Abstract
Calcium ions, primarily introduced through flotation reagents and mineral dissolution, progressively accumulate a considerable amount due to the process of water recycling, significantly impacting the flotation behavior of minerals. In this paper, the adsorption of calcium ions on a calcite surface was initially studied by surface characteristic analysis, and then further evaluated for its influence on the separation of scheelite from calcite using single mineral flotation and atomic force microscopy (AFM) measurements. The results indicate that calcium ions significantly reduce the hydrophobicity of calcite surface induced by sodium oleate (NaOL) adsorption, while enhancing the adsorption of sodium silicate (SS). In addition, SS forms a strong chemical adsorption on calcite, rendering the surface negatively charged. However, the surface charge diminishes under the combined influence of calcium and silicate ions. AFM measurements further reveal that the adhesion forces between scheelite and calcite are weakened by silicate adsorption. Nevertheless, these forces are markedly restored in the presence of calcium ions, thereby considerably reducing the selectivity of SS and hindering effective particle separation. These findings align with the results of mixed binary flotation, confirming that calcium ions indeed interfere with the separation of scheelite from calcite.
1. Introduction
Calcite is a significant rock-forming mineral that commonly occurs as a carbonate gangue mineral associated with other valuable minerals [1,2,3,4]. Owing to the Ca and O active sites contained on its crystal surface, typical anionic or cationic collectors used for oxidation ore flotation commonly exhibit strong affinity for calcite, thereby adversely affecting beneficiation efficiency [5,6,7]. To separate scheelite from calcite, the addition of acidified water glass [8,9] or camphor leaf extract [10] could effectively reduce calcite recovery under specific conditions. Cationic RNH3+ ionized from dodecylamine in an aqueous solution would be adsorbed on a negatively charged scheelite surface through electrostatic adsorption. However, on a positively charged calcite surface it would be adsorbed through chemical adsorption [11]. Meanwhile, RNH3+ could react with anionic species released from scheelite and calcite to form precipitates on both mineral surfaces through physical absorption, as well as the adsorption of neutral species RNH2. Thus, the complex precipitates would lead to different mineral surface hydrophobicity and flotation responses, thereby enabling the effective separation of scheelite from calcite [12].
The challenge of separating calcium-bearing minerals persists due to their similar surface characteristics [13,14,15]. The crystal structures of calcium-bearing minerals have been investigated using molecular simulations to explore anisotropy in commonly exposed surfaces [16,17]. The surfaces of {112} and {001} are the most frequently exposed surfaces of scheelite crystals. The {001} surface of calcite crystal exhibits lower surface energy compared to other surfaces, whereas the {104} surface is more frequently exposed [18]. For fluorite, the {111} surface is the most stable cleavage plane due to its minimum surface energy compared to other commonly exposed surfaces [19]. Meanwhile, the interaction mechanisms between flotation reagents and calcium-bearing minerals have been elucidated at the atom–molecule reaction scale. Thus, novel flotation reagents could be designed and assembled according to the characteristics of solid affinity functional group and hydrophobic chain [20]. Wang et al. [21] found that Al-starch, synthesized by the reaction of aluminum ions and starch, could selectively adsorb on O active sites of calcite, altering its surface charge and atomic characteristics. In contrast, Al-starch would not interact with O active sites on a scheelite surface, thereby achieving selective inhibition. The combined development of novel reagents and flotation processes could continually improve the technical aspects of scheelite ore flotation [22,23,24].
In addition, unavoidable metal ions, primarily derived from mineral dissolution, grinding media, and process water, would also deteriorate the flotation pulp environment by regulating the reagent composition or altering mineral surface properties. These effects would impair the performance of flotation agents and reduce the separation efficiency of mixed minerals [13,25,26,27,28]. In the common flotation process, calcium ions in pulp primarily originate from the extensive use of lime and recycled process water, which leads to a considerable concentration. Due to the interaction between Ca2+ and flotation agents, the role of Ca2+ would vary with the reagent regime [29,30]. When sodium carbonate is used to adjust pulp pH, nano calcium carbonate precipitate will be formed first and will compete with the collector for adsorption. In contrast, sodium hydroxide adjustment would exert a positive effect. The subsequent addition of sodium fluoride promotes the transformation of nano calcium carbonate into calcium fluoride. This transformation would reduce the adsorption of nano calcium carbonate and the adverse effect on scheelite flotation would be eliminated [31,32]. However, the presence of calcium ions would induce surface conversion among scheelite, fluorite, and calcite, rendering their surface properties more similar, and diminishing the selectivity of flotation reagents [33].
Therefore, the adsorption of calcium ions on calcite will be investigated through zeta potential, XPS, and contact angle measurements, to reveal its influence on reagent adsorption. Furthermore, the influence of calcium ions on the separation between calcite and scheelite are be studied through single mineral flotation tests and AFM measurements. Then, the mechanism of calcium ions adsorption on flotation separation of calcite and scheelite will be clarified.
