Parametric Study on APTES Silanization of Coal Fly Ash for Enhanced Rubber Composite Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
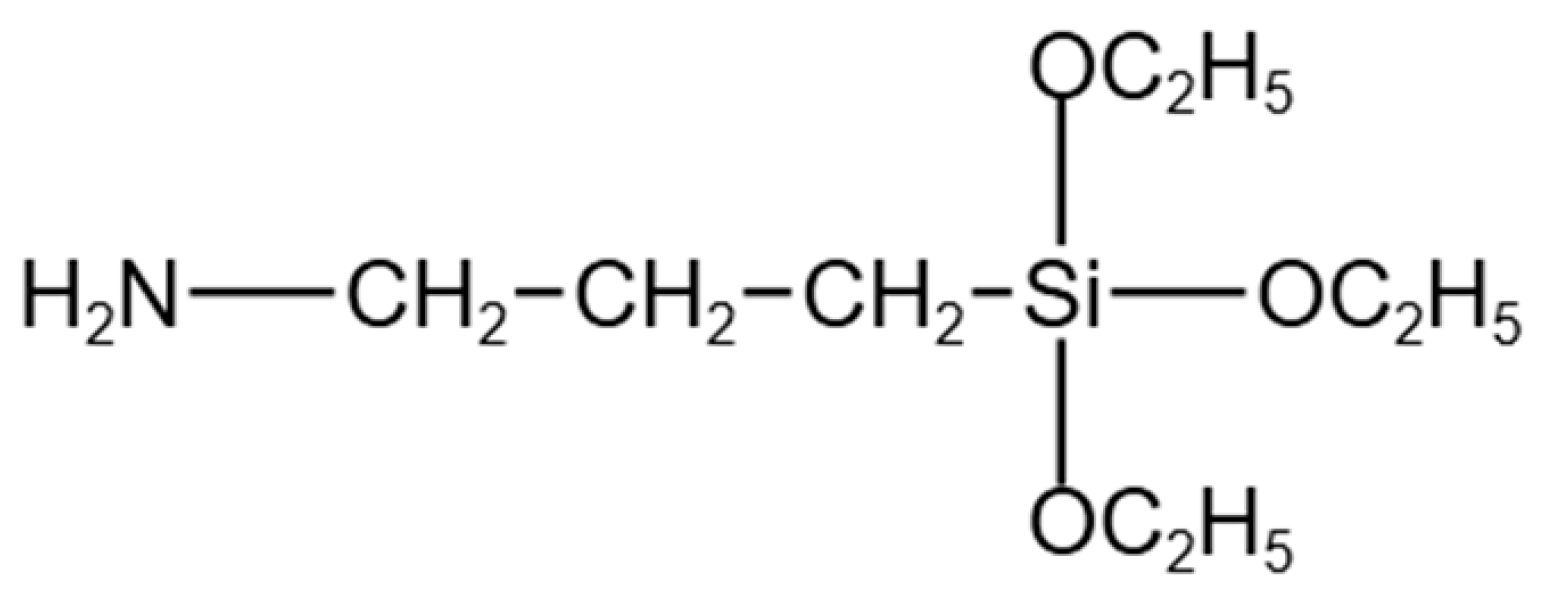
2.2. Silane Coupling of CFA with APTES
2.3. Characterization
3. Results and Discussion
3.1. Chemical and Mineralogical Compositions of Untreated CFA
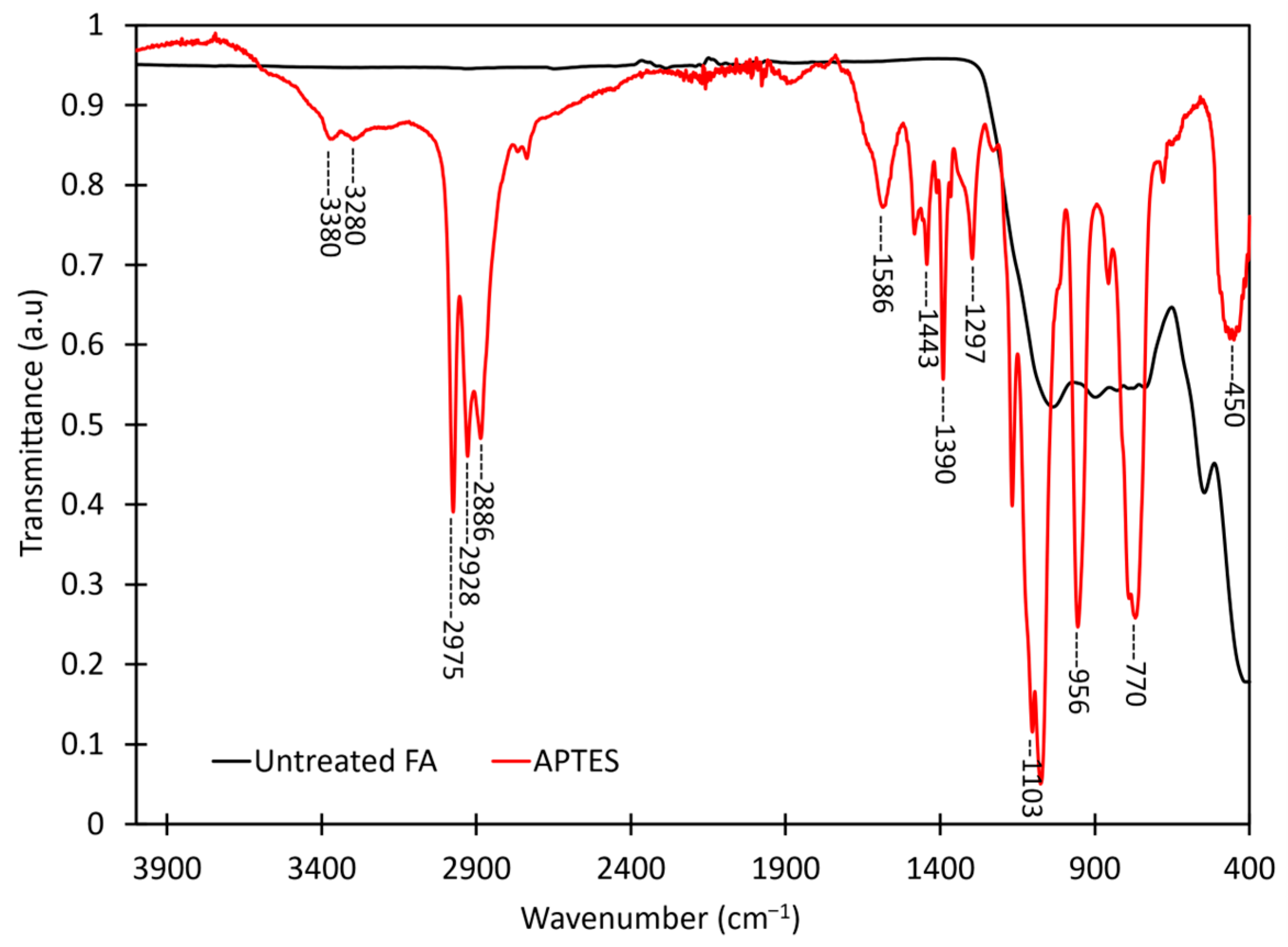
3.2. FTIR Investigation of CFA Surface Silanization with APTES
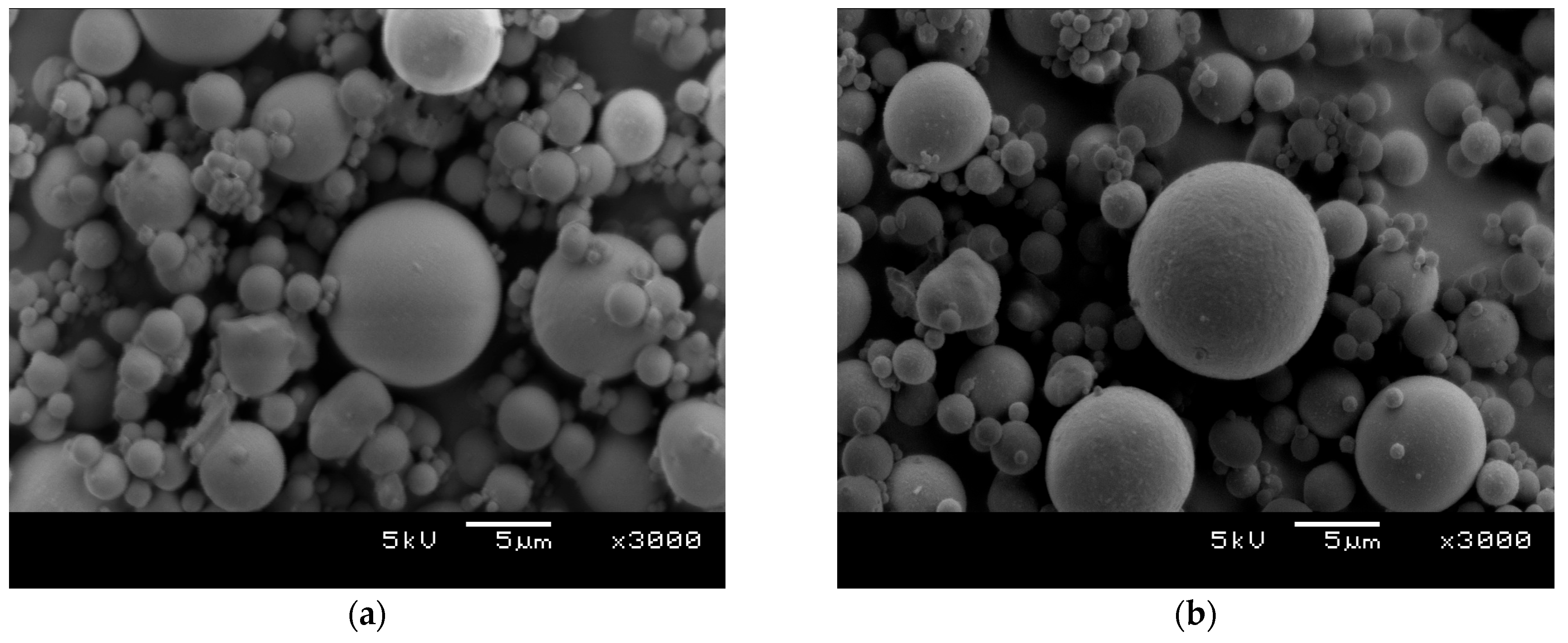
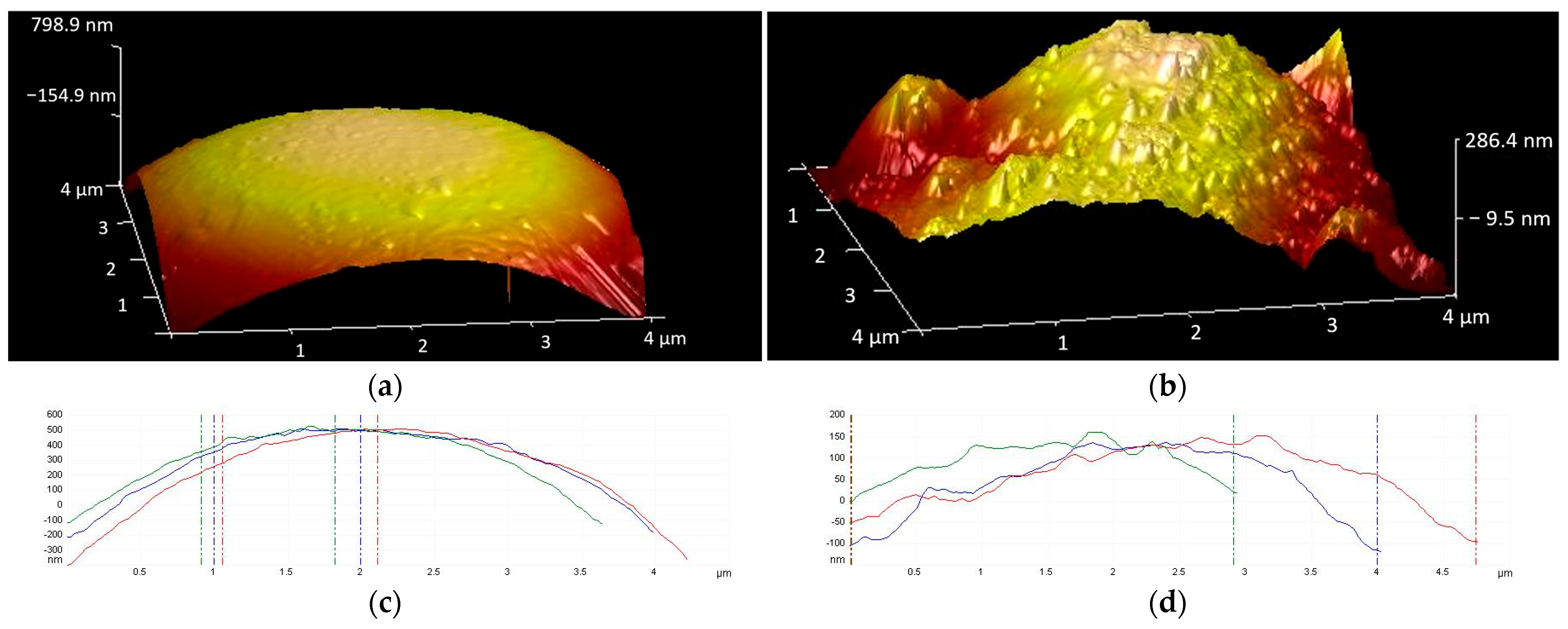
3.3. Direct Visualization of CFA Surface Following Silanization with APTES
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFA | coal fly ash |
| APTES | 3-aminopropyltriethoxysilane |
| FTIR | Fourier-transform infrared spectroscopy |
| NR | natural rubber |
| SBR | styrene–butadiene rubber |
| phr | parts per hundred rubber |
| OFAT | one-factor-at-a-Time |
| SEM | scanning electron microscopy |
| AFM | atomic force microscopy |
References
- Mancheno-Posso, P.; Dittler, R.F.; Lewis, D.; Juang, P.; Ji, X.; Xu, X.H.; Lynch, D.C. Review of status, trends, and challenges in working with silane and functional silanes. In Silane: Chemistry, Applications and Performance; Moriguchi, K., Utagawa, S.S., Eds.; Nova Science Publishers: New York, NY, USA, 2013; pp. 66–87. [Google Scholar]
- Indumathy, B.; Sathiyanathan, P.; Prasad, G.; Reza, M.S.; Prabu, A.A.; Kim, H. A comprehensive review on processing, development and applications of organofunctional silanes and silane-based hyperbranched polymers. Polymers 2023, 15, 2517. [Google Scholar] [CrossRef] [PubMed]
- Ahangaran, F.; Navarchian, A.H. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Presto, D.; Meyerhofer, J.; Kippenbrock, G.; Narayanan, S.; Ilavsky, J.; Moctezuma, S.; Sutton, M.; Foster, M.D. Influence of silane coupling agents on filler network structure and stress-induced particle rearrangement in elastomer nanocomposites. ACS Appl. Mater. Interfaces 2020, 12, 47891–47901. [Google Scholar] [CrossRef]
