Abstract
This research is based on the deposition of ceramic membranes made from Algerian clays within tubular supports. The major objective is to compare the mechanical strength and water permeability of the developed supports. The membranes made from the same clays are then examined in terms of their application areas and efficacy in treating a local-cheese effluent. The study of these clays demonstrates that the tubular supports made from Aomar clay are more robust than those obtained from kaolin and bentonite. This was due to the higher calcination temperature, which was 1000 °C for Aomar and kaolin clays and 800 °C for bentonite. However, the tubular support based on kaolin has the maximum water permeability (1460.09 L/m2.h.bar). In addition, the permeability tests performed on the membranes deposited on these clays indicate that those of bentonite and Aomar clay are ultrafiltration membranes, whereas the membrane obtained from kaolin is a microfiltration membrane. We demonstrated that the three membranes show high efficiency for the clarification and retention of multiple-pollutant loads of a local-cheese effluent.
1. Introduction
Water is a vital resource for human existence and progress in a variety of industries, such as agriculture and manufacturing. Therefore, it must be maintained clean and functional. Nonetheless, the most technologically advanced human activities have led to its contamination, and unfortunately it is discharged into the environment as effluents without any prior treatment [1,2]. According to their composition, these effluents are poisonous, and their use is hazardous to ordinary living. While certain effluents (pickling bath, surface treatment, etc.), pose major pollution concerns, others, such as those from the agrifood industry, are highly polluting yet readily biodegradable and profitable (dairies, sugar factories, starch processing, fruit and vegetable conversion, etc.) [3]. The color of other effluents, such as those from the paper and/or textile industries, has a considerable aesthetic influence on the aquatic environment. Consequently, it is essential to treat them prior to their release into the natural environment.
In the dairy industry, rejected waters are contaminated by cleaning, washing, and disinfecting chemicals, sterilizers, and other dairy products [4,5]; all of this pollution demonstrates that dairy effluents are substantially polluted with both mineral and organic pollutants, posing a significant threat to the environment when discharged untreated into aquatic receiving media [4,6].
The purification of wastewaters entails enhancing their physicochemical and biological properties so that the treated water fulfills the needed criteria [7,8]. In this regard, a number of procedures have been used for the treatment of wastewater, including physicochemical processes such as adsorption [9,10,11,12], coagulation–flocculation [13,14,15,16], and advanced oxidation processes [17]; microorganism-based biological treatment was also used to reduce carbon, nitrogen, and phosphate contamination, notably in dairy effluents [18], methods of membrane separation using the osmosis phenomena [19], and membrane-filtration processes, such as nanofiltration, ultrafiltration and microfiltration membranes [20].
Membrane filtration is an increasingly popular technology for treating wastewater, due to its high efficiency and versatility in removing various contaminants. This technology involves the use of semi-permeable membranes to separate solids and dissolved substances from wastewater. Over the past few decades, significant progress has been made in the development and application of membrane-filtration systems, making it a viable alternative to traditional treatment methods such as sedimentation and chemical precipitation. According to recent studies [21,22], membrane filtration has shown promising results in terms of water-quality improvement and cost effectiveness.
The separation mechanism in membrane filtration is based on the size-exclusion principle, where the pores in the membrane act as a physical barrier to separate contaminants from the wastewater. The pore size of the membrane can be adjusted to target specific contaminants, such as bacteria, viruses, and organic matter. The separation mechanism can be further enhanced by combining membrane filtration with other treatment processes, such as coagulation and flocculation [23]. The efficiency of the separation process is also influenced by various factors, including the pressure difference across the membrane, the temperature and pH of the wastewater, and the type of membrane material used [24].
Studies have shown that the use of membrane filtration in wastewater treatment can result in high removal rates for various contaminants, including pathogens (e.g., Escherichia coli and coliphages) [22], nutrients (e.g., nitrogen and phosphorus) [25], and emerging contaminants (e.g., pharmaceuticals and personal-care products) [26]. The separation mechanism in membrane filtration provides a sustainable and cost-effective solution for addressing the growing challenges in wastewater treatment. The increasing demand for clean water and the stringent regulations for water quality have driven the need for further research and development in the field of membrane filtration.
Microfiltration (MF) and ultrafiltration (UF) are two commonly used membrane-filtration processes for wastewater treatment. MF is a low-pressure filtration process that uses membranes with pore sizes ranging from 0.1 to 10 μm. The main goal of MF is to remove suspended solids and colloidal particles from the wastewater, resulting in the improvement of water clarity and turbidity [27]. UF, on the other hand, uses membranes with pore sizes ranging from 0.01 to 0.1 μm and operates at higher pressures than MF. The objective of UF is to remove dissolved substances, including proteins, organic molecules, and pathogens, from the wastewater [28].
Both MF and UF have been widely applied in various wastewater-treatment applications, such as municipal-wastewater treatment, industrial-wastewater treatment, and desalination. MF and UF are also compatible with other treatment processes, such as adsorption and oxidation, which can further improve the removal efficiency of contaminants [29]. The use of MF and UF in wastewater treatment has been shown to result in high removal rates for various contaminants, such as bacteria, organic matter, and nutrients. The combination of MF and UF provides a flexible and cost-effective solution for addressing the complex challenges in wastewater treatment.
Ceramic membranes are a type of membrane-filtration technology that is gaining increasing attention for its potential in wastewater treatment. Ceramic membranes are made from inorganic materials, such as alumina, zirconia, and titania, and are known for their high mechanical strength and chemical stability [30]. Compared to polymeric membranes, ceramic membranes offer several advantages, such as higher temperature and pH tolerance, better resistance to fouling, and longer membrane life [31]. However, ceramic membranes also have some disadvantages, such as high cost, low flexibility, and limited availability of pore sizes [32]. Furthermore, ceramic membranes have a relatively high permeation resistance, which can result in lower permeate flux compared to polymeric membranes [33]. Despite these limitations, ceramic membranes have shown promising results in wastewater-treatment applications, particularly in the removal of challenging contaminants, such as heavy metals, organics, and pathogens [30]. In comparison to polymeric membranes, ceramic membranes have been shown to provide higher removal efficiency and long-term stability in various wastewater-treatment processes. Overall, ceramic membranes are a promising technology for wastewater treatment, offering high performance and durability, while also addressing some of the challenges associated with polymeric membranes.
Recently, the use of clay as a raw material for synthesizing ceramic membranes has gained attention as a sustainable and low-cost alternative to traditional ceramic materials. Clay is abundant and widely available, making it an attractive option for large-scale production of ceramic membranes. Additionally, clay has good plasticity and can be molded into various shapes and sizes, providing flexibility in the design of ceramic membranes [34]. Studies have shown that clay-based ceramic membranes can provide high performance in various wastewater-treatment applications, including the removal of pathogens, organic pollutants, and heavy metals [35]. Furthermore, clay-based ceramic membranes have demonstrated good mechanical strength and chemical stability, making them a promising alternative to traditional ceramic membranes. The use of clay as a raw material for synthesizing ceramic membranes has the potential to make this technology more accessible and cost-effective, while also providing high performance and durability in wastewater-treatment applications. Further research is needed to optimize the synthesis process and enhance the performance of clay-based ceramic membranes, making them a viable option for wider implementation in wastewater treatment.