2. Materials and Methods
2.1. Materials
Pure scheelite and calcite samples were first handpicked and dry-ground in a mill using agate ball as the medium. Then, the sample was divided into a fraction of −74 + 20 μm for micro-flotation and −20 + 0 μm for analysis tests. Chemical composition analysis (Table 1) and X-ray diffraction (XRD, D8 Advance, Bruker, Billerica, MA, USA, Cu Kα source, Figure 1) confirmed a purity of 97.97% for scheelite and 96.70% for calcite. The purity was calculated based on the content of the main mineral component (WO3 for scheelite, CaO and CO3 for calcite), considering the stoichiometry and ignoring minor impurities.

Table 1.
The chemical composition purity of minerals (wt%).
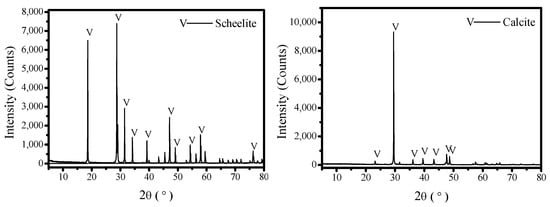
Figure 1.
XRD analysis of pure scheelite and calcite.
Calcium ions were sourced from calcium chloride (CaCl2) with the maximum ion concentration of 11.8 × 10−3 mol/L determined by the saturation concentration of the gypsum solution [34]. All reagents such as sodium oleate (NaOL, C18H33O2Na), sodium silicate (SS, Na2SiO3·9H2O), and CaCl2 were chemically pure. NaOH and HCl stock solutions were analytical grade pure to adjust the pH during experiments. Deionized water with a resistivity of 18 MΩ × cm was used in all experiments.
2.2. Flotation Tests
Micro-flotation tests were conducted in a 40 mL plexiglass cell using an XFG flotation machine at 1600 rpm. Each mineral suspension was prepared by combining 2.0 g mineral with 35 mL deionized water, while the mixed binary mineral suspensions were created with a 1:1 ratio. The feed WO3 content for the mixed binary system was approximately 40.26%. After adding the desired reagent, the suspension was stirred for 3 min, followed by the regulation of required pH. Manual scraping was conducted for 3 min to collect the froth product. Then, concentrate and tailings were individually filtered, dried, and weighed. Each test was conducted three times to evaluate the product’s grade and recovery. The schematic diagram of the flotation process is shown in Figure 2.
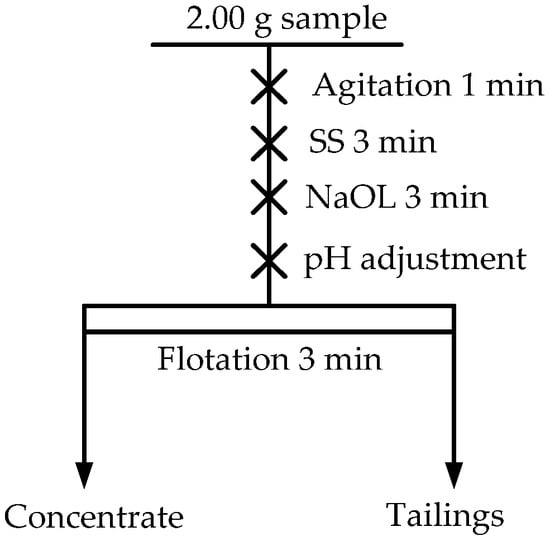
Figure 2.
Schematic diagram of the flotation process.
2.3. Contact Angles Measurements
Mineral contact angles were measured using a Drop Shape Analyzer (DSA100, KRUSS, Hamburg, Germany) with the sessile drop method. The samples (−20 μm) were initially conditioned in calcium ion solutions of varying concentrations. Following this, they were treated with an optimal concentration of sodium oleate (as determined by flotation tests) at pH of 9, with agitation for 3 min. The final samples were then filtered, washed, and dried at a low temperature. Subsequently, the treated samples were compressed into pellets under a pressure of 50 MPa.
2.4. Zeta Potential Measurements
Zeta potentials of scheelite and calcite were determined in a 1 × 10−3 mol/L KCl electrolyte solution with a Brookhaven Zetaplus analyzer (Brookhaven, Holtsville, NY, USA). Suspensions were prepared by dispersing 0.05 g mineral in 50 mL electrolyte solution. After condition adjustment, the suspension was stirred for 20 min and the pH was regulated. The fine particle suspension was collected for zeta potential measurements. Each sample was measured three times with typical variation within 5%, and average values were calculated.
2.5. X-Ray Photoelectron Spectroscopy (XPS) Analysis
XPS spectra of minerals were recorded using an ESCALAB 250Xi (Thermo Fisher Scientific, Waltham, MA, USA, Al Kα source). Spectra were calibrated against the C 1s binding energy at 284.8 eV for charge compensation. Samples were prepared by mixing 1.0 g mineral with 100 mL deionized water under specific conditions. The suspension pH was adjusted and stirred for 20 min. After settling, suspensions were filtrated, rinsed three times with deionized water, and dried in a vacuum oven.