- Goyal, S. Silanes: Chemistry and applications. J. Indian Prosthodont. Soc. 2006, 6, 14–18. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lassila, L.V.; Ozcan, M.; Yli-Urpo, A.; Vallittu, P.K. An introduction to silanes and their clinical applications in dentistry. Int. J. Prosthodont. 2004, 17, 155–164. [Google Scholar] [PubMed]
- Naviroj, S.; Culler, S.; Koenig, J.; Ishida, H. Structure and adsorption characteristics of silane coupling agents on silica and E-glass fiber; dependence on pH. J. Colloid Interface Sci. 1984, 97, 308–317. [Google Scholar] [CrossRef]
- Guillet, A. Treatment of fillers with organofunctional silanes, technology and applications. In Macromolecular Symposia; Wiley: Hoboken, NJ, USA, 2003; pp. 63–74. [Google Scholar]
- Thongsang, S.; Sombatsompop, N. Effect of NaOH and Si69 treatments on the properties of fly ash/natural rubber composites. Polym. Compos. 2006, 27, 30–40. [Google Scholar] [CrossRef]
- Abdelsalam, A.A.; Ward, A.A.; Abdel-Naeem, G.; Mohamed, W.S.; El-Sabbagh, S.H. Effect of alumina modified by silane on the mechanical, swelling and dielectric properties of Al2O3/EPDM/SBR blend composites. Silicon 2023, 15, 3609–3621. [Google Scholar] [CrossRef]
- Yangthong, H.; Nun-Anan, P.; Krainoi, A.; Chaisrikhwun, B.; Karrila, S.; Limhengha, S. Hybrid Alumina–Silica Filler for Thermally Conductive Epoxidized Natural Rubber. Polymers 2024, 16, 3362. [Google Scholar] [CrossRef]
- Van der Merwe, E.M.; Prinsloo, L.C.; Mathebula, C.L.; Swart, H.; Coetsee, E.; Doucet, F. Surface and bulk characterization of an ultrafine South African coal fly ash with reference to polymer applications. Appl. Surf. Sci. 2014, 317, 73–83. [Google Scholar] [CrossRef]