The aim of this work is to evaluate the effectiveness of three synthetized clay-based ceramic membranes in purifying cheese effluent, based on their performance compared to the standards set by the World Health Organization (WHO), as referenced in the official journal of the Algerian Republic. For this purpose, three Algerian clays, namely, bentonite from northwestern Algeria, kaolin from northeastern Algeria, and clay from Aomar (in the central north of the country) were selected for the study; to our knowledge, such work has never been undertaken before.
2. Materials and Methods
2.1. Raw Materials
Local clays (Algerian) from various regions are used to develop tubular supports, including bentonite from Maghnia (located in northwestern Algeria), kaolin from Tamazert (located in northeastern Algeria), and clay from Aomar (in the central north of the country), which is used in the production of red brick.
An X-ray-fluorescence-spectroscopy study using a spectrometer (S8 TIGER, Bruker, Germany) was undertaken, to identify the chemical composition of each kind of clay.
2.2. Preparation of Ceramic Paste
The optimal formulation of ceramic pastes, using various types of clay, has been developed to possess the requisite rheological characteristics, including homogeneity, cohesion, porosity, and extrusion-deformation capacity, ensuring their suitability for intended applications [36].
Each type of clay is sieved through a 75 µm sieve, mixed with water and organic additives (starch with the chemical formula (C6H10O5)n—from FlukaBiochemika, methocel which is a cellulosic derivative with the chemical name hydroxypropyl methylcellulose, and from The Dow Chemical Company, amijel, which is a derivative product consisting of pre-gelled starch Cplus12,072, cerestar. These organic additives play a vital role in tubular-support shaping and providing acceptable physical and mechanical qualities after sintering [37].
2.3. Extrusion of Ceramic Paste
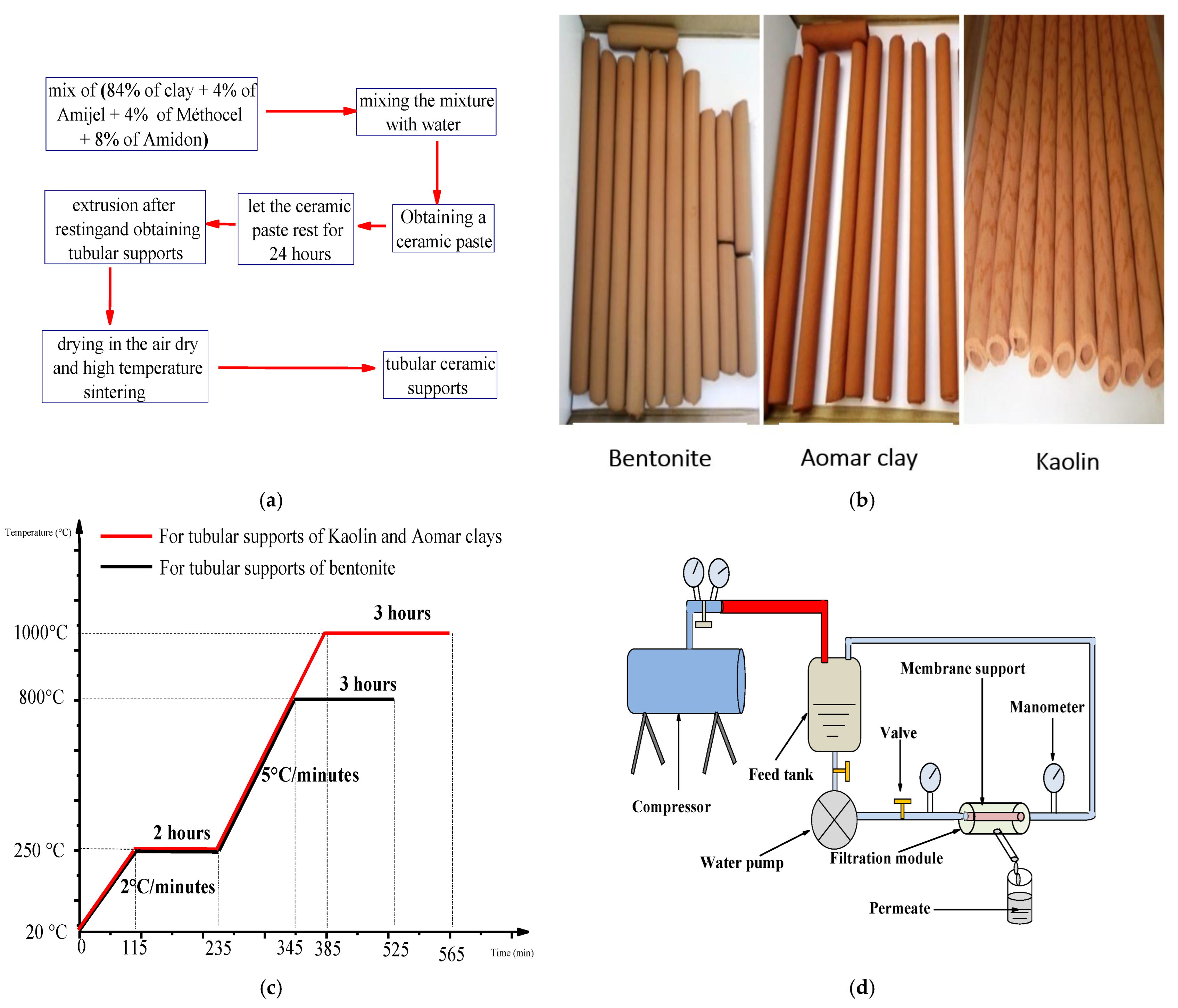
Extrusion of the previously produced ceramic paste is used to construct tubular supports. It is based on the idea of compressing the paste in a cylinder which is put on another cylindrical molded component to produce single-channel tubular supports with well-defined diameters [38,39]. Following extrusion, the supports are air dried for 6 days before being heat treated in a furnace (Nabertherm GmbH, Lilienthal, Germany), using a two-stage thermal program. Figure 1a–c show the experimental procedures for this preparation, including all of the tubular supports produced for each kind of clay, the thermal program used for sintering them, and a schematic depiction of the thermal program used for sintering the tubular supports.
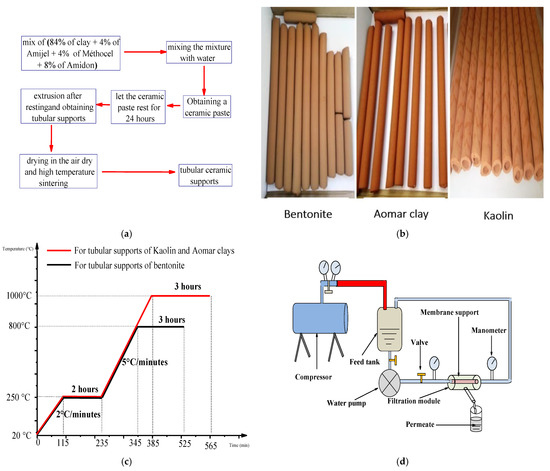
Figure 1.
Representative figures of the (a) flowchart of the main procedures followed for the elaboration of tubular ceramic supports, (b) tubular supports obtained for each type of clay, (c) schematic representation of the thermal program followed for the sintering of the tubular supports and (d) schematic representation of used filtration pilot.
2.4. Mechanical and Physical Properties of Tubular Supports before and after Sintering
The outer and inner diameters, lengths, and thicknesses of the elaborated tubular supports were measured with a caliper before, during, and after drying and sintering, to determine their physical properties.