2.6. Atomic Force Microscope (AFM) Measurements
Interaction force measurements and AFM images were acquired using a Multimode 8 AFM (Bruker). A calcite colloid probe for interaction force tests was prepared using an N-type silicon probe (CSC37/Tipless/No Al, MikroMasch, Tallinn, Estonia). The scheelite cleavage surface in Figure 3 was obtained by cutting a natural scheelite crystal. AFM images of the scheelite surface were captured in ScanAsyst-air mode at 1 Hz. The average surface roughness (Ra) of scheelite was 1.29 nm over a 0.25 μm2 area, making it appropriate for AFM force measurements. The calcite colloidal probe in Figure 4 was prepared by crushing and screening fine calcite particles. Cantilever beam images were obtained under a light microscope. The spring constant (k0) of the probe cantilever was calibrated on hard SiO2 in testing liquids. The experimental details and principles have been described by Ducker et al. [35,36] and Xing et al. [37].
Figure 3.
AFM images of scheelite.
Figure 4.
Images of the calcite colloidal probe.
3. Results and Discussion
3.1. Flotation Behavior of Scheelite and Calcite
Single mineral flotation tests were performed to study the effect of pH, NaOL concentration, and calcium concentration on scheelite and calcite flotation. The results in Figure 5 revealed that both scheelite and calcite reached maximum recovery at pH of 9–10 with NaOL concentration of 2.5 × 10−4 mol/L. Meanwhile, the recoveries of both minerals decreased significantly with the increase of calcium concentration, and stabilized at calcium concentration 6 × 10−3 mol/L. These results indicate that both minerals’ surface hydrophobicity and flotation recovery would be reduced dramatically by the adsorption of calcium ions and the consumption of NaOL.
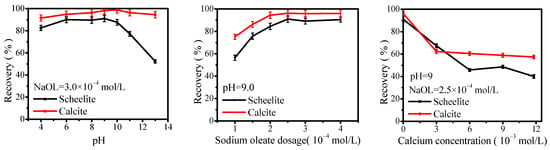
Figure 5.
Effect of pH, NaOL concentration, and calcium concentration on scheelite and calcite flotation.
As shown in Figure 6a, SS commonly showed selective inhibition of calcite flotation, reducing its recovery to 65% at an SS concentration of 4 × 10−3 mol/L while exhibiting little influence on the recovery of scheelite with the increase in SS concentration. In the mixed mineral systems shown in Figure 6b, the WO3 content in concentrate increased gradually and the recovery decreased slightly. When the concentration of SS reaches 8 × 10−3 mol/L, the WO3 content reaches 64.24% with a recovery of 82.32%, which indicates that SS could achieve effective separation of scheelite and calcite to some extent.
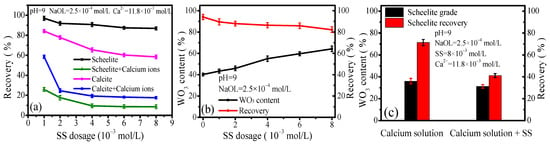
Figure 6.
Effect of SS on single mineral and mixed binary mineral flotation at pH of 9.
However, in the presence of calcium ions, the recovery of scheelite and calcite were reduced to below 17.67% and 24.67%, respectively, when SS concentration reached at 2.0 × 10−3 mol/L (Figure 6a). In mixed mineral systems, flotation selectivity deteriorated; both WO3 content and recovery decreased markedly when adding SS of 8 × 10−3 mol/L to the calcium solution (Figure 6c). Obviously, the synergetic effects between calcium ions and SS coexisting in solution would promote the adsorption of silicate on both scheelite and calcite, which indicates that calcium ions could disrupt the selective inhibition effect of SS, making scheelite–calcite separation ineffective [38].
3.2. Influence of Calcium Ions Adsorption on Calcite Hydrophobicity
Contact angles experiments were conducted to evaluate the influence of calcium ions’ adsorption on calcite hydrophobicity. The contact angle results in Figure 7 indicate that the untreated calcite exhibited weak hydrophobicity with angle results of 33.45° ± 0.19. After being treated with NaOL in deionized water, the hydrophobicity of calcite increased significantly with angle results reaching 131.69° ± 0.39, indicating strong collector adsorption. Conversely, the contact angles of calcite treated with NaOL were negatively correlated with the concentration of calcium ions, and decreased to 115.21° ± 0.86 with a calcium concentration of 11.8 × 10−3 mol/L. Supporting this finding, similar conclusions were obtained in previous research on scheelite under identical experimental conditions [39]. These results suggest that calcium ions would hinder the adsorption of NaOL on the mineral surface, thereby depressing the hydrophobicity of calcite and scheelite. Notably, the inhibition mechanism differs from that induced by SS [40].
