Abstract
In this work, the effects of dodecylamine storage state on the adsorption behavior of calcium sulfate dihydrate and silica were systematically investigated by using Raman detection, solution equilibrium calculation, and calculation based on density functional theory. The results show that the selective adsorption behavior of dodecylamine with calcium sulfate dihydrate and silica is closely related to its occurrence state. The adsorption of dodecylamine in the ionic state with calcium sulfate dihydrate and silica is dominated by the strong electrostatic adsorption between the H-O atoms under acidic conditions, while that of dodecylamine in the molecular state is dominated by the weak electrostatic adsorption between the Ca-N or Si-N atoms under alkaline conditions. Finally, by comparing the distribution coefficients and adsorption energies of the ionic/molecular states of dodecylamine with the change in pH, the reason why dodecylamine adsorbs calcium sulfate dihydrate more readily under acidic conditions was explained at the atomic level.
1. Introduction
Phosphogypsum is a large waste byproduct of the phosphochemical industry. For every ton of phosphoric acid produced, about 5 tons of phosphogypsum are produced [1]. The global inventory of phosphogypsum has exceeded 6 billion tons [2,3], and the cumulative storage of phosphogypsum in China has exceeded 830 million tons, with an annual utilization rate of about 45% [4]. Long term storage not only occupies land, but also easily causes comprehensive pollution of water, soil, and air [5]. The main component of phosphogypsum is calcium sulfate dihydrate (CaSO4·2H2O, 80%–90%), and the main impurity is silicon dioxide (SiO2, 4%–20%) [4]. The industrial application of phosphogypsum is greatly restricted by coexisting impurities [6,7,8,9,10,11,12,13]. Selective separation of calcium sulfate dihydrate and silicon dioxide is the key to impurity removal.
The methods for removing impurities from phosphogypsum include water washing (to remove water-soluble fluorine/phosphorus, etc.) [14], acid leaching (to remove impurities such as sulfuric acid/hydrochloric acid, etc.) [15], and organic amine flotation (to remove insoluble impurities such as silicon) [16,17]. The organic amine flotation method is becoming a research hotspot due to its ability to effectively remove silicon and prepare high-purity calcium sulfate dihydrate, as shown in Table 1. The reason why amine collectors are effective is that they have different adsorption properties on the surfaces of calcium sulfate dihydrate and silica, which changes the hydrophobicity of calcium sulfate dihydrate and silica in aqueous solution and promotes the separation of silica and gypsum. As shown by Qi et al. [18], before and after the addition of the dodecylamine hydrochloride (DH) collector, gypsum exhibits strong N-H bond stretching vibration peaks in FTIR, while quartz only shows very weak N-H bond stretching vibration peaks. At the same time, the zeta potential of gypsum and quartz changes with pH. At pH 2.0, the contact angle of gypsum increases from 34.70° to 62.97°, and the contact angle of quartz increases from 34.41° to 42.44°. After interacting with the collector, gypsum becomes more hydrophobic and floats in water by binding with bubbles through hydrophobic interactions, while quartz still maintains hydrophilicity and cannot stably bind with bubbles, thus achieving selective separation of gypsum and quartz.

Table 1.
Previous research on the flotation process to remove silica from phosphogypsum.
The organic amine collectors used in the experimental study of silicon removal by phosphogypsum flotation include dodecyl dimethyl ethylbenzyl ammonium chloride (DDEA), dodecylbis(2-hydroxyethyl) methylammonium chloride (2HEAC-12), dodecylamine hydrochloride (DH), dodecylamine (DDA), octadecylamine (ODA), dodecyltrimethylammonium chloride (DTAC), mixed amine, etc. Among them, the direct flotation of phosphogypsum by dodecylamine has the most significant effect and the widest source. Zhang et al. used dodecylamine as a collector to obtain a concentrate product with a gypsum purity of 97.50% and a silica content of 1.17%, which meets the first-grade requirements of the Chinese national standard.
