufficiently high removal efficiency of iron with a very low loss of chromium is difficult to achieve.
4.2. Recovery of Chromium by Salting-Out
In the mixed system of organic phase (water-soluble) and water, the solubility of the purple salt ([Cr(H2O)6]2(SO4)3) is much smaller than that of ferric sulfate. This property is utilized for salting-out chromium from the mixed solvent to separate chromium and iron. Wei et al. [65] used this method to separate chromium from iron in the leachate of ferrochromium alloy and the results showed that almost all Cr3+ was converted to [Cr(H2O)6]2(SO4)3. After the addition of ethanol to the leachate and vibration for a period of time, the purple salt precipitated. Chromium sulfate was obtained after drying in 97.1% purity.
4.3. Removal of Iron by Precipitation Methods
To improve the separation, different precipitation methods for iron or chromium have been proposed.
4.3.1. Iron Hydrolysis
The pH value is different when hydrolysis reactions of different metal ions are at equilibrium. Compared to other metal ions, dissolved iron is more prone to hydrolyze and precipitate as ferric hydroxide Fe(OH)3 [66]. Souza et al. [67] used hydrochloric acid to dissolve electroplating sludge and obtained an acid leaching solution containing Cr3+, Fe3+ and other metallic ions. After the addition of H2O2 to the solution, Cr3+ was oxidized to Cr6+. Then, by adjusting the pH value, Fe3+ and other metal ions (except Cr6+) precipitated in the form of hydroxides. Because of the strong adsorption by Fe(OH)3, the concentration of iron in the solution was very low, and the loss of chromium decreased to ~8%.
When the concentration of Fe3+ is sufficiently low, Fe3+ can be precipitated as goethite (FeOOH) by adjusting the pH value [68,69]. The chemical reaction is shown in Equation (4).
Chen et al. [70] carried out an experimental study on iron removal from the sulfuric acid leaching solution of chrome cake using the goethite process. Oxygen was blown into the leaching solution, and the oxidation rate was controlled by tuning the rate of oxygen addition. The concentration of Fe3+ in solution was controlled to <1.5 g·L−1 during the entire process. The iron removal was 99.5%, but the total chromium loss reached 17%. Hu et al. [71] controlled the oxidation rate of Fe2+ in the leachate of ferrochromium alloy by injecting H2O2 slowly to ensure that the concentration of Fe3+ was <1.0 g·L−1. Finally, the removal of iron reached 99%, and the chromium loss was 15%. The above studies indicate some limitations to the oxidation rate of Fe2+ to ensure that the concentration of Fe3+ is maintained at a low level. To control the concentration of Fe3+ at <1.0 g·L−1, the production efficiency of goethite becomes low. In addition, the chromium loss is usually >15% because of the strong adsorption by goethite at the pH values used to form goethite.
4.3.2. Jarosite Process
According to the equilibrium diagram for the Fe2O3-SO3-H2O system [68], by adjusting the pH value and temperature, Fe3+ can be precipitated from the sulfate solution in the form of a basic salt, normal salt or acidic salt. Among these salts, the basic salt (3Fe2O3·4SO3·9(H2O)) is the most stable and can also be written as (H3O)2·3Fe2O3·4SO3·6H2O. When Na+, K+ or NH4+ is present in the solution, (H3O)2·3Fe2O3·4SO3·6H2O can be transformed into jarosite analogs (Na,K,NH4)2Fe6(SO4)4(OH)12, which are usually very stable. The reaction is shown by Equation (5) [72].
Zhang [73] combined the goethite and jarosite processes to separate Fe3+ from the Cr-rich solution from chromite at room temperature and atmospheric pressure. The ferrous removal reached 97%, while the loss of chromium was 15%. Shi [74] studied the ferrous removal from chromite leachate using the jarosite process under hydrothermal conditions in an autoclave. The ferrous removal was >99% and, at the same time, the chromium loss was 5.9%.
4.3.3. Formation of Mohr’s Salt
The principle of Mohr’s salt process is to crystallize ammonium chromium sulfate and ammonium ferrous sulfate (Mohr’s salt) by adding ammonium sulphate into the solution containing Cr3+ and Fe2+. The crystallization reactions are shown in Equations (6) and (7).