- Sombatsompop, N.; Thongsang, S.; Markpin, T.; Wimolmala, E. Fly ash particles and precipitated silica as fillers in rubbers. I. Untreated fillers in natural rubber and styrene–butadiene rubber compounds. J. Appl. Polym. Sci. 2004, 93, 2119–2130. [Google Scholar] [CrossRef]
- Orczykowski, W.; Bieliński, D.M.; Anyszka, R.; Pędzich, Z. Fly Ash from Lignite Combustion as a Filler for Rubber Mixes. Part I: Physical Valorization of Fly Ash. Materials 2022, 15, 4869. [Google Scholar] [CrossRef] [PubMed]
- Moyo, D.S.; Doucet, F.J.; Hlangothi, S.P.; Woolard, C.D.; Reynolds-Clausen, K.; Kruger, R.A.; van der Merwe, E.M. Physicochemical Surface Modification and Characterisation of Coal Fly Ash for Application in Rubber Composites. Minerals 2024, 14, 1258. [Google Scholar] [CrossRef]
- Branda, F.; Parida, D.; Pauer, R.; Durante, M.; Gaan, S.; Malucelli, G.; Bifulco, A. Effect of the coupling agent (3-aminopropyl) triethoxysilane on the structure and fire behavior of solvent-free one-pot synthesized silica-epoxy nanocomposites. Polymers 2022, 14, 3853. [Google Scholar] [CrossRef]
- Okhrimenko, D.V.; Budi, A.; Ceccato, M.; Cárdenas, M.; Johansson, D.B.; Lybye, D.; Bechgaard, K.; Andersson, M.P.; Stipp, S.L. Hydrolytic stability of 3-aminopropylsilane coupling agent on silica and silicate surfaces at elevated temperatures. ACS Appl. Mater. Interfaces 2017, 9, 8344–8353. [Google Scholar] [CrossRef]
- Caban, R.; Gnatowski, A. Analysis of the Impact of Waste Fly Ash on Changes in the Structure and Thermal Properties of the Produced Recycled Materials Based on Polyethylene. Materials 2024, 17, 3453. [Google Scholar] [CrossRef]