Previous work has shown that pH significantly affects the flotation efficiency of dodecylamine. For example, Qi et al. treated phosphogypsum with dodecylamine (30 mg/L) at pH 2.0, which increased the purity of gypsum from 84.44% to 96.33% and reduced the silica content from 9.08% to 0.62% [22]. Guo et al. found that at pH 3–11, the purity of gypsum concentrate decreased from 90.03% to 82.55%, and the silica content increased from 2.60% to 6.50% [23]. The acid flotation effect of dodecylamine on phosphogypsum was significantly better than that of alkaline flotation. Current research has mostly focused on describing experimental phenomena and lacks in-depth analysis at the atomic level of the correlation between pH and the existence state (ionic/molecular) of dodecylamine and its adsorption mechanism.
This study investigated the changes in the occurrence state of dodecylamine at different pH values and its effects on the adsorption mechanism of calcium sulfate dihydrate and silica from the perspective of interaction by combining Raman detection, solution equilibrium calculation, and density functional calculation. The reason why dodecylamine is more prone to adsorb calcium sulfate dihydrate under acidic conditions was identified at the atomic level.
2. Materials and Methods
All chemical reagents (dodecylamine, calcium sulfate dihydrate, silica, and deionized water) were commercially purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China), analytically pure, and used without further purification. A certain amount of analytical pure dodecylamine liquid was measured and dissolved in deionized water to prepare the dodecylamine solution with the concentration of 2% wt. The pH of the solution was adjusted with sulfuric acid or sodium hydroxide solution to obtain dodecylamine solutions at different pH values as Raman samples. X-ray powder diffraction (XRD, D8 Advance, Bruker Instruments, Karlsruhe, Germany) was used to confirm the crystalline structures of the samples. The Raman analysis was performed using the LabRAM HR Evolution Raman spectrometer from HORIBA (Montpellier, France).
Density functional theory (DFT) calculations were performed using the DMol [3]program with the spin unrestricted approach [24,25]. The generalized gradient approximation functional externalized using the Perdew–Burke–Ernzerhof [26] and DFT semi-core pseudopots were employed in all calculations. The cutoff energy was set as 500.0 eV. Reciprocal space integration was performed with a Monkhorst–Pack grid of 2 × 2 × 1. The unit cell parameters of calcium sulfate dihydrate are a = 6.284 Å, b = 15.200 Å, c = 6.523 Å; α = 90.000°, β = 127.414°, γ = 90.000° [27]; the unit cell parameters of silica are a = 4.913 Å, b = 4.913 Å, c = 5.405 Å; α = 90.000°, β = 90.000°, γ = 120.000°. The thickness of the vacuum layer was set to 30 Å.
The adsorption energy (Eabs) of dodecylamine adsorbed on the surface can be calculated as:
where Esurface+dodecylamine is the total energy of the surface with a dodecylamine molecule or ion adsorbed, Esurface is the energy of the blank surface (calcium sulfate dihydrate or silica), and Edodecylamine is the energy of a dodecylamine molecule or ion.
3. Results
3.1. The Effect of pH on the Occurrence State of Dodecylamine
Figure 1 shows the effect of pH on the Raman spectra of a 2% solution of dodecylamine. Spectral lines in the 400–600 cm−1 region represent the vibrational peaks of dodecylamine carbon skeleton deformation; peaks in the 800–1200 cm−1 region are the rocking vibration peaks of dodecylamine CH2 and CH3, as well as the stretching vibration peaks of CH2-CH2 and CH2-CH3; peaks in the 1300–1450 cm−1 region consist of the CH2 shear vibration peak and the CH3 asymmetric deformation vibration peak of dodecylamine; and the shear peaks near 1600 cm−1 represent NH2 or NH3+ [28]. Among them, the 1589 cm−1 peak corresponds to the NH3+ shear vibration peak, the 1587 cm−1 peak corresponds to the NH3+ and NH2 shear vibration peaks, and the 1577 cm−1 peak corresponds to the NH2 shear vibration peak. The peak in the range of 400–1450 cm−1 did not show a significant shift when the pH of the solution decreased from 12.0 to 2.0, but the NH2 group gradually protonated to form NH3+, causing the 1577 cm−1 shear vibration peak to shift towards the higher wave number (1589 cm−1 peak). The Raman spectroscopy detection results in Figure 1 indicate that under acidic conditions, dodecylamine in the solution is mainly in the ionic state of NH3+. Under alkaline conditions, the dodecylamine in solution is mostly in the molecular state NH2.