When the temperature is 0 °C, the solubility of ammonium ferrous sulfate is 0.4 g·L−1, and the solubility of ammonium chromium sulfate is 70 g·L−1. The difference in the solubility can be used to precipitate ferrous to remove iron from the solution. Some researchers [75,76,77] carried out ferrous removal experiments from the sulfuric acid solution of ferrochromium alloy by using the Mohr’s salt process. The concentration of ferrous ion was decreased to ~0.1 g·L−1, and the ferrous removal tops out at ~95%. Because of the theoretical limitation of this method, ferrous ions cannot be precipitated completely; therefore, clean separation is hard to achieve.
4.3.4. Ferrous Oxalate Precipitation
Ferrous oxalate precipitates can be formed by adding oxalic acid into the Fe2+-bearing solution. The reaction is shown in Equation (8).
Hu [78] and Wang [79] both studied the removal of Fe2+ from a leachate of carbon ferrochrome by ferrous oxalate precipitation. The content of chromium in the ferrous oxalate product was very low. However, the ferrous removal was not satisfactory and the content of ferrous in chromium oxide powders was up to ~0.5%. Moreover, the oxalic acid is expensive, increasing the process cost.
As mentioned above, the separation of iron and chromium has been improved through on-going efforts. As a result, a clean hydrometallurgical process can be designed, taking the preparation process of basic chromium sulfate as an example [80]. As shown in Figure 4, chromite is first decomposed by sulfuric acid solution with the addition of an oxidant, and Cr3+ is extracted into the acid solution. The major component of the residue is SiO2, which can be directly applied to ceramic preparations and in the construction industry. For the removal of iron, seed crystal is added to the acid solution, and jarosite is precipitated by the hydrothermal treatment, which can be used for the preparation of pigment and magnetic material. Notably, jarosite is potentially unstable and can be releached under suitable conditions if not impounded correctly. After the removal of iron, basic chromium sulfate is obtained by adjusting the basicity of the solution by adding sodium carbonate followed by drying. Some preliminary experimental studies have shown that no hexavalent chromium is generated in the entire process. Moreover, the mass fraction of iron in the basic chromium sulfate is <0.1% [75]. However, the chromium loss is also high (≥4%).
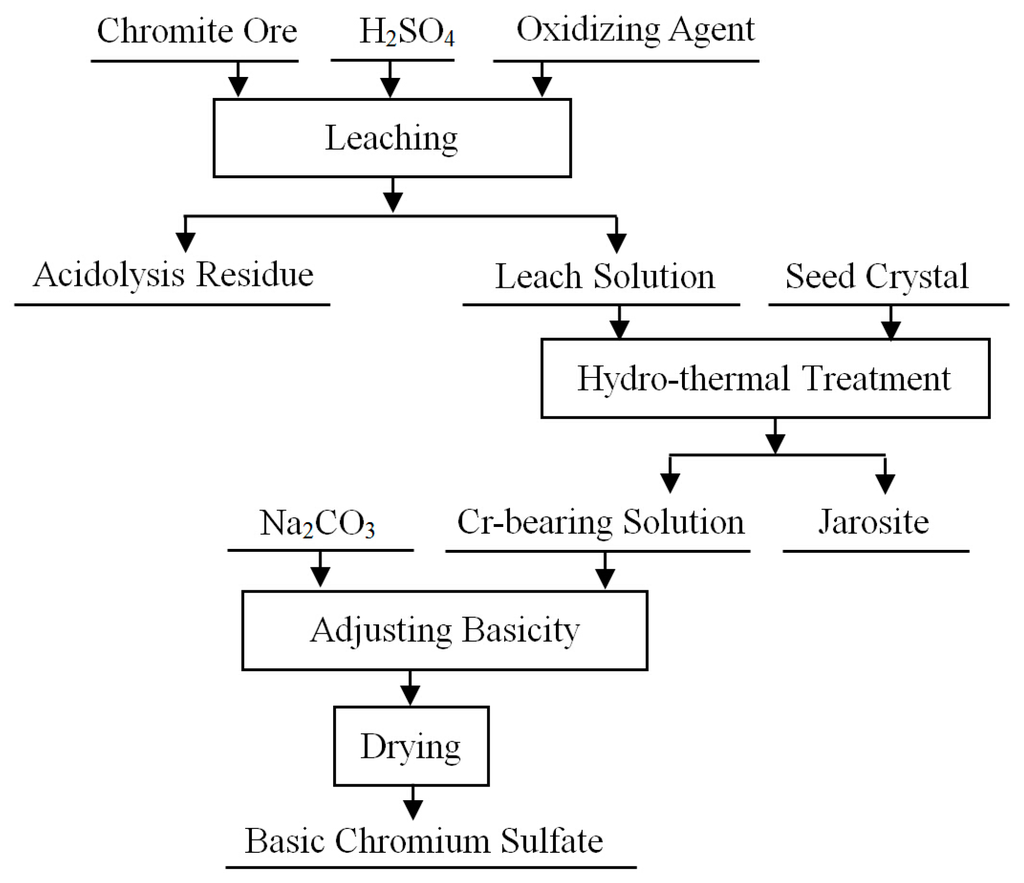
Figure 4.
Process flow chart for the production of a chromium salt from chromite ore.
5. Conclusions and Perspectives