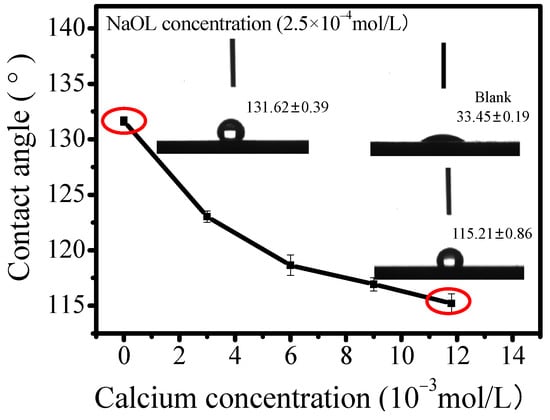
Figure 7.
Influence of calcium ion concentration on calcite contact angle treated with NaOL.
3.3. XPS Analysis of Adsorption Mechanisms
XPS measurements were conducted to examine the adsorption mechanism of SS and calcium ions on the calcite surface. The atomic concentration of the main elements and the full scan spectra are shown in Figure 8 and Table 2, respectively. According to these results, there are no new characteristic peaks generated on the mineral’s surface after being treated in calcium solution. Meanwhile, silicate could be easily adsorbed on calcite surface in the presence of calcium ions, based on the elements’ analysis results and new characteristic Si 2p peak generated in Figure 8d.
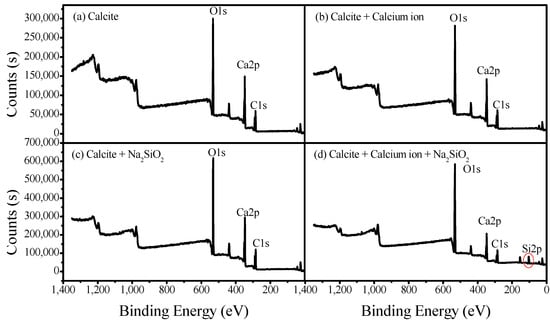
Figure 8.
XPS spectra of calcite before and after it was treated with SS and calcium solution.

Table 2.
Atomic concentration of the main elements on calcite surfaces.
Figure 9 presents the Ca 2p spectra of calcite treated with SS and calcium solution. For untreated calcite, the Ca 2p 1/2 and 3/2 peaks appear at 350.24 eV and 346.70 eV, respectively. In calcium solution, the two peaks both increased from 0.07 eV to 350.17 eV and 346.63 eV, respectively. This shift is considered to be within the instrument’s error range. After it was treated with SS, the two peaks still appeared nearby their original position. Furthermore, in the synergetic effects between calcium ions and SS, the Ca 2p 1/2 and 3/2 peaks shift to bigger values by 0.11 eV and 0.12 eV, respectively.
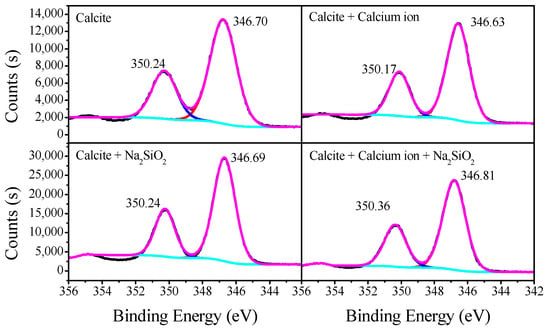
Figure 9.
Ca 2p spectra of calcite treated with SS and calcium solution.
Figure 10 presents the O 1s spectra of calcite treated with SS and calcium solution. The O 1s peak of untreated calcite appears at 531.17 eV, and it decreased 0.04 eV to 531.13 eV after being treated with SS. It was still considered to be within the error range of the instrument’s sensitivity. When treated in the calcium solution, the O 1s peak decreased by 0.10 eV to 531.07 eV, meaning that there was a weak interaction with calcium ions. Meanwhile, after being treated with SS in a calcium solution, O 1s peak shifted to a larger value by 0.43 eV to 531.60 eV. This indicates that silicate interacts with Ca2+ in a solution more easily and adsorbs on the mineral’s surface.
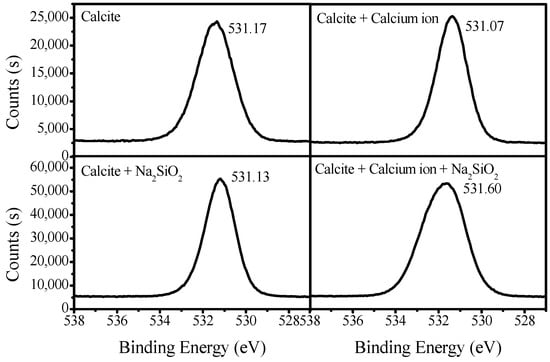
Figure 10.
O 1s spectra of calcite treated with SS and calcium solution.
Figure 11 presents the Si 2p spectra for calcite treated with SS and calcium solution. After being treated with SS, a weak characteristic peak assigned to Si 2p 1/2 at 103.07 eV and Si 2p 3/2 at 102.13 eV could be detected on calcite’s surface. In the presence of calcium ions, an obvious characteristic peak assigned to Si 2p 1/2 at 103.35 eV and Si 2p 3/2 at 102.57 eV could be detected after treatment with SS. This indicates that silicate can adsorb on calcite’s surface due to the reactions between sodium silicate and the active Ca atom on the mineral’s surface [41,42], while calcium ions can strengthen silicate adsorption on calcite’s surface through chemical adsorption.