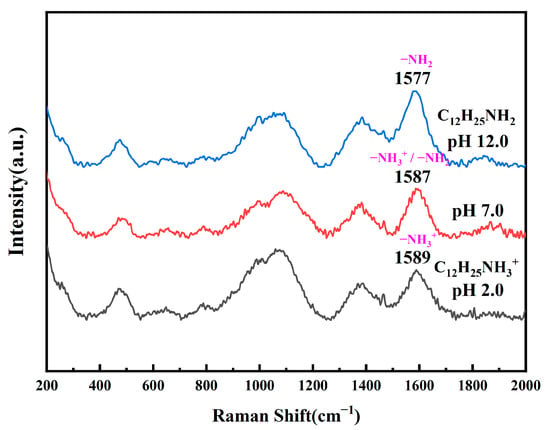
Figure 1.
Raman spectra of dodecylamine aqueous solution at pH = 2.0, 7.0, and 12.0.
3.2. Calculation
Figure 2 shows the XRD patterns of pure calcium sulfate dihydrate and silica, with diffraction peaks located at 11.75°, 23.50°, and 29.21°, which correspond well to the (020), (040), and (041) crystal planes of CaSO4·2H2O (PCPDF 33-0311). The peaks located at 20.83°, 26.61°, and 50.11° correspond well to the (100), (101), and (112) crystal planes of SiO2 (PCPDF 46-1045). The (020) and (101) crystal planes are the strongest peaks in XRD, so the (020) surface of calcium sulfate dihydrate and the (101) surface of silicon dioxide were selected to conduct further calculations.
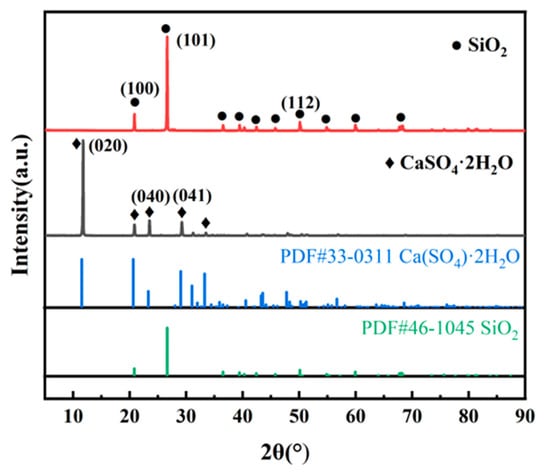
Figure 2.
XRD patterns of calcium sulfate dihydrate and silica.
Figure 3a–d show the stable adsorption configuration of CaSO4·2H2O (020) to C12H25NH3+, CaSO4·2H2O (020) to C12H25NH2, SiO2 (101) to C12H25NH3+, and SiO2 (101) to C12H25NH2, respectively. Calculation results show that the adsorption energies are E1 = −20.105 eV, E2 = −1.998 eV, E3 = −13.036 eV, and E4 = −3.169 eV, respectively. The isoelectric point of calcium sulfate dihydrate and silicon dioxide is approximately pH = 2.0 [29,30,31]. When pH > 2.0, both calcium sulfate dihydrate (020) and silicon dioxide (101) crystal surfaces are negatively charged. The amino group head of the dodecylamine ion (C12H25NH3+) is an electron deficient group with a positive charge, while the amino group head of the dodecylamine molecule (C12H25NH2) is an electron rich group with a negative charge. Dodecylamine adsorbs onto the crystal faces of calcium sulfate dihydrate (020) and silicon dioxide (101) through the amino group head.