- Nookala, R. Mechanistic Study of Silane Assisted Rubber to Brass Bonding and the Effect of Alkaline Pre Treatment of Aluminum on Silane Performance. Master’s Thesis, University of Cincinnati, Cincinnati, OH, USA, 2006. [Google Scholar]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. In situ ATR-FTIR study of the early stages of fly ash geopolymer gel formation. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef]
- Çomo, A.; Ylli, F. FTIR spectroscopic investigation of alkali-activated fly ash: Atest study. Zast. Mater. 2018, 59, 539–542. [Google Scholar] [CrossRef]
- Boerio, F.; Schoenlein, L.; Greivenkamp, J. Adsorption of γ-aminopropyltriethoxysilane onto bulk iron from aqueous solutions. J. Appl. Polym. Sci. 1978, 22, 203–213. [Google Scholar] [CrossRef]
- Pantoja, M.; Martínez, M.; Abenojar, J.; Encinas, N.; Ballesteros, Y. Effect of EtOH/H2O ratio and pH on bis-sulfur silane solutions for electrogalvanized steel joints based on anaerobic adhesives. J. Adhes. 2011, 87, 688–708. [Google Scholar] [CrossRef][Green Version]
- Aissaoui, N.; Bergaoui, L.; Landoulsi, J.; Lambert, J.-F.; Boujday, S. Silane layers on silicon surfaces: Mechanism of interaction, stability, and influence on protein adsorption. Langmuir 2012, 28, 656–665. [Google Scholar] [CrossRef]
- Boerio, F.; Armogan, L.; Cheng, S. The structure of γ-aminopropyltriethoxysilane films on iron mirrors. J. Colloid Interface Sci. 1980, 73, 416–424. [Google Scholar] [CrossRef]
- Acres, R.G.; Ellis, A.V.; Alvino, J.; Lenahan, C.E.; Khodakov, D.A.; Metha, G.F.; Andersson, G.G. Molecular structure of 3-aminopropyltriethoxysilane layers formed on silanol-terminated silicon surfaces. J. Phys. Chem. C 2012, 116, 6289–6297. [Google Scholar] [CrossRef]
- Miranda, A.; Martínez, L.; De Beule, P.A. Facile synthesis of an aminopropylsilane layer on Si/SiO2 substrates using ethanol as APTES solvent. MethodsX 2020, 7, 100931. [Google Scholar] [CrossRef]