Because of the acute toxicity of Cr(VI)-bearing substances, the pollution problem caused by the residue from the preparation process of chromium salts with chromite as the raw material has become a worldwide concern. For the development of a clean hydrometallurgical process of chromite, a large number of studies focusing on the fundamentals and technology developments have been conducted. Although a significant progress has been made, the technologies based on the alkali treatment cannot fundamentally resolve the pollution problem because they require the oxidation of Cr(III) to Cr(VI).
As Cr(III) is soluble in sulfuric acid solution as chromium sulfate, oxidizing Cr(III) to Cr(VI) is not required in the sulfuric acid treatment process of chromite. The Cr(VI) pollution can therefore be eliminated from the original source of production. With the on-going efforts, the resolutions of the key obstacles hindering the development of the sulfuric acid treatment process have achieved significant progress. A clean hydrometallurgical process without hexavalent chromium is demonstrated in this study. First, the chromite is decomposed in sulfuric acid solution with oxidation, and Cr3+ is extracted into the acid solution. Seed crystal is added to acid solution, and the jarosite is precipitated by the hydrothermal treatment. After the removal of iron, the basic chromium sulfate is obtained by adjusting the basicity of the solution with sodium carbonate, separation, and drying. This clean technology for the preparation of chromium salts based on the sulfuric acid leaching process is believed to be suitable for commercialization, achieving industrialization in the near future.
This study, focused on the leaching of chromite in sulfuric acid, achieved chromium extractions exceeding 90% under optimal conditions, and the thermodynamic and kinetic mechanisms of chromite spinel decomposition were clarified. To further improve the extraction of chromium, more studies are required to explore the choice of oxidant and its amount with the premise that no Cr(VI) is generated. Furthermore, jarosite is potentially unstable and subsequent chromium loss is high in the iron removal process; therefore, further study is required to impound jarosite correctly, minimizing the chromium loss. Finally, to obtain a detailed and thorough assessment of the economical efficiency and potential for contamination, scaled-up test work should be carried out.
Acknowledgments
Financial support to this project was provided by the National Natural Science Foundation of China (51304042), the Fundamental Research Funds for the Central Universities (N140204010), and the Postdoctoral Science Foundation of Northeastern University.