To regulate the resistance of the tubular supports, the mechanical properties of the supports produced after sintering were determined by measuring their mechanical resistance using the three-point bending technique with a TLS-Techlab-system instrument (Lezo, Spain). The objective of this measurement is to position a sample of a solid material on two simple supports and apply a force to the sample’s center until it fractures, and then read the breaking-strength data.
2.5. Test of Water Permeability for Tubular Supports
Following sintering, the tubular supports were cut to 15 cm and tested for permeability using a closed-circuit filtering pilot (see Figure 1d). This pilot consists of a feed tank, a water pump, a filter module including the support, two pressure regulators, and a compressor used to apply varying pressures.
2.6. Membrane Preparation and Deposition
Our membranes were manufactured by preparing a slip consisting of a mass percentage (W%) combination of 30 W% polyvinyl alcohol gel (12 W% PVA in water), 65 W% water, and 5 W% clay powder sieved through 40 µm [39].
Slip-casting is the technique used for the deposition of membranes of each type of clay. It consists of placing the tubular supports, which are blocked at one end, in a vertical position and then filling them with the slip during a specific time of engobing, then allowing them to air dry for 24 h, to allow the excess slip to drip off. The membranes placed on the inner surface of the tubular supports are consolidated using heat treatment, at the optimal sintering temperature for each membrane, 750 °C for the bentonite-based membrane and 900 °C for the kaolin- and Aomar-clay-based membranes. It is essential to note that the optimal sintering temperature for each membrane was determined based on their homogeneity and adhesion to the inner walls of the tubular supports.
2.7. Determination of the Field of Application of Each Membrane
Determining the area of application for ceramic membranes is a crucial stage in the membrane-filtration process in order to determine the type of the effluent that will be filtered on each membrane [37]. Figure 1d depicts a filtration pilot used to test the permeability to pure water of our newly designed membranes. We were able to identify the area of application for our membranes based on the findings obtained by comparing the volume-flow-density order with that of different membrane techniques described in the literature [37].
2.8. The Efficiency of Membranes for Filtration of a Cheese Effluent
Filtration of a cheese effluent from the TARTINO cheese situated in the Rouiba industrial zone was used to evaluate the performance of our newly designed membranes (central north of Algeria). The effluent was injected into the feed tank while the support containing the ceramic membrane was installed within the module (Figure 1d) and exposed to varying pressures of effluent circulation.