- Munguía-Cortés, L.; Pérez-Hermosillo, I.; Ojeda-López, R.; Esparza-Schulz, J.M.; Felipe-Mendoza, C.; Cervantes-Uribe, A.; Domínguez-Ortiz, A. APTES-functionalization of SBA-15 using ethanol or toluene: Textural characterization and sorption performance of carbon dioxide. J. Mex. Chem. Soc. 2017, 61, 273–281. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S. Measurement of the condensation temperature of nanosilica powder organically modified by a silane coupling agent and its effect evaluation. J. Appl. Polym. Sci. 2008, 108, 3038–3045. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.-q.; Wang, Z.-m.; Shen, C.-j. Study on the preparation and characterization of high-dispersibility nanosilica. Sci. Eng. Compos. Mater. 2016, 23, 401–406. [Google Scholar] [CrossRef]
- Gunasekara, C.; Law, D.W.; Setunge, S.; Sanjayan, J.G. Zeta potential, gel formation and compressive strength of low calcium fly ash geopolymers. Constr. Build. Mater. 2015, 95, 592–599. [Google Scholar] [CrossRef]
- Veeramasuneni, S.; Yalamanchili, M.; Miller, J. Measurement of interaction forces between silica and α-alumina by atomic force microscopy. J. Colloid Interface Sci. 1996, 184, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Sypabekova, M.; Hagemann, A.; Rho, D.; Kim, S. 3-Aminopropyltriethoxysilane (APTES) deposition methods on oxide surfaces in solution and vapor phases for biosensing applications. Biosensors 2022, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, D.; Mittal, K. Effect of pH of silane solution on the adhesion of polyimide to a silica substrate. J. Appl. Polym. Sci. 1984, 29, 2039–2043. [Google Scholar] [CrossRef]

| Parameter | Variable |
|---|---|