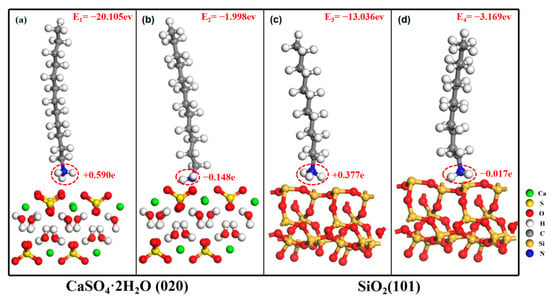
Figure 3.
Stable adsorption configuration of ionic/molecular dodecylamine with calcium sulfate dihydrate and silica. (a) CaSO4·2H2O (020)-C12H25NH3+, (b) CaSO4·2H2O (020)-C12H25NH2, (c) SiO2(101)-C12H25NH3+, (d) SiO2 (101)-C12H25NH2.
Figure 4 shows the key atomic interaction distance of dodecylamine in ion/molecular state adsorbed with calcium sulfate dihydrate and silica, as well as the charge density difference. The blue area in Figure 4 represents the region where the electron density increases, while the yellow area represents the region where the electron density decreases. The isovalue is 0.01 e/Å3. The atoms exposed on the (020) surface of calcium sulfate dihydrate are Ca atoms and O atoms of sulfate ions, while the atoms exposed on the (101) surface of silicon dioxide are Si atoms and O atoms.
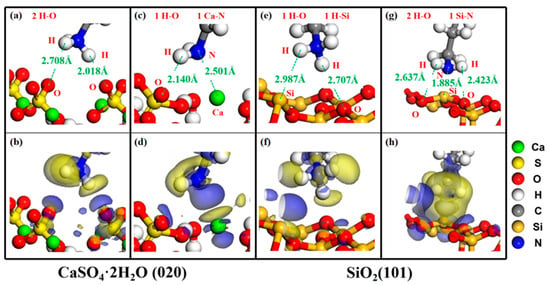
Figure 4.
Adsorption charge density difference and key atomic interaction distance of ion/molecular dodecylamine with calcium sulfate dihydrate and silica. Isovalue = 0.01 e/Å [3]. Blue and yellow areas represent the areas of increased/decreased electron density, respectively. (a,b) CaSO4·2H2O (020)-C12H25NH3+, (c,d) CaSO4·2H2O (020)-C12H25NH2, (e,f) SiO2(101)-C12H25NH3+, (g,h) SiO2 (101)-C12H25NH2.
Figure 4a,b show that there is a significant electronic interaction between two H-O atoms at the CaSO4·2H2O (020)-C12H25NH3+ adsorption interface, with distances of 2.018 Å and 2.708 Å, respectively. The length of the H-O bond in the O-H…O hydrogen bond is approximately 1.200–1.215 Å [32], and the length of the H-O bond in the N-H…O hydrogen bond is approximately 1.95 Å [33], indicating electrostatic interaction between H-O atoms. Figure 4c,d show that there is a significant electronic interaction between one Ca-N atom and one H-O atom at the CaSO4·2H2O (020)-C12H25NH2 adsorption interface. The distances between Ca-N atoms and H-O atoms are 2.140 Å and 2.501 Å, respectively. The bond length of Ca-N is approximately 2.31–2.51 Å [34], indicating the formation of coordination bonds between Ca-N atoms. Figure 4e,f show that there is a significant electronic interaction between one H-O atom and one H-Si atom at the SiO2(101)-C12H25NH3+ adsorption interface. The distances between H-O atoms and H-Si atoms are 2.707 Å and 2.987 Å, respectively, both of which are electrostatic interactions. Figure 4g,h show that there is a significant electronic interaction between one Si-N atom and two H-O atoms at the SiO2 (101)-C12H25NH2 adsorption interface. The distances between Si-N atoms and H-O atoms are 1.885 Å, 2.423 Å, and 2.637 Å, respectively. The Si-N bond length is approximately 1.70–1.78 Å [35], indicating that there is electrostatic interaction between Si-N atoms.