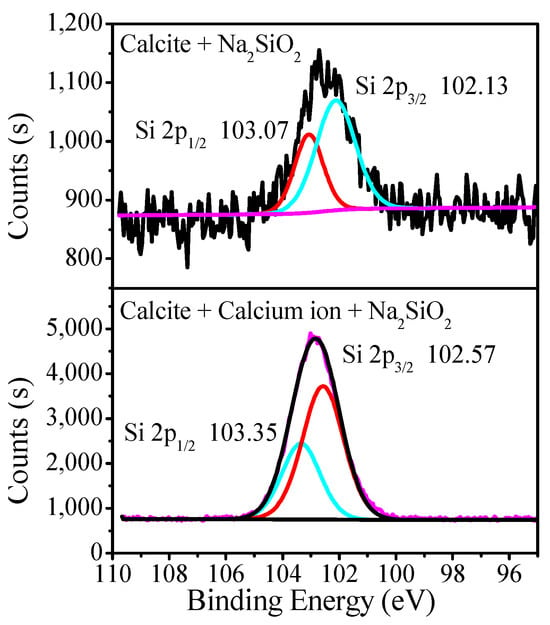
Figure 11.
Si 2p spectra for calcite treated with SS in different solutions.
3.4. Effect of Calcium Ions on Surface Zeta Potential
When a mineral surface is covered by ions, its surface zeta potential is affected by the new covering layer. Figure 12 presents the effect of calcium concentration on the zeta potential of calcite at a pH of 9. The results indicate that calcium ion adsorption raises the surface potential of calcite, maintaining a positive charge as calcium concentration increases, suggesting a limited effect by calcium ions. The findings suggest that calcium ion adsorption primarily occurs through chemisorption or hydrogen bonding [43], rather than solely through electrostatic adsorption under specific conditions.
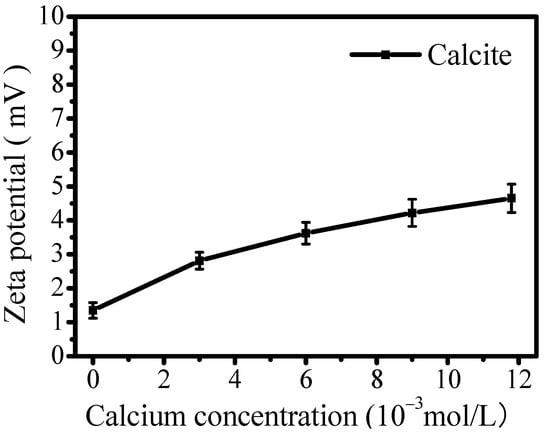
Figure 12.
Influence of calcium concentration on zeta potential of calcite at pH of 9.
Figure 13 presents the zeta potential of calcite treated with SS and calcium solution as a function of pH. The zeta potential of calcite in deionized water decreases with the increase in pH value and obtains the isoelectric point (IEP) at pH of 9.2 [44,45]. In the presence of calcium ions, the zeta potential behavior of calcite resembles that in deionized water, but the IEP shifts to pH of 9.7. SS treatment could alter the surface charge of calcite by the adsorption of silicate, which shifts the zeta potential from positive to negative. The presence of calcium ions could alter the adsorption state of SS on calcite’s surface due to its interaction with calcium ions. In this case, the zeta potential of calcite remains negatively charged after being treated with silicate sodium, but the amount of surface charge has been reduced. The adsorption of calcium ions on calcite’s surface neutralizes the negative charge brought by sodium silicate, thereby reducing the electrostatic repulsion between particles.
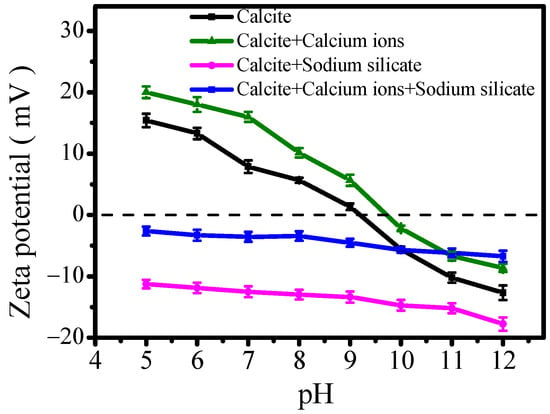
Figure 13.
Zeta potential of calcite treated in different solutions as a function of pH.
3.5. AFM Force Measurements Between Scheelite and Calcite
In order to investigate the challenges of separating scheelite and calcite particles in a calcium ions solution, the colloidal probe technique was utilized to explore their interaction under various solution conditions.