Following filtration, various physicochemical pollution characteristics were analyzed for each permeate collected in Erlenmeyer flasks for each applied pressure, as well as the unfiltered effluent returned to the feed tank. Table 1 depicts the pollutant metrics evaluated and their limit levels regarding Algerian industrial discharges.

Table 1.
Limit values of some physicochemical parameters of pollution in industrial rejection in Algeria.
3. Results and Discussions
3.1. Characterization of the Raw Materials
The findings produced by the X-ray-fluorescence analysis are stated in the oxide equivalent for each atom present in each clay (see Table 2).

Table 2.
Chemical composition of the clays in mass percentage(W%).
The X-ray-fluorescence analysis of the three clays showed that they are composed of several metal oxides with different proportions. The silicates and alumina form the main composition of each clay studied. This outcome aligns with the findings in the study of natural zeolite-based clay ceramic membranes [40].
3.2. Physical and Mechanical Characteristics of Tubular Supports
Table 3 shows the physical and mechanical properties of tubular supports produced (without membrane) from each kind of clay.

Table 3.
Physical-mechanical characteristics of the tubular supports obtained from each type of clay.
The table demonstrates that the fabricated tubular supports underwent volume shrinkage during air drying, represented by VSd, and volume shrinkage during sintering, represented by VSs. The tubular supports prepared on the basis of bentonite have a higher volume shrinkage compared to those based on the kaolin and Aomar clays. These two phenomena, linked to VSd and VSs, have been explained in the literature by the disappearance of the water used for the production of ceramic pastes during drying, in addition to the removal of organic additives included in the paste during sintering [41]. Furthermore, mechanical resistance to ceramic while drying, as well as the disappearance of bending, reveal that tubular supports made of Aomar clay are more robust than those made of kaolin and bentonite (21.02 Mpa, 18.6 Mpa and 13.84 Mpa, respectively). This finding is explained by the difference in the rate of lime (CaO) in the three clays, which is larger in Aomar clay (13.18%) compared to Kaolin (6.15%) and bentonite (2.37%). Indeed, recent studies have demonstrated that increasing the quantity of lime in clays enhances thermal stability and mechanical strength [42,43].
3.3. Determination of the Water Permeability of Tubular Supports
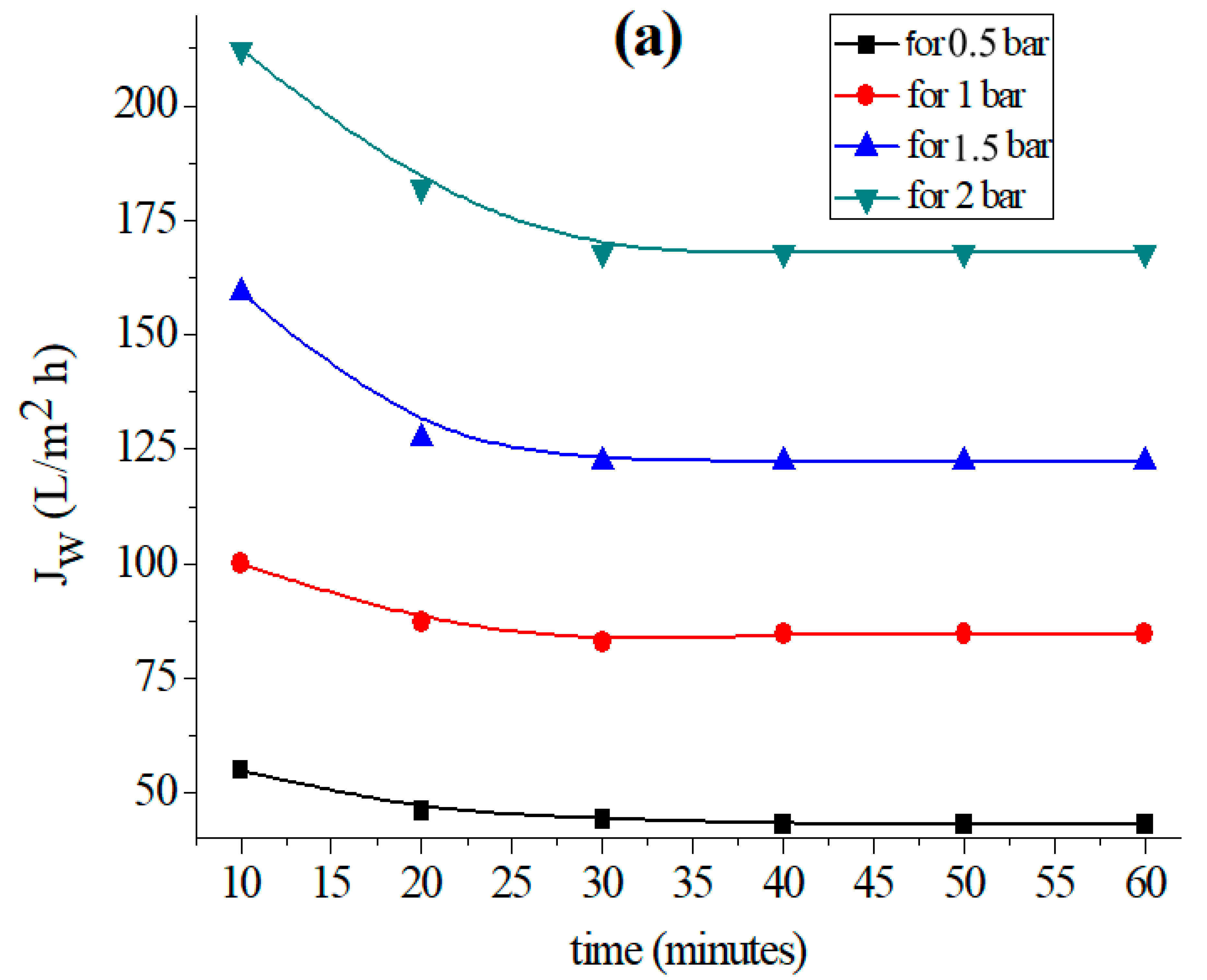
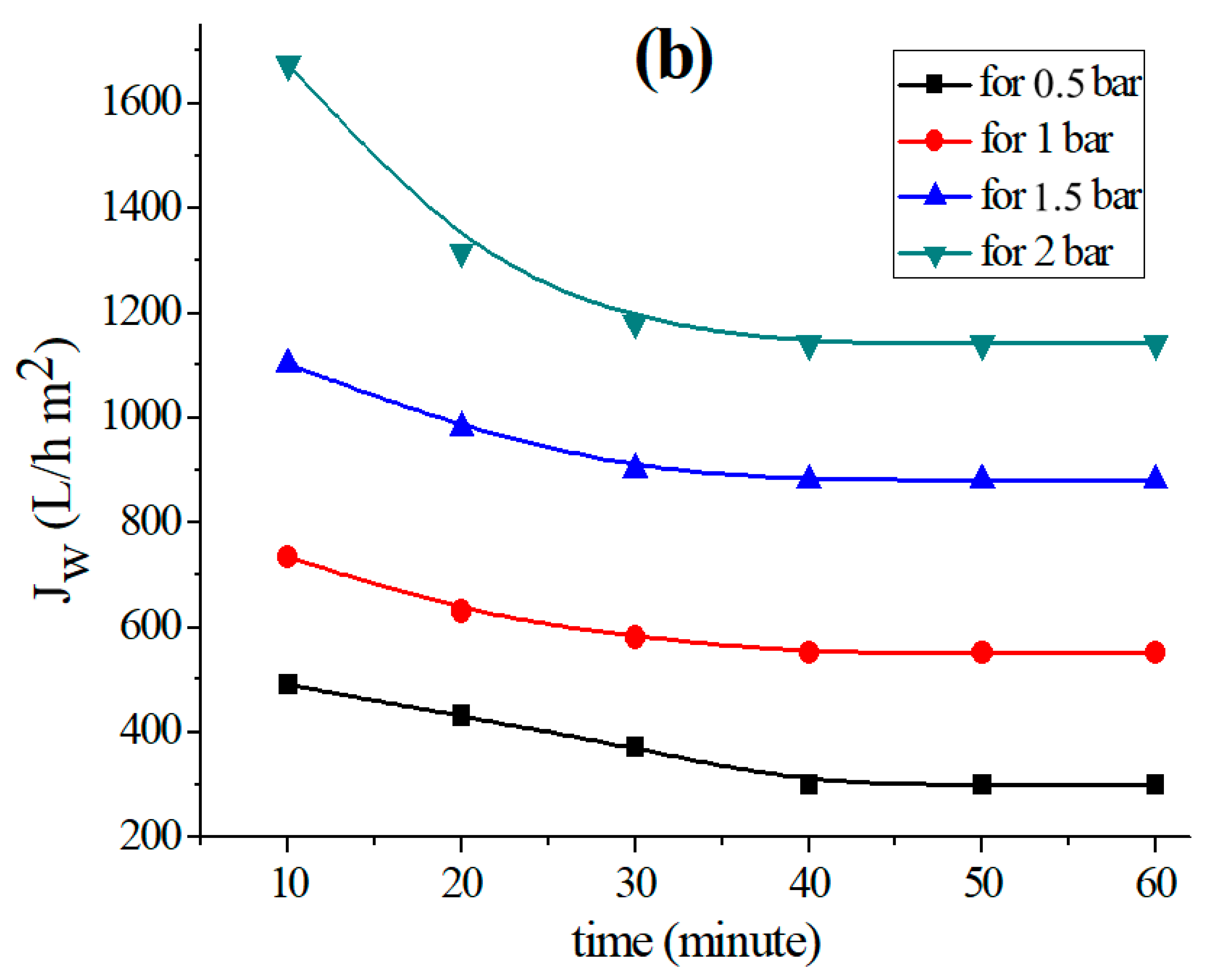
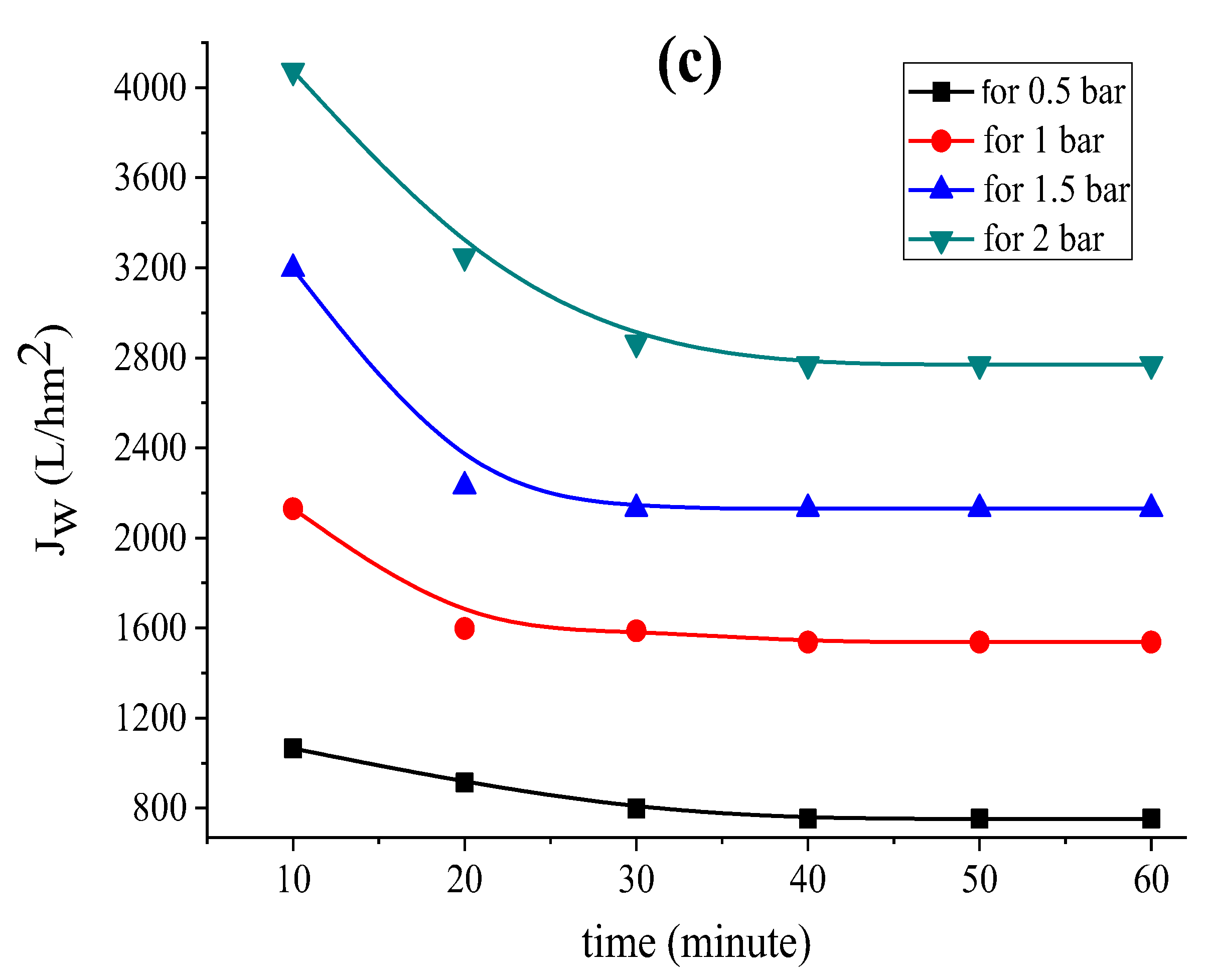
The permeability of the supports based on each clay was measured by applying the well-known relationship (1) [44] to investigate the fluctuation of the permeation flux with distilled water through the support until it reaches stability. Figure 2 shows the Study’s findings for each kind of clay.