Author Contributions
Bo Zhang, Peiyang Shi and Maofa Jiang contributed to the conception of this study. Bo Zhang contributed significantly to the writing and analysis of the materials included in this review. All authors read and approved the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- Yan, J.X.; Chen, J.X.; Hu, L. Metallurgy of Chromium; Metallurgical Industry Press: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Li, Z.Y. The current status and development suggestion of chromium salt industry. Inorg. Salt Ind. 2004, 38, 1–5. (In Chinese) [Google Scholar]
- Meegoda, J.N.; Partymiller, K.; Richards, M.K.; Kamolpornwijit, W.; Librizzi, W.; Noval, B.A.; Mueller, R.T.; Santora, S. Remediation of chromium-contaminated soils: Pilot-scale investigation. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2000, 4, 7–15. [Google Scholar] [CrossRef]
- Wang, T.G.; He, M.L.; Pan, Q. A new method for the treatment of chromite ore processing residues. J. Hazard. Mater. 2007, 149, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.H.; Wang, Z.K.; Chen, J.Y. Green chemistry and chromium salt industry a new generation of industrial revolution. Chem. Ind. Eng. Prog. 1986, 10, 172–178. (In Chinese) [Google Scholar]
- Tinjum, J.M.; Benson, C.H.; Edil, T.B. Mobilization of Cr(VI) from chromite ore processing residue through acid treatment. Sci. Total Environ. 2008, 391, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wazne, M.; Jagupilla, S.C.; Moon, D.H.; Christodoulatos, C.; Koutsospyros, A. Leaching mechanisms of Cr(VI) from chromite ore processing residue. J. Environ. Qual. 2008, 37, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Meegoda, J.N.; Kamolpornwijit, W.; Vaccari, D.A.; Ezeldin, A.S.; Noval, B.A.; Mueller, R.T.; Santora, S. Remediation of chromium-contaminated soils: Bench-scale investigation. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 1999, 3, 124–131. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Chemistry Handbook Version; Science Press: Beijing, China, 1991. (In Chinese) [Google Scholar]
- Zheng, S.; Zhang, Y.; Li, Z.; Qi, T.; Li, H.; Xu, H. Green metallurgical processing of chromite. Hydrometallurgy 2006, 82, 157–163. [Google Scholar] [CrossRef]
- Amer, A.M.; Ibrahim, I.A. Leaching of a low grade Egyptian chromite ore. Hydrometallurgy 1996, 43, 307–316. [Google Scholar] [CrossRef]
- Amadta, U.; Batza, M.; Bellinghaousena, R. Method for manufacturing alkali chromates from chromics ore. Miner. Eng. 1996, 9, 1183. [Google Scholar]
- Burke, T.; Fagliano, J.; Goldoft, M.; Hazen, R.E.; Tglewicz, R.; Mckee, T. Chromite ore processing residue in Hudson County, New Jersey. Environ. Health Perspect. 1991, 92, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.A.; Salem, H. The Biological and Environmental Chemistry of Chromium; VCH Publishers: New York, NY, USA, 2000. [Google Scholar]
- Wilbur, S.B. Toxicological Profile for Chromium; Department of Health and Human Services: Washington, DC, USA, 2000.
- Cheng, S.W.; Ding, Y.; Yang, C.R. Chromate Production Process; Chemical Industry Press: Beijing, China, 1987. (In Chinese) [Google Scholar]
- Weber, R.; Rosenow, B.; Block, H.D. Process for the Preparation of Sodium Dichromate. U.S. Patent 5273735, 28 December 1993. [Google Scholar]
- Kapland, M.A.; Annapolis, M.D.; Robinson, M.W. Process for Treating and Stabilizing Chromium Ore Waste. U.S. Patent 4504321, 12 March 1985. [Google Scholar]