For the CaSO4·2H2O (020) surface, the smaller the distance between H-O atoms, the greater the electrostatic attraction and adsorption energy. For the SiO2 (101) surface, the nitrogen atom at the head of the dodecylamine group has one lone pair electron, which coordinates or electrostatically attracts positively charged Ca and Si atoms on the crystal plane. The smaller the distance between the atoms, the greater the attraction and the higher the adsorption energy. The electronegativity of oxygen (O) atoms is 3.44, and the electronegativity of nitrogen (N) atoms is 3.04 [36]. Since the electronegativity of O atoms is greater than that of N atoms, the electrostatic attraction between H-O atoms is greater than that between Si-N atoms and Ca-N atoms. Therefore, the order of the adsorption forces on the surface is: CaSO4·2H2O (020)-C12H25NH3+ > SiO2(101)-C12H25NH3+ > SiO2 (101)-C12H25NH2 > CaSO4·2H2O (020)-C12H25NH2. This is in good agreement with the calculated results of the head charge and adsorption energy of dodecylamine, where the more positive the charge, the greater the adsorption energy; The more negative the charge, the smaller the adsorption energy, indicating that the ionic dodecylamine has a stronger adsorption effect on calcium sulfate dihydrate and silica, and is more easily adsorbed with calcium sulfate dihydrate. The adsorption energy, atomic distance, and interaction types of dodecylamine of ion/molecular states on the surface of calcium sulfate dihydrate and silica are summarized in Table 2. The data in Table 2 indicate that CaSO4·2H2O (020)-C12H25NH3+ is the dominant form of adsorption in competitive adsorption.

Table 2.
The summary of adsorption properties of dodecylamine in ionic/molecular states on the surface of calcium sulfate dihydrate and silica.
3.3. The Effect of pH on Selective Adsorption of Dodecylamine
As dodecylamine exists in the form of both ion and molecule in the aqueous solution, and the ion/molecule proportion is closely related to pH, the concept of mixed adsorption energy is proposed to investigate the effect of pH on selective adsorption of dodecylamine. The process is as follows.
In the aqueous solution of dodecylamine, dodecylamine will dissolve in water:
with and [37].
Combined with , the distribution coefficient of ionic dodecylamine
and the distribution coefficient of molecular dodecylamine
could be derived.
The mixed adsorption energy of dodecylamine on calcium sulfate dihydrate under different pH conditions is defined as
the mixed adsorption energy of dodecylamine on silica under different pH conditions as
and the difference in adsorption energy of calcium sulfate dihydrate and silica mixture by dodecylamine as
The distribution coefficient of ion/molecular state dodecylamine varies with pH as shown in Figure 5a. When pH < 10.63, dodecylamine mainly exists in an ionic state. When pH > 10.63, dodecylamine mainly exists in molecular form. At pH 10.63, the distribution coefficients of dodecylamine in both molecular and ionic states are equal, both being 0.5.
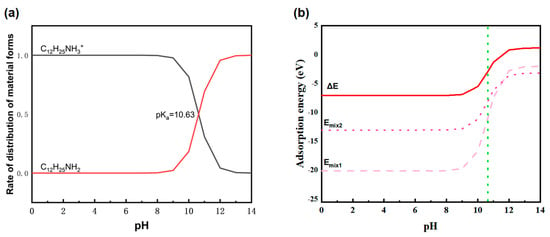
Figure 5.
(a) Distribution coefficient of dodecylamine in ion/molecular state and (b) variation in mixed adsorption energy with pH.