where Jw is the water permeate flux, V the volume collected after each 10 min, S represents the support surface (S = 2πrL) and t is the time required to collect the same volume of water after every 10 min.
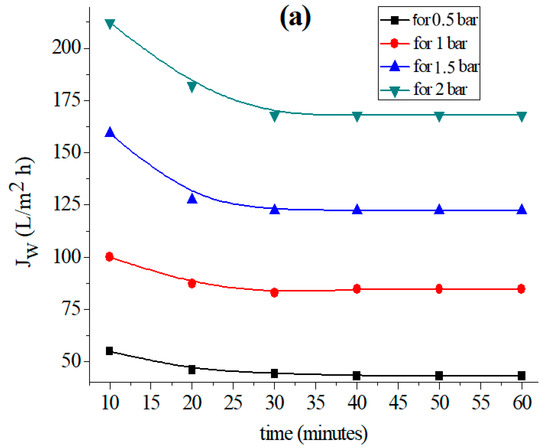
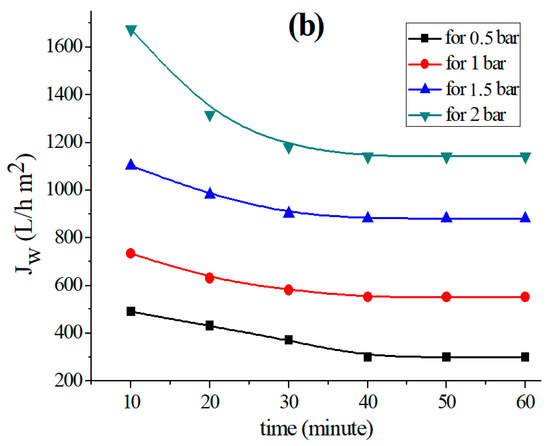
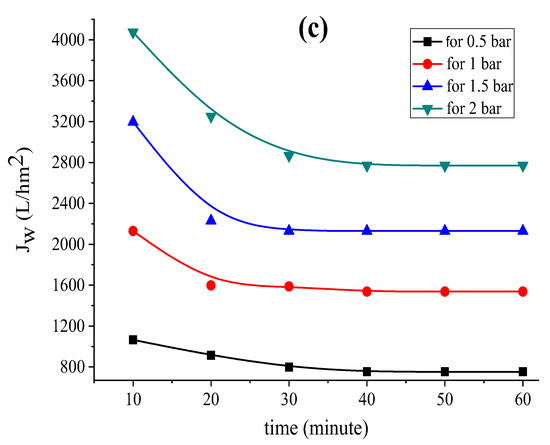
Figure 2.
Variation in the permeation flux with distilled water as a function of time for the (a) support based on bentonite, (b) support based on Aomar clay and (c) support based on kaolin.
The data in Figure 2 clearly illustrate the fact that the permeate flow diminishes with time in all examined tubular supports, and stabilizes after 40 min for each applied pressure. This flux-stability finding is consistent with previous research, which reveals that the permeation flux often stabilizes within 30 or 40 min [37,39,45].
Using the relation (2) [46], we were able to calculate the water permeability (Lp) for the tubular supports that corresponded to each kind of clay via the graphical depiction of the flux fluctuation as a function of applied pressure Jw = f(P).
were ΔP is the effective transmembrane-pressure difference and Jw the steady flow of the applied pressure.
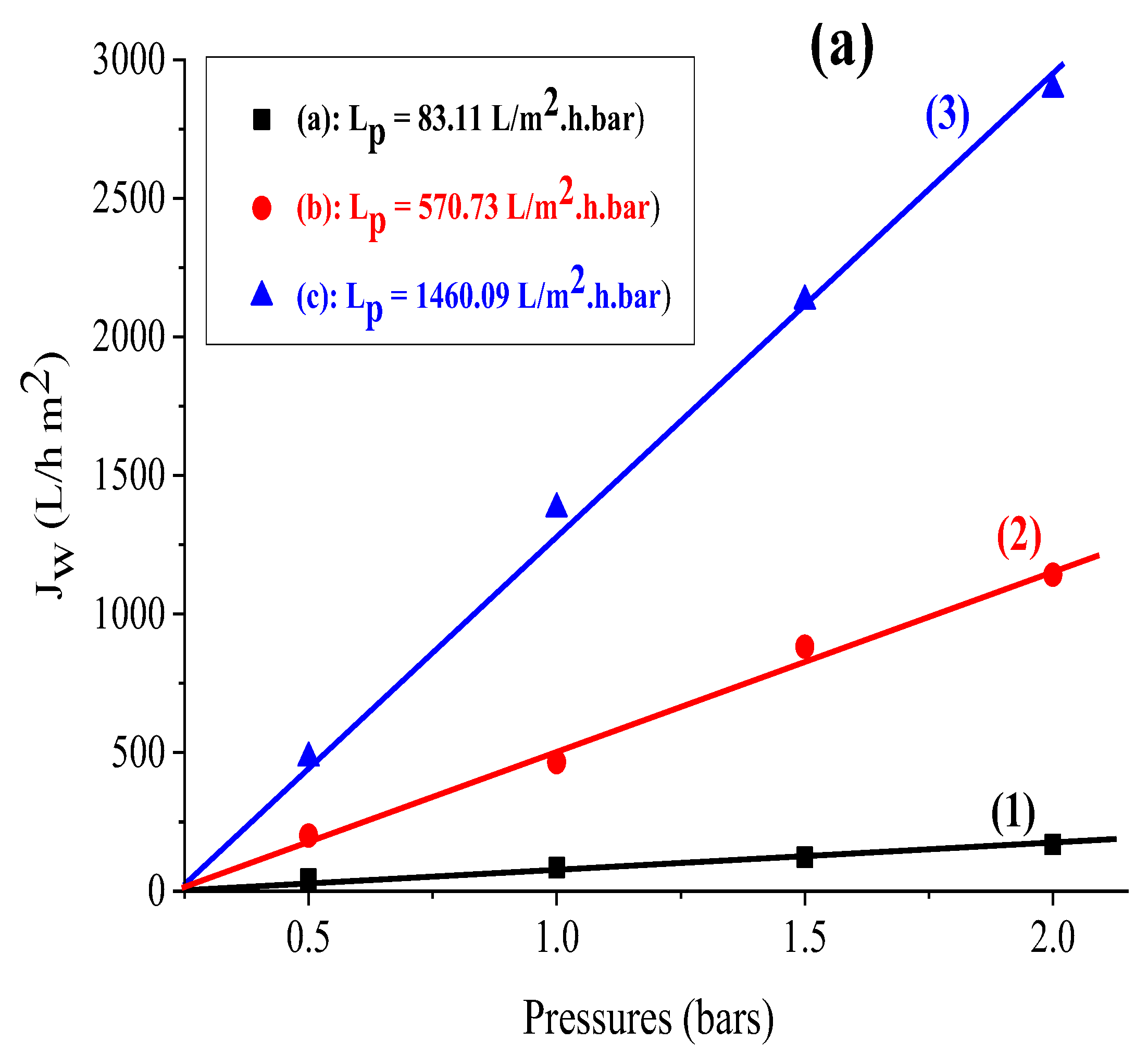
The findings reveal that the flux variation as a function of pressure is linear for all of the supports investigated. Furthermore, the permeability computed for each support (see Figure 3a) shows that kaolin has a larger permeability than the Aomar and bentonite clays (1460.09 L/m2 bar, 570.73 L/m2 h bar, and 83.11 L/m2 bar, respectively).