Figure 14 illustrates the influence of SS on the interaction forces between scheelite’s surface and calcite particles at pH of 9. When the space between the calcite particle probe and the scheelite base surface in deionized water, the inter-particle force is negligible until the surfaces come into contact, at which point a repulsion force was observed. During retraction, an adhesion force of 8.80 nN at a separation distance of 34.21 nm was obtained, primarily due to the electrostatic interaction between the polar mineral particles. When approaching in SS solution, repulsion force is the main force between minerals when the distance reduces. In a 3 × 10−3 mol/L SS solution, a maximum adhesion force of 0.61 nN was measured at a separation distance of 171.23 nm. Meanwhile, the adhesion forces decreased significantly as SS concentration increased. SS as a dispersant could selectively adsorb on the calcite surface and reduce the adhesion between mineral particles, thereby facilitating the effective separation of scheelite and calcite particles.
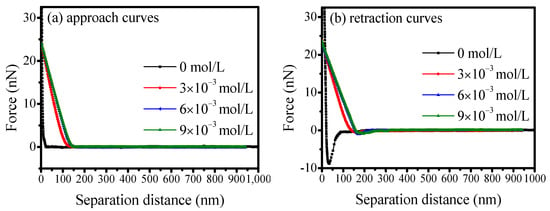
Figure 14.
Effect of SS on the interaction forces between scheelite and calcite.
Figure 15 illustrates the interaction forces between the scheelite surface and calcite particle before and after treatment in a calcium solution at pH of 9. The results found that the interaction forces between scheelite and calcite changed to strong adhesion forces and increased as calcium concentration increased. Meanwhile, a similar variation trend of the corresponding jump-in distance and separation distance was be obtained. When treated in calcium solution of 1 × 10−3 mol/L, a maximum adhesion force of 14.48 nN at a separation distance of 30.72 nm was obtained. Furthermore, the adhesion force increased to 44.72 nN as the calcium solution increased to 11.8 × 10−3 mol/L.
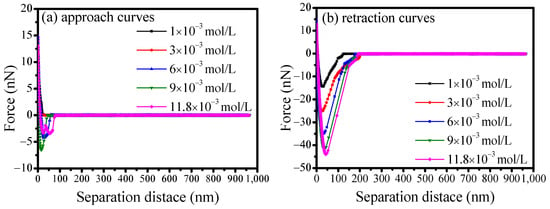
Figure 15.
Effect of calcium concentration on the interaction forces between scheelite and calcite.
Figure 16 illustrates the interaction forces between the scheelite surface and calcite particle in SS and calcium solution at pH of 9. In order to prevent the prior reaction of precipitate between silicate and calcium ions, the scheelite surface and calcite particle were pre-treated in a calcium solution of 11.8 × 10−3 mol/L before conducting measurements in the silicate solution. The concentration of SS was determined as 8 × 10−3 mol/L based on the mixed binary mineral flotation. According to the results, a maximum adhesion force of 43.62 nN at a separation distance of 89.29 nm was obtained, suggesting that the presence of calcium ions could significantly disrupt the selective depression of SS on calcite. Consequently, adhesion forces would continue to dominate the interaction between scheelite and calcite under the combined interaction of silicate and calcium ions. The presence of calcium ions promotes the connection between the surfaces of scheelite and calcite through calcium silicate substances, thereby enhancing adhesion.
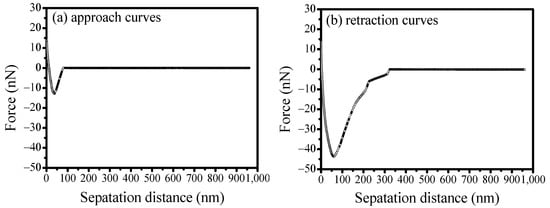
Figure 16.
Effect of SS on the interaction forces between scheelite and calcite after being treated in calcium solution.
4. Conclusions
This study investigated the effect of calcium ions released from mineral dissolution on calcite’s surface properties. Then, the influence of calcium ions on the separation of scheelite from calcite was studied through flotation behavior and interparticle interactions. The main conclusions are as follows:
- (1)
- Calcium ions dissolved from calcium-bearing minerals would adsorb on calcite’s surface, leading to increased surface positive charge. This behavior is consistent with previous findings on scheelite and fluorite surface adsorption. Furthermore, competitive adsorption between calcium ions and oleate at surface the Ca site would reduce oleate adsorption capacity, thereby increasing the hydrophilicity of both minerals.
- (2)
- Results from both mixed binary mineral flotation and adhesion force measurements suggest that calcium ions would enhance SS adsorption on scheelite and calcite surfaces, thereby eliminating its selectivity for calcite. The adhesion forces would increase with the adsorption of calcium ions on mineral surfaces, hindering the effective separation of scheelite from calcite. Therefore, to achieve efficient separation of scheelite from calcite, the pulp environment and reagent regime should be carefully optimized to mitigate the detrimental effects of unavoidable calcium ion accumulation.