Figure 5b shows the variation in Emix1, Emix2, and ΔE with pH under different pH conditions. When pH < 10.63, the negative value of ∆E is large, indicating that dodecylamine has a much higher adsorption energy for calcium sulfate dihydrate than for silica, indicating that dodecylamine is more likely to adsorb calcium sulfate dihydrate at this time. After pH > 10.63, the negative value of ∆E sharply decreases, indicating that the difference in adsorption energy between dodecylamine and dodecylamine becomes smaller, and the selective adsorption becomes worse.
4. Conclusions
This work investigates the effect of the occurrence state of dodecylamine on the adsorption properties of calcium sulfate dihydrate and silica by combining Raman analysis, solution equilibrium calculation, and DFT calculation. At pH > 2.0, the calcium sulfate dihydrate (020) surface and silica (101) surface are negatively charged and adsorb with the head of the dodecylamine group. Computational results show that under acidic conditions, dodecylamine is mainly in an ionic state, and the head of the amine group is positively charged. The electrostatic adsorption between the H-O atoms of calcium sulfate dihydrate and silica is stronger, making it easier to adsorb calcium sulfate dihydrate. Under alkaline conditions, dodecylamine is mainly in a molecular state, with a negative charge on the head of the amine group. The adsorption between Ca-N and Si-N atoms of dihydrate calcium sulfate and silica is weak and the difference is not significant. By comparing the distribution coefficient of ion/molecular state dodecylamine and the variation in adsorption energy with pH, the reason why dodecylamine is more prone to adsorb dihydrate calcium sulfate under acidic conditions was identified at the atomic level, providing theoretical guidance for phosphogypsum flotation and process optimization.
Author Contributions
Conceptualization, X.L. and R.C.; methodology, X.L. and R.C.; software, L.X.; validation, X.L.; formal analysis, X.L. and R.C.; investigation, X.L. and R.C.; resources, L.X.; data curation, X.L.; writing—original draft preparation, X.L.; writing—review and editing, R.C. and L.X.; visualization, X.L.; supervision, L.X.; project administration, L.X.; funding acquisition, L.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Research and Development Program of China (No. 2023YFC3707600) and the National Natural Science Foundation of China (No. 52274410).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, Y.; Liu, Y.; Huang, J.; Mei, Y. Improved Length of Calcium Sulfate Crystal Seeds and Whiskers via Ball Milling and Hydration Treatment. Chin. J. Chem. Eng. 2024, 71, 102–109. [Google Scholar] [CrossRef]
- Akfas, F.; Elghali, A.; Aboulaich, A.; Munoz, M.; Benzaazoua, M.; Bodinier, J.-L. Exploring the potential reuse of phosphogypsum: A waste or a resource? Sci. Total Environ. 2024, 908, 168196. [Google Scholar] [CrossRef] [PubMed]
- Murali, G.; Azab, M. Recent research in utilization of phosphogypsum as building materials: Review. J. Mater. Res. Technol. 2023, 25, 960–987. [Google Scholar] [CrossRef]
- Li, X.; Lv, X.; Xiang, L. Review of the State of Impurity Occurrences and Impurity Removal Technology in Phosphogypsum. Materials 2023, 16, 5630. [Google Scholar] [CrossRef]
- Jia, W.; Li, J.; Shen, C.; Li, G.; Li, H.; Fan, G.; Zhou, G.; Cao, Y. Research advances in phosphogypsum flotation purification: Current status and prospects. Sep. Purif. Technol. 2025, 354, 129244. [Google Scholar] [CrossRef]