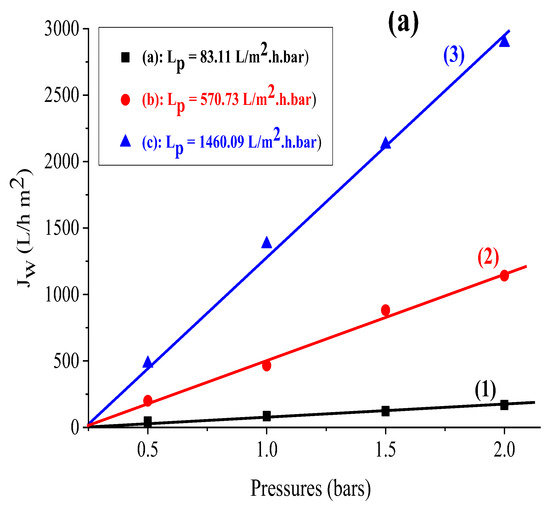
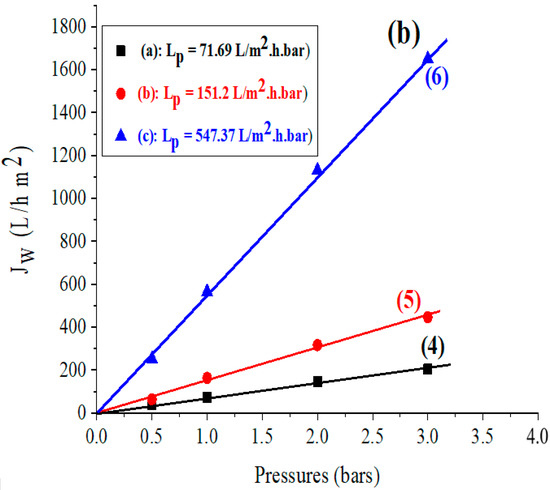
Figure 3.
(a) Variation of the flux as a function of the applied pressure for the support based on (1) bentonite, (2) Aomar clay, (3) kaolin, and for the membranes developed based on (b) (4) bentonite, (5) Aomar clay and (6) kaolin.
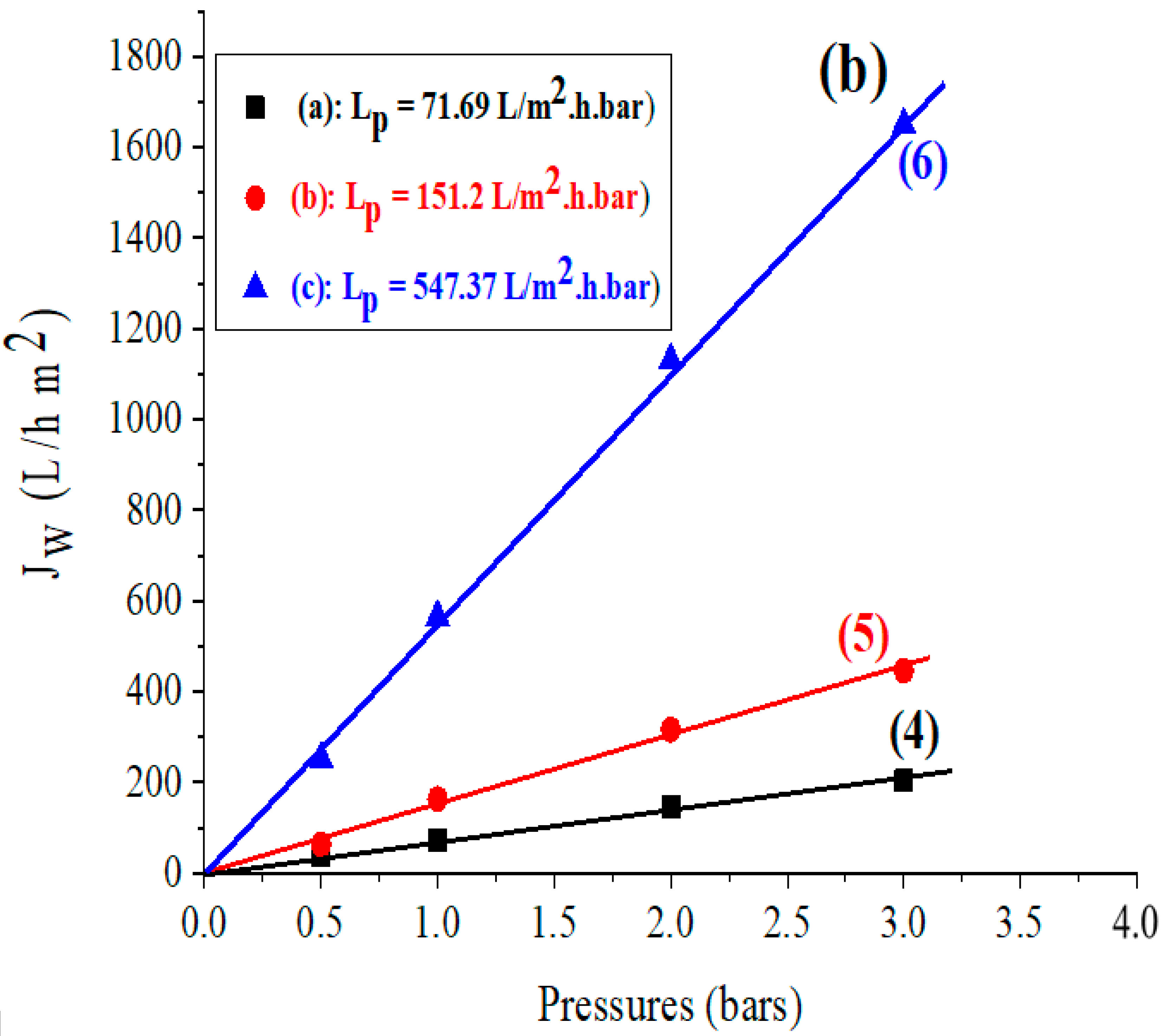
3.4. The Water Permeability of Membranes Developed
The permeability of membranes produced from the investigated clays was calculated using the same approach as the permeability of the previously indicated supports. The acquired findings are shown in Figure 3b.