Author Contributions
Conceptualization, Z.Z. and C.S.; methodology, X.Z.; formal analysis, Z.Z.; investigation, X.Z.; data curation, Z.Z.; writing—original draft preparation, Z.Z.; writing—review and editing, Z.Z. and X.D.; project administration, C.S. and B.X.; funding acquisition, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (52504290); the Key Lab of Critical Metals Minerals Supernormal Enrichment and Extraction, Ministry of Education (GJJSKFYB202504); the Fundamental Research Funds for the Universities of Henan Province (NSFRF2502046).
Data Availability Statement
Data are contained within the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gu, X.; Zhu, Y.; Li, Y.; Han, Y. Selective flotation of siderite and quartz from a carbonate-containing refractory iron ore using a novel amino-acid-based collector. Physicochem. Probl. Mineral Process. 2018, 54, 803–813. [Google Scholar] [CrossRef]
- Foucaud, Y.; Filippov, L.; Filippova, I.; Badawi, M. The challenge of tungsten skarn processing by froth flotation: A review. Front. Chem. 2020, 8, 230. [Google Scholar] [CrossRef]
- Wang, J.; Liu, R.; Jing, M.; Han, S. Research progress on mineral interaction in flotation system composed of calcite and its associated minerals. Nonferrous Met. Eng. 2023, 13, 78–87. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.; Wen, S.; Ma, M.; Sun, C.; Yin, W.; Ma, Y. Effect of carbonate minerals on quartz flotation behavior under conditions of reverse anionic flotation of iron ores. Int. J. Miner. Process. 2016, 152, 1–6. [Google Scholar] [CrossRef]
- Lu, Y.; Ding, R.; Li, G.; Song, X.; Cao, Y.; Jia, K. Research Progress with Scheelite Flotation Reagents: A Review. Minerals 2023, 13, 1257. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Duverger, A.; Severov, V.V. Synergetic effect of a mixture of anionic and nonionic reagents: Ca mineral contrast separation by flotation at neutral pH. Miner. Eng. 2014, 66–68, 135–144. [Google Scholar] [CrossRef]
- Xu, L.; Liu, D.; Sun, R.; Wang, Y.; Liu, Z.; Shao, P.; Wang, C.; Wen, S. Flotation separation of smithsonite and calcite in sodium oleate system using soluble starch as depressant. Miner. Eng. 2024, 205, 108490. [Google Scholar] [CrossRef]
- Kupka, N.; Kaden, P.; Jantschke, A.; Schach, E.; Rudolph, M. Acidified water glass in the selective flotation of scheelite from calcite, part II: Species in solution and related mechanism of the depressant. Physicochem. Probl. Mineral Pro. 2020, 56, 797–817. [Google Scholar] [CrossRef]
- Kupka, N.; Mockel, R.; Rudolph, M. Acidified water glass in the selective flotation of scheelite from calcite, Part I: Performance and impact of the acid type. Physicochem. Probl. Mineral Process. 2020, 56, 238–251. [Google Scholar]
- Pan, Z.; Zhang, Y.; Hu, J.; Jiao, F.; Qin, W. Camph or leaf extract as neoteric and environmentally friendly depressant in flotation separation of scheelite and calcite. Trans. Nonferrous Met. Soc. China 2023, 33, 275–284. [Google Scholar] [CrossRef]
- Dong, L.; Qin, W.; Jiao, F.; Zhu, H. Flotation separation of scheelite and calcite using mixed cationic-anionic collectors. Min. Metall. Eng. 2018, 38, 61–64. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, W.; Hu, Y. New insights into the dodecylamine adsorption on scheelite and calcite: An adsorption model. Miner. Eng. 2015, 79, 54–61. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Han, H.; Gui, X.; Xing, Y. Flotation chemistry of scheelite and its practice: A comprehensive review. Miner. Eng. 2023, 204, 108404. [Google Scholar] [CrossRef]
- Dong, L.; Cui, Y.; Qiao, L.; Lan, S.; Zheng, Q.; Shen, P.; Liu, D. A critical review on the flotation of calcium-containing minerals. Sep. Purif. Technol. 2025, 360, 131082. [Google Scholar] [CrossRef]
- Sun, W.; Wei, Z.; Han, H.; Gao, Z.; Wang, J.; Wang, R. Flotation chemistry of tungsten ore and its practice. Met. Mine 2021, 535, 24–41. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, R.; Ralston, J.; Sun, W.; Hu, Y. Surface broken bonds: An efficient way to assess the surface behaviour of fluorite. Miner. Eng. 2019, 130, 15–23. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Sun, W.; Hu, Y. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, C.; Sun, W.; Gao, Y.; Kowalczuk, P.B. Froth flotation of fluorite: A review. Adv. Colloid Interfac. 2021, 290, 102382. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, B.; Chen, Y. Flotation separation depressants for scheelite and calcium-bearing minerals: A review. Int. J. Miner. Metall. Mater. 2023, 30, 1621–1632. [Google Scholar] [CrossRef]