- Zhu, H.S.; Xie, G. The study of the behavior of chromium ore in roasting process. Min. Metall. Eng. 2006, 26, 57–60. (In Chinese) [Google Scholar]
- Ji, Z. Overview of non-calcium roasting technology for chromite. Chromium Salt Ind. 1996, 2, 12–19. (In Chinese) [Google Scholar]
- Korallus, U. The significance of ascorbic acid and glutathione for chromate metabolism in man. Toxicol. Environ. Chem. 1989, 12, 332–340. [Google Scholar]
- Zhang, D.W.; Li, X.; Ji, Z. Study on parameter control of chromite roasting with non-calcium process. Inorg. Chem. Ind. 2012, 6, 37–39. (In Chinese) [Google Scholar]
- Ji, Z.; Tian, Q.; Zhao, Q.Z. The phase composition of chromite calcium-free roasting clinker and slag. Inorg. Chem. Ind. 1984, 6, 31–32. (In Chinese) [Google Scholar]
- William, W.L.; Douglas, G.F.; Alan, B.G. Method for the Conversion of Chromite Ore to Sodium Chromate. U.S. Patent 3816094, 11 June 1974. [Google Scholar]
- Charles, P.B.; William, W.L.; Edmund, W.S. Method for Recovering Chromite Values from Chromite Ore. U.S. Patent 3816095, 11 June 1974. [Google Scholar]
- Charles, P.B.; Douglas, G.F. Production of Sodium Chromate. G.B. Patent 1454125, 30 June 1976. [Google Scholar]
- Wolfgang, B.; Hans, G.N.; Hans, N. Alkaline Disintegration of Chromites. U.S. Patent 4066734, 3 January 1978. [Google Scholar]
- Somanahalli, N.S.; Thomas, R.M.; Douglas, G.F. Method for Production of Alkali Metal Chromates from Chrome Ores. U.S. Patent 4244925, 13 January 1981. [Google Scholar]
- Heinrich, S. Alkali Treatment of Chromium Ores. U.S. Patent 3510256, 5 May 1970. [Google Scholar]
- Johann, N.M.; Hans, N.; Hans, G.N. Disintegration of Chromates. U.S. Patent 4500350, 19 February 1979. [Google Scholar]
- Cooper, D.; Hugh, S.; Rand, E.; Henry, J. Process for Producing Alkali Metal Chromates and Dichromates. U.S. Patent 3932598, 13 January 1976. [Google Scholar]
- Ji, Z. Reaction mechanism of calcium-free roasting of chromite. Inorg. Chem. Ind. 1997, 1, 18–21. (In Chinese) [Google Scholar]
- Yu, K.P.; Zhang, H.L.; Chen, B.; Xu, H.B.; Zhang, Y. Investigation of reaction mechanism on the lime-free roasting of chromium-containing slag. Metal. Mater. Trans. B 2015, 46, 2553–2563. [Google Scholar] [CrossRef]
- Reid, R.D.; Schneidmiller, D. Resolution of the mixed waste issue for EDTA-based steam generator chemical cleaning waste solutions. Waste Manag. 1996, 16, 271–276. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.H.; Wang, Z.K.; Chen, J.Y. Green chemistry and new revolution of chromic salts industry. Prog. Chem. 1998, 10, 172–178. (In Chinese) [Google Scholar]
- Sun, Z.; Zheng, S.L.; Zhang, Y. Thermodynamics study on the decomposition of chromite with KOH. Acta Metal. Sin. 2007, 20, 187–192. [Google Scholar] [CrossRef]
- Zheng, S.L.; Zhang, Y. Thermodynamic analysis on new reaction system of liquid phase oxidation of chromite in molten salt. Chin. J. Nonferr. Metals 1999, 9, 800–804. [Google Scholar]
- Sun, Z.; Zheng, S.L.; Xu, H.B.; Zhang, Y. Oxidation decomposition of chromite ore in molten potassium hydroxide. Int. J. Miner. Process. 2007, 83, 60–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, S.L.; Xu, H.B.; Du, H.; Zhang, Y. Decomposition of chromite ore by oxygen in molten NaOH-NaNO3. Int. J. Miner. Process. 2010, 95, 10–17. [Google Scholar] [CrossRef]
- Xu, H.B.; Zheng, S.L.; Zhang, Y.; Li, Z.H.; Wang, Z.K. Oxidative leaching of a vietnamese chromite ore in highly concentrated potassium hydroxide aqueous solution at 300 °C and atmospheric pressure. Miner. Eng. 2005, 18, 527–535. [Google Scholar] [CrossRef]