The various domains of application of membranes (ultrafiltration, microfiltration, and nanofiltration) have been characterized in the literature based on permeability to distilled water and applied-pressure-value intervals [37,47].
In this regard, the values obtained for the Lp permeability of different membranes in Figure 3b indicate that the membranes based on bentonite and Aomar clays are ultrafiltration membranes with values of Lp = 71.69 and 151.2 L/m2.h.bar, respectively, and the membrane based on kaolin is a microfiltration membrane with a value of Lp = 547.37 L/m2.h.bar. The comparison of our findings with prior studies on natural zeolite-based ceramic membranes highlights the application of these membranes for ultrafiltration and nanofiltration [35,44,48]. These studies have demonstrated their efficacy in filtering saline water and retaining monovalent and bivalent metals.
3.5. The Study of the Efficiency of Our Membranes in the Filtration of a Local Effluent
Our filtering membranes are specifically aimed at filtration of a local-cheese effluent. This is because the effluent needs to undergo special treatment to reduce its organic and inorganic contaminant levels, prior to being released into the environment [4,7,49].
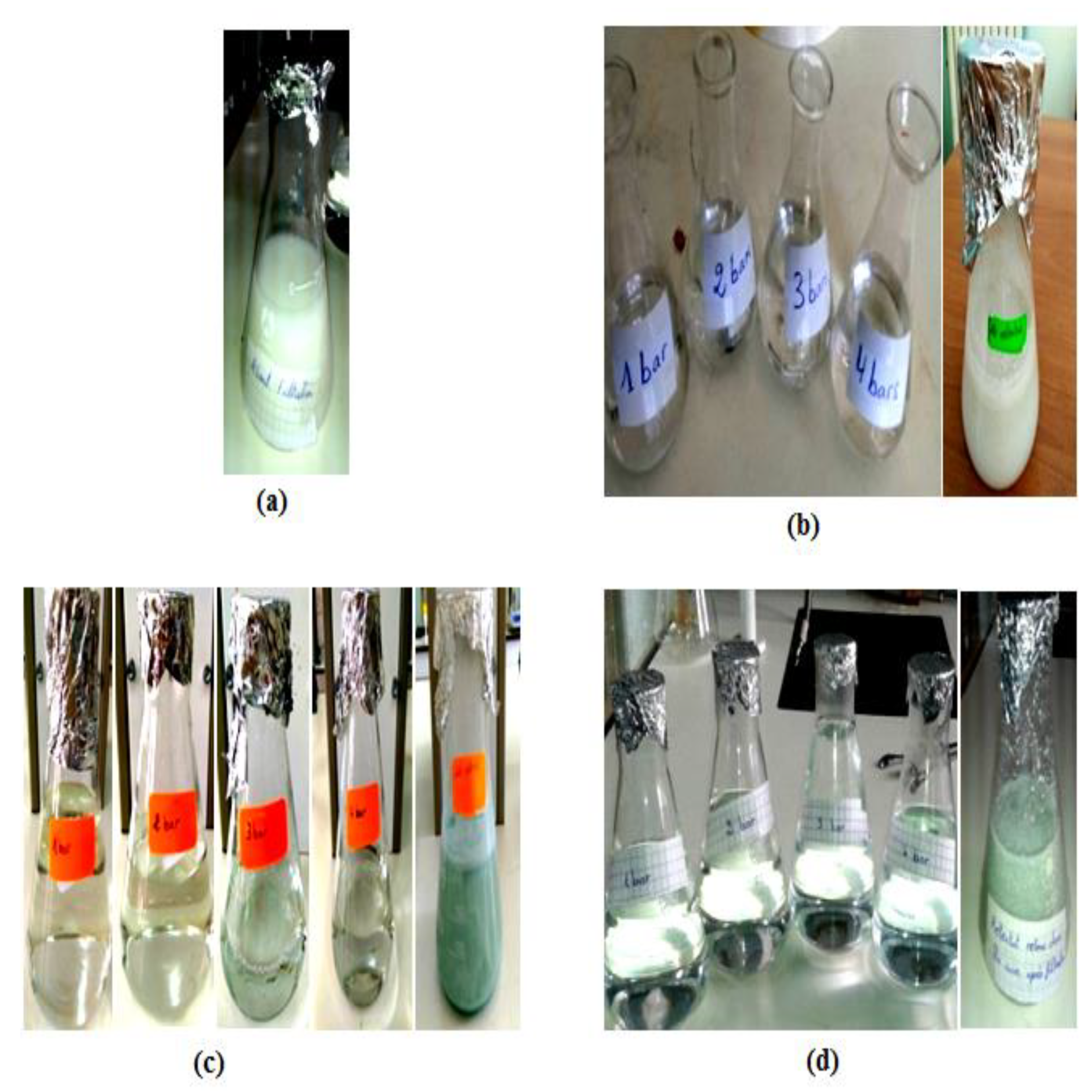
Figure 4 shows the difference in appearance of the effluent before and after filtration, with varying transmembrane pressures on the developed membranes, as well as the liquid in the feed tank. The images reveal that before filtration, the effluent has a white tint with high turbidity. However, after filtration through the microfiltration and ultrafiltration membranes at various pressures, the resulting permeate is clear and transparent, indicating the effectiveness of the membraned in clarifying the effluent. The dark color and high turbidity of the liquid in the feed tank also supports this result.
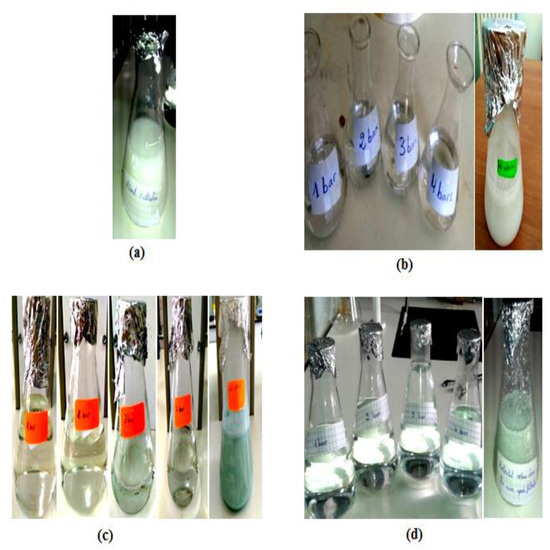
Figure 4.
Representative image of the visual appearance of the effluent, (a) before filtration on the developed membranes, (b) after filtration on the developed membrane based on bentonite, (c) after filtration on the developed membrane based on Aomar clay, and (d) after filtration on the developed membrane based on kaolin.