- Wang, R.; Han, H.; Sun, W.; Sun, W.; Zhang, H.; Cheng, Y. Selective inhibition behavior and mechanism of Al-starch complex on ultrafine calcite in scheelite flotation. Conserv. Util. Miner. Resour. 2023, 43, 1–10. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Q.; Wang, S.; Wang, C.; Chen, M.; Li, H. Efficient selective flotation separation of fluorite from calcite using ferrous and ferric species as combined depressant. Miner. Eng. 2024, 205, 108451. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Fu, J.; Zhang, Z.; Li, W.; Han, H.; Wei, Z.; Chen, W.; Liu, W. Recycle of Pb-BHA collectors based on the adsorption and desorption mechanism on scheelite surface. J. Clean Prod. 2024, 435, 140555. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Wang, P.; Liu, D.; Han, H. A novel metal-organic complex surfactant for high-efficiency mineral flotation. Chem. Eng. J. 2021, 426, 130853. [Google Scholar] [CrossRef]
- Gao, Z.; Jiang, Z.; Sun, W.; Gao, Y. Typical roles of metal ions in mineral flotation: A review. Trans. Nonferrous Met. Soc. China 2021, 31, 2081–2101. [Google Scholar] [CrossRef]
- Corin, K.C.; Charamba, A.; Manono, M.S. Water quality impact on flotation response: A focus on specific ions and temperature. Miner. Eng. 2024, 207, 108549. [Google Scholar] [CrossRef]
- Chen, J. The interaction of flotation reagents with metal ions in mineral surfaces: A perspective from coordination chemistry. Miner. Eng. 2021, 171, 107067. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Xu, Y.; Shu, K.; Fang, S.; Xu, L. The effect of dissolved calcite species on the flotation of bastnaesite using sodium oleate. Miner. Eng. 2020, 145, 106095. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, Y.; Ma, Z.; Liao, Y. Impact of calcium and gypsum on separation of scheelite from fluorite using sodium silicate as depressant. Sep. Purif. Technol. 2019, 215, 249–258. [Google Scholar] [CrossRef]
- Guo, W.; Chang, J.; Wang, S.; Liu, Q.; Zhang, H. Probing the interaction of calcium and magnesium ions on scheelite surface by atomic force microscopy. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131200. [Google Scholar] [CrossRef]
- Sun, W.; Han, H.; Zou, Q.; Chen, W. Study on the effect of calcium ion on the flotation behaviors of scheelite and its regulation. Met. Mine 2023, 567, 90–97. [Google Scholar] [CrossRef]
- Sun, W.; Han, H.; Sun, W.; Wang, R.; Wei, Z. Novel insights into the role of colloidal calcium dioleate in the flotation of calcium minerals. Miner. Eng. 2022, 175, 107274. [Google Scholar] [CrossRef]
- Sun, W.; Han, H.; Sun, W.; Wang, R. Novel insights into the mechanism of lime method based on calcium dioleate and mineral surface transformation. J. Cent. South Univ. 2023, 30, 2983–2992. [Google Scholar] [CrossRef]
- Filippov, L.O.; Duverger, A.; Filippova, I.V.; Kasaini, H.; Thiry, J. Selective flotation of silicates and Ca-bearing minerals: The role of non-ionic reagent on cationic flotation. Miner. Eng. 2012, 36–38, 314–323. [Google Scholar] [CrossRef]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Direct measurement of colloidal forces using an atomic force microscope. Nature 1991, 353, 239–241. [Google Scholar] [CrossRef]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Measurement of forces in liquids using a force microscope. Langmuir 1992, 8, 1831–1836. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, M.; Gui, X.; Cao, Y.; Babel, B.; Rudolph, M.; Weber, S.; Kappl, M.; Butt, H. The application of atomic force microscopy in mineral flotation. Adv. Colloid Interfac. 2018, 256, 373–392. [Google Scholar] [CrossRef]
- Cao, Z.; Cheng, Z.; Wang, J.; Cao, Y. Synergistic depression mechanism of Ca2+ ions and sodium silicate on bastnaesite flotation. J. Rare Earth 2022, 40, 988–995. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, Y.; Liao, Y.; Ma, Z. Impact of gypsum on flotation of scheelite and fluorite using sodium oleate as collector. Sep. Sci. Technol. 2020, 55, 2528–2537. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Zhang, G.; Chen, W.; Chen, Y. Effect of depressants in the selective flotation of scheelite and calcite using oxidized paraffin soap as collector. Int. J. Miner. Process. 2016, 157, 210–215. [Google Scholar] [CrossRef]
- Feng, B.; Guo, W.; Xu, H.; Peng, J.; Luo, X.; Zhu, X. The combined effect of lead ion and sodium silicate in the flotation separation of scheelite from calcite. Sep. Sci. Technol. 2017, 52, 567–573. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Shi, J.; Ning, J. Effects of Pb2+ ions on the flotation behavior of scheelite, calcite, and fluorite in the presence of water glass. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127826. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Liao, Y.; Jin, C.; Ma, Z. Effect of unavoidable ion (Ca2+) in pulp on the dispersion behavior of fine smithsonite. Molecules 2022, 27, 9026. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, Z. Interactions of amphoteric amino phosphoric acids with calcium-containing minerals and selective flotation. Int. J. Miner. Process. 2003, 72, 87–94. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).