| Solvent (v/v) | Ethanol/water (80:20), Acetone/water (40:60), Sulphuric acid/water (10:90), Toluene (100:0) |
| Temperature (°C) | 20, 40, 60, 80, 100 |
| pH | 2, 3, 4, 8, 9, 10 |
| Wavenumber (cm−1) | Assignment |
|---|---|
| 3380 | symmetric and asymmetric NH stretch |
| 3280 | symmetric and asymmetric NH stretch |
| 2975 | C–H, SiOCH2CH3 |
| 2928 | C–H, SiOCH2CH3 |
| 2886 | C–H, SiOCH2CH3 |
| 1586 | NH2 scissor vibrations, H2N–CH2CH2CH2 |
| 1443 | C–H, CH2 |
| 1390 | C–H stretching vibrations in CH3 and CH2 |
| 1297 | C–H stretching vibrations in CH3 and CH2 |
| 1131 | CH2 rocking of unreacted (Si–OCH2CH3) |
| 1103 | Si–OC, Si–OCH2CH3 |
| 1076 | Si–OC, Si–OCH2CH3 |
| 956 | Si–OC, Si–OCH2CH3 |
| 770 | Si–OC, Si–OCH2CH3 |
| 450 | Si–O rocking |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyo, D.S.; Kleinhans, G.; Wei, X.; Doucet, F.J.; van der Merwe, E.M. Parametric Study on APTES Silanization of Coal Fly Ash for Enhanced Rubber Composite Performance. Minerals 2025, 15, 1198. https://doi.org/10.3390/min15111198
Moyo DS, Kleinhans G, Wei X, Doucet FJ, van der Merwe EM. Parametric Study on APTES Silanization of Coal Fly Ash for Enhanced Rubber Composite Performance. Minerals. 2025; 15(11):1198. https://doi.org/10.3390/min15111198
Chicago/Turabian StyleMoyo, Dennis S., George Kleinhans, Xueting Wei, Frédéric J. Doucet, and Elizabet M. van der Merwe. 2025. "Parametric Study on APTES Silanization of Coal Fly Ash for Enhanced Rubber Composite Performance" Minerals 15, no. 11: 1198. https://doi.org/10.3390/min15111198
APA StyleMoyo, D. S., Kleinhans, G., Wei, X., Doucet, F. J., & van der Merwe, E. M. (2025). Parametric Study on APTES Silanization of Coal Fly Ash for Enhanced Rubber Composite Performance. Minerals, 15(11), 1198. https://doi.org/10.3390/min15111198