Table 4, Table 5 and Table 6 shows the results of physicochemical-pollutant-parameter analyses performed on our samples before and after membrane filtration.

Table 4.
Results of the physicochemical- pollution parameters measured on the studied effluent using membrane based on bentonite (UF).

Table 5.
Results of the physicochemical-pollution parameters measured on the studied effluent using membrane based on Aomar clay (UF).

Table 6.
Results of the physicochemical pollution parameters measured on the studied effluent using membrane based on kaolin (MF).
The turbidity level of the examined effluent before filtering was 2400 NTU, which is substantially higher than the recommended limit in Algeria (80 NTU). The rise in turbidity might be attributed to the mobilization of organic and inorganic particles in suspension [50].
The turbidity in the permeate collected at different pressures was reduced to values well below the required standard after filtration, in contrast to the highest turbidity observed in the liquid retained in the feed tank; this result indicates that the suspended matter was retained by the three membranes studied. These findings are consistent with previous studies on the measuring of turbidity before and after membrane filtration [37,45,51]. A reduction in turbidity has been observed with the rise of transmembrane pressure up to 3 bars, after which they progressively increase as indicated in Table 4, Table 5 and Table 6, which may be explained by a partial fouling of the membranes caused by transmembrane pressures of more than 3 bars [52].
3.5.1. Conductivity
The recorded electrical-conductivity values from both the effluent before filtration and the permeates after filtration, at various transmembrane pressures between 1 and 4 bars, are better than the Algerian standard cited in Table 2.
These findings explain why the samples studied had substantial salt even after membrane filtration (less than 2500 μS/cm). This salinity is caused by salts, washing chemicals, detergents, and disinfectants [53,54]. It is critical to note here that the values of electrical conductivity steadily rise as the transmembrane pressure reaches 3 bars, which corresponds to the turbidity values reached beyond 3 bars. These conductivity findings in Table 4, Table 5 and Table 6 suggest that ultrafiltration membranes are more effective than microfiltration membranes for mineral-salt retention, as reported in the literature [51]. This result, of the successful retention of mineral salts by our two ultrafiltration membranes, is in line with previous research findings on the filtration of saline water using ceramic membranes based on natural zeolites [35,44,48].
3.5.2. Ammonium NH4+
Ammonia nitrogen is a reliable indication of urban-effluent contamination in water systems. The findings in Table 4, Table 5 and Table 6 reveal that the quantity of NH4+ ions in the unfiltered effluent is excessively high, reaching 59.2 mg/L. After ultrafiltration and microfiltration by the membrane at various pressures, a considerable drop in the quantity of these ions was noticed, which was followed by an increase in the retained liquid, showing that these ions were successfully retained by filtration on all three membranes. Indeed, better values for NH4+ ions were found.
After ultrafiltration with the bentonite and Aomar clay membranes, the result was consistent with the necessary standard-limit value (≤5 mg/L), even when the applied pressure was increased from 1 to 4 bars. However, the amount of NH4+ produced via membrane microfiltration based on kaolin is substantially greater than the Algerian standard. This outcome is due to the excessively high permeability achieved by the kaolin-based membrane at 547.37 L/m2.h.bar (see Figure 3b), which is approximately four times greater than the permeability values of the bentonite- and Aomar-clay-based membranes. This explains why the porosity of the kaolin-based membrane is significant, allowing a great amount of ammonium ions to pass through its pores even at low transmembrane pressure (refer to the results in Table 6 of the revised version of the manuscript).
3.5.3. Nitrates NO3− and Nitrite NO2−
Nitrates and nitrites are both oxidized forms of nitrogen pollution found in wastewater [55]. The presence of lactating proteins, mineral nitrogen in milk, the bacterial oxidation of ammonia and/or organic-matter decomposition, and the usage of nitric acid during washing all contribute to the high concentration of these ions that define nitrogen pollution [53]. Table 4, Table 5 and Table 6 shows that the measured nitrate and nitrite levels in the unfiltered effluent are much higher than the necessary requirements (30 mg/L for NO3− ions and 03 mg/L for NO2− ions).
Even at high transmembrane pressures, we detect a drop in these values after filtration on our UF and MF membranes. Indeed, the amounts of NO3− recovered after filtering are less than the acceptable requirements, allowing us to conclude that these ions were partly retained by the two filtration procedures in all membranes tested. Moreover, the concentration of NO2− ions obtained after filtering using the two procedures of UF and MF is lower than the necessary standard. This conclusion is explained by the fact that NO2− is an intermediate molecule that is unstable in the presence of oxygen and has a lower concentration than the two other forms, namely nitrate and ammonium ions [56].
3.5.4. Phosphates PO4−3
The amount of ions in orthophosphates obtained in Table 4, Table 5 and Table 6 for the unfiltered effluent is higher than the Algerian standard (10 mg/L), which is most likely due to the usage of H3PO4 for machine cleaning at the level of cheese manufacturers in general. Furthermore, phosphorus compounds such as soluble orthophosphates and organophosphorus derivatives may be found in natural waters and wastewater [55].
The number of orthophosphate Ions Is lowered below the acceptable level after filtering using ultrafiltration membranes based on bentonite and Aomar clays, regardless of the applied transmembrane pressure (1–4 bars). However, the quantities of these ions following filtration on kaolin-based microfiltration membranes remain too high at all pressures employed. These findings suggest that a membrane-ultrafiltration method can achieve orthophosphate retention, but not a microfiltration procedure.
3.5.5. pH and Temperature
pH is an effective indication of pollution; it fluctuates depending on whether the effluent is basic or acidic. The biological pH range is 6.5 to 8.5 [57]. Indeed, the pH values obtained before and after filtration for the three kinds of membranes demonstrate that all of the samples studied had pH values between 6.8 and 7.3. These results are consistent with those of the rejected effluents in Algeria, where the pH must be in the range (6.5–8.5).
Temperature changes have a significant impact on the formation of microorganism colonies [58,59]. Indeed, increasing the temperature of industrial effluents promotes their growth and hence the consumption of huge amounts of oxygen, while decreasing the amount of dissolved oxygen in these effluents [60].
According to Table 4, Table 5 and Table 6, the observed temperatures for all ultrafiltration- and microfiltration-membrane samples are almost consistent, and fall below the acceptable limit (30 °C). This result indicates that the examined samples do not constitute a thermal-pollution concern to the receiving natural environment. Values over 30 °C, on the other hand, contribute to the acceleration of biological processes for the treatment of industrial effluents by increasing the kinetics of organic degrading matter [61].
3.5.6. The Chemical Oxygen Demand COD
The COD data (Table 4, Table 5 and Table 6) demonstrate that the unfiltered effluent is highly contaminated with organic matter, with a value of 5928 mg/L, which is much more than the necessary limit (120 mg/L). The COD value reported in this unfiltered effluent is three times that found in study work on wastewater-COD analysis [62]. This conclusion may be explained by the fact that cheese effluents include residues of milk and chemical products used for machine cleaning at the cheese-factory level, resulting in an increase in the quantity of organic matter responsible for the growth of aerobic bacteria [63].
After filtering at pressures ranging from 1 to 4 bars, the COD value drops to between 665.5 and 476.8 mg/L (approximately 