- Xu, H.B.; Zhang, Y.; Li, Z.H. A Pressure Leaching of Chromite Sodium Chromate Cleaner Production Methods. China Patent 101817561A, 1 September 2010. [Google Scholar]
- Wang, Z.H.; Du, H.; Wang, S.N.; Zheng, S.L.; Zhang, Y.; Hwang, S.; Kim, N.S.; Jeong, T.E. Electrochemical enhanced oxidative decomposition of chromite ore in highly concentrated KOH solution. Miner. Eng. 2014, 57, 16–24. [Google Scholar] [CrossRef]
- Kamolpornwijit, W.; Meegoda, J.N.; Hu, Z. Characterization of chromite ore processing residue. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2007, 11, 234–239. [Google Scholar] [CrossRef]
- Ji, Z. Preparation of trivalent chromium compounds from chromite by acid-leaching technique. Inorg. Chem. Ind. 2012, 44, 1–5. (In Chinese) [Google Scholar]
- Zhao, Q. Fundamental Research on the Cleaner Preparation Process of Basic Chromium Sulfate. Ph.D. Thesis, Northeastern University, Shenyang, China, 2015. [Google Scholar]
- Kanari, N.; Gaballah, I.; Allain, E. A study of chromite carbochlorination kinetics. Metal. Mater. Trans. B 1999, 30, 577–587. [Google Scholar] [CrossRef]
- Hussein, M.K.; Winterhager, H.; Kammel, R. Chlorination behavior of the main oxide components chromite ores. Trans. Inst. Min. Metal. Sect. C 1974, 83, 154–160. [Google Scholar]
- Bale, C.W.; Chartrand, P.; Decterov, S.A. FactSage thermochemical software and databases. Calphad 2002, 62, 189–228. [Google Scholar] [CrossRef]
- Biermann, W.J.; Heinrichs, M. The attack of chromite by sulphuric acid. Can. J. Chem. 1960, 38, 1449–1454. [Google Scholar] [CrossRef]
- Geveci, A.; Topkaya, Y.; Ayhan, E. Sulfuric acid leaching of Turkish chromite concentrate. Miner. Eng. 2002, 15, 885–888. [Google Scholar] [CrossRef]
- Vardar, E; Eric, R.H.; Letowski, F.K. Acid leaching of chromite. Miner. Eng. 1994, 7, 605–617. [Google Scholar]
- Shi, P.Y.; Jiang, M.F.; Liu, C.J.; Liu, S.L. A Method for Preparing Basic Chrome Sulphate. China Patent 1264755C, 19 July 2006. [Google Scholar]
- Shi, P.Y.; Liu, S.L. Experimental study on sulphuric acid leaching of chromite. J. Chin. Rare Earth Soc. 2002, 20, 472–474. (In Chinese) [Google Scholar]
- Liu, C.J.; Shi, P.Y.; Jiang, M.F. A Method of Sulfuric acid Leaching to Process Chromite. China Patent 101979679A, 23 February 2011. [Google Scholar]
- Liu, C.J.; Sun, L.F.; Shi, P.Y.; Jiang, M.F. Study on sulfuric acid leaching technology from chromite. J. Ecotechnol. Res. 2006, 12, 177–180. [Google Scholar]
- Liu, C.J.; Qi, J.; Jiang, M.F. Experimental study on sulfuric acid leaching behavior of chromite with different temperature. Adv. Mater. Res. 2012, 361, 628–631. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, C.J.; Shi, P.Y.; Zhang, B.; Jiang, M.F.; Zhang, Q.S.; Zevenhoven, H.S.R. Sulfuric acid leaching of South African chromite. Part 1: Study on leaching behavior. Int. J. Miner. Process. 2014, 130, 95–101. [Google Scholar] [CrossRef]
- Jiang, M.F.; Zhao, Q.; Liu, C.J.; Shi, P.Y.; Zhang, B.; Yang, D.P.; Saxén, H.; Zevenhoven, R. Sulfuric acid leaching of South African chromite. Part 2: Optimization of leaching conditions. Int. J. Miner. Process. 2014, 130, 102–107. [Google Scholar] [CrossRef]
- Liu, S.Y. Industry standard profile of chromium oxides. Chem. Stand. Qual. Surveill. 1997, 1, 7–9. (In Chinese) [Google Scholar]