- Mymrin, V.; Aibuldinov, E.K.; Avanci, M.A.; Alekseev, K.; Argenda, M.A.; Carvalho, K.Q.; Erbs, A.; Catai, R.E. Material cycle realization by hazardous phosphogypsum waste, ferrous slag, and lime production waste application to produce sustainable construction materials. J. Mater. Cycles Waste Manag. 2021, 23, 591–603. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Z.; Ma, X.; Yang, X.; Zhang, X.; Pan, H.; Wu, J.; Xu, M.; Lin, L.; Zhang, Y.; et al. Promoting coordinative development of phosphogypsum resources reuse through a novel integrated approach: A case study from China. J. Clean. Prod. 2022, 374, 134078. [Google Scholar] [CrossRef]
- Sun, T.; Li, W.; Xu, F.; Yu, Z.; Wang, Z.; Ouyang, G.; Xu, D. A new eco-friendly concrete made of high content phosphogypsum based aggregates and binder: Mechanical properties and environmental benefits. J. Clean. Prod. 2023, 400, 136555. [Google Scholar] [CrossRef]
- Costa, E.T.d.S.; Lopes, G.; Carvalho, G.S.; Penha, H.G.V.; Curi, N.; Guilherme, L.R.G. Phytoremediation of Arsenic-Contaminated Soils Amended with Red Mud Combined with Phosphogypsum. Water Air Soil Pollut. 2021, 232, 417. [Google Scholar] [CrossRef]
- Borges, W.L.B.; Juliano, P.H.G.; de Souza, I.M.D.; Rodrigues, L.N.F.; Hipólito, J.L.; Andreotti, M. New Methodologies for the Surface Application of Limestone and Gypsum in Different Crop Systems. Sustainability 2022, 14, 8926. [Google Scholar] [CrossRef]
- Oszako, T.; Pasławski, T.; Szulc, W.; Rutkowska, B.; Rutkiewicz, A.; Kukina, O.; Bakier, S.; Borowik, P. Short-Term Growth Response of Young Pine (Pinus silvestris) Seedlings to the Different Types of Soil Media Mixture with Phosphogypsum Formulations under Poland Forest Environmental Conditions. Forests 2023, 14, 518. [Google Scholar] [CrossRef]
- Liu, H.; Jin, X.; Chen, L.; Chang, X.; Li, J.; An, Y.; Liu, J.; Pang, C.; Gao, Y. Effects of phosphogypsum whiskers modification with calcium stearate and their impacts on properties of bleached softwood paper sheets. TAPPI J. 2021, 20, 567–578. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Nie, C.; Xie, G.; Che, Z.; Zhu, D.; Guo, L.; Xiang, Y.; Shi, W. PMMA-Grafted Calcium Sulfate Whiskers for Applications as Fillers in PVC. Polymers 2022, 14, 4199. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Ge, Y.; Chen, Z.; Xing, B.; Bao, S.; Yong, Q.; Chi, R.; Yang, S.; Ni, B.-J. Flotation purification of waste high-silica phosphogypsum. J. Environ. Manag. 2022, 320, 115824. [Google Scholar] [CrossRef]
- Lv, X.; Xiang, L. The Generation Process, Impurity Removal and High-Value Utilization of Phosphogypsum Material. Nanomaterials 2022, 12, 3021. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, F.; Wang, Z.; Yang, F.; Du, M.; Fu, K.; Wang, Z. A novel method for purification of phosphogypsum. Physicochem. Probl. Miner. Process. 2020, 56, 975–983. [Google Scholar] [CrossRef]
- Mweene, L.; Khanal, G.P. New insights into the flotation of quartz in presence of polyoxirane as a novel biotite depressant: Experimental and theoretical approach. Inorg. Chem. Commun. 2025, 177, 114423. [Google Scholar] [CrossRef]
- Qi, M.; Peng, W.; Wang, W.; Cao, Y.; Fan, G.; Huang, Y. Selective flotation separation of gypsum and quartz using dodecyl amine hydrochloride as collector: Mechanism and application. Surf. Interface Anal. 2024, 56, 603–615. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, M.; Ke, X.; Yu, Y.; Tan, X.; Ye, H.; Chen, S. Novel ethylbenzyl and hydroxyethyl quaternary ammonium collectors for co-reverse flotation desilication and impurity removal from phosphogypsum: Flotation performance and mechanism. Sep. Purif. Technol. 2025, 358, 130403. [Google Scholar] [CrossRef]