- Cheng, J.Y. Hydrometallurgy Manual; Metallurgical Industry Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Stauter, J.C.; Richard, T. Recovery of Chromium Values. U.S. Patent 4029734, 14 June 1977. [Google Scholar]
- Akash, D.; Paulo, F.M.; Jorge, M.R. Selective recoveries of Fe(III) and Cr(III) from a tannery filtrate using Cyanex 923. Anal. Chim. Acta 2006, 558, 254–260. [Google Scholar]
- Shi, P.Y.; Liu, C.J.; Jiang, M.F. One Method to Separate Iron and Chromium from Multi-Component Solution. China Patent 101974688A, 16 February 2011. [Google Scholar]
- Ma, H.R.; Li, D.X.; Shi, X.F. Solvent extraction of iron and chromium from bio-leachate derived from tannery sludge. Environ. Chem. 2007, 26, 508–511. (In Chinese) [Google Scholar]
- Wei, Y.Z.; Kumaya, M.; Takashima, Y. A Separation and Recovery Method of Chromium. Japan Patent 157219, 18 June 1996. [Google Scholar]
- Speight, J.G. Langec’s Handbook of Chemistry; Science Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Souza, E.S.; Mello, N.T.; Menezes, D.M.; Monteneqro, M.C.; Araujo, A.N.; Barros, N.B.; Silva, A.L. Extraction and recovery of chromium from electroplating sludge. J. Hazard. Mater. 2006, 128, 39–43. [Google Scholar]
- Chen, J.Y.; Yu, S.Q.; Wu, Z.C. Separation and Utilization of Iron in Hydrometallurgy; Metallurgical Industry Press: Beijing, China, 1991. (In Chinese) [Google Scholar]
- Jiang, H.Y. Hydrometallurgy Process of Physical Chemistry; Metallurgical Industry Press: Beijing, China, 1984. (In Chinese) [Google Scholar]
- Chen, J.; Wang, Y.C.; Yang, H. Technical study on iron removal from basic chromium sulfate produced by patty recovered. Chem. Eng. 2009, 12, 60–71. (In Chinese) [Google Scholar]
- Hu, G.R.; Li, G.; Deng, X.R. Removal of iron sulfuric acid leaching solution of ferrochromium alloy by goethite. Hydrometal. China 2006, 25, 198–201. (In Chinese) [Google Scholar]
- Zhu, C.S.; Sun, Z.Y.; Gong, W.Q.; Chen, H.S. Study on treatment of chromium-containing wastewater with biomineralized goethite. Res. Environ. Sci. 2003, 16, 57–58. (In Chinese) [Google Scholar]
- Zhang, Q.S. Exploratory Study on the Separation of Chromium and Iron in Multicomponent Acid Solution System. Master’s Thesis, Northeastern University, Shenyang, China, 2014. [Google Scholar]
- Shi, P.Y. Study on Leaching Process of Preparation of Alkaline Chromium Sulphate by Sulphuric Acid Leaching. Master’s Thesis, Northeastern University, Shenyang, China, 2001. [Google Scholar]
- Wu, J.H.; Yang, L.Z.; Zhang, J. Research on separation of chromium and iron in waste ferrochromium alloy. Hydrometal. China 2011, 30, 51–56. (In Chinese) [Google Scholar]
- Fan, J.Y. The research of technology on producing chrome by electrolysis method. J. Kunming Metal. Coll. 2001, 17, 27–30. (In Chinese) [Google Scholar]
- Jiang, X.J. Separation of iron from chromium electrolyte with DDTC-Na. Ferro Alloys 2000, 5, 19–21. (In Chinese) [Google Scholar]
- Hu, G.H. One Method for the Production of Chromium Oxide and Ferrous Oxlalate from Carbon Ferrochrome. China Patent 101041466A, 26 September 2007. [Google Scholar]
- Wang, Q.M. Study on Effective Separation and Comprehensive Utilization of Elements from Carbon Ferrochromium. Master’s Thesis, Central South University, Changsha, China, 2011. [Google Scholar]
- Zhang, B.; Chi, W.H.; Shi, P.Y.; Liu, C.J.; Jiang, M.F. Study on the clean production process of basic chromium sulphate. Adv. Mater. Res. 2014, 888, 651–656. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).