- Xiao, J.; Lu, T.; Zhuang, Y.; Jin, H. A Novel Process to Recover Gypsum from Phosphogypsum. Materials 2022, 15, 1944. [Google Scholar] [CrossRef]
- Zhang, L.; Lü, Z.; Zhang, Y.; Wu, Z.; Zhang, X.; Tan, X. Experimental study on improving quality and reducing impurity of phosphogypsum. Inorg. Chem. Ind. 2021, 53, 171–173. [Google Scholar] [CrossRef]
- Qi, M.; Peng, W.; Wang, W.; Cao, Y.; Fan, G.; Huang, Y. Simple and efficient method for purification and recovery of gypsum from phosphogypsum: Reverse-direct flotation and mechanism. J. Mol. Liq. 2023, 371, 121111. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Fan, P.; Li, H.; Chen, C.; Du, L.; Xu, S. Experimental Study on a New Flotation Desilication Process for Phosphogypsum from Yunnan. Non-Met. Mines 2022, 45, 53–56. [Google Scholar]
- Delley, B. An All-Electron Numerical Method for Solving the Local Density Functional for Poly-Atomic Molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Boeyens, J.C.A.; Ichharam, V.V.H. Redetermination of the crystal structure of calcium sulphate dihydrate, CaSO4 • 2H2O. Z. Krist.-New Cryst. Struct. 2002, 217, 9–10. [Google Scholar]
- Teixeira-Dias, J.; de Carvalho, L.; da Costa, A.; Lampreia, I.M.; Barbosa, E.F. Conformational studies by Raman spectroscopy and statistical analysis of gauche interactions in n-butylamine. Spectrochim. Acta Part A Mol. Spectrosc. 1986, 42, 589–597. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Walls, H.J.; Khan, S.A. Rheology of Silica Dispersions in Organic Liquids: New Evidence for Solvation Forces Dictated by Hydrogen Bonding. Langmuir 2000, 16, 7920–7930. [Google Scholar] [CrossRef]
- Zhukov, A.N.; Varzhel, V.I. Specific Surface Conductivity of Quartz in the Solution of Electrolytes in N-Butanol Close to the Isoelectric Points. Vestn. Leningr. Univ. Seriya Fiz. Khimiya 1985, 2, 108–110. [Google Scholar]
- Kong, X.-F.; Li, C.-X.; Jiang, J.; Huang, L.-B.; Hartley, W.; Wu, C.; Xue, S.-G. Improvement of alkaline electrochemical characteristics of bauxite residue amendment with organic acid and gypsum. J. Cent. South Univ. 2019, 26, 430–439. [Google Scholar] [CrossRef]
- Tarakanova, E.G.; Yukhnevich, G.V. Relationship between the bond lengths in N-H…N, O-H…O, F-H…F, and Cl-H…Cl hydrogen bridges. J. Struct. Chem. 2009, 50, 1015–1020. [Google Scholar] [CrossRef]
- Bleckmann, P.; Breitenbach, P.; Dickhut, K.U.; Keller, D.; Schwittek, C. Intermolecular potentials and force constants from ab initio energies—Application to the N-H…O=C hydrogen bonds in formamide dimers. Anal. Bioanal. Chem. 1997, 359, 115–120. [Google Scholar] [CrossRef]
- Chen, Y.H.; Kang, L.; Zhang, C.R.; Luo, Y.C.; Yuan, L.H.; Li, Y.L. Density functional theory study on the structures and properties of (Ca3N2)n (n = 1–4) clusters. Acta Phys. Sin. 2008, 57, 6265–6270. [Google Scholar] [CrossRef]
- Kohatsu, I.; McCauley, J.W. Re-examination of the crystal structure of α-Si3N4. Mater. Res. Bull. 1974, 9, 917–920. [Google Scholar] [CrossRef]
- Allred, A. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 1961, 17, 215–221. [Google Scholar] [CrossRef]
- Somasundaran, P.; Wang, D. Solution Chemistry: Minerals and Reagents; Developments in Mineral Processing Series; Wills, B.A., Ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 2006; Volume 17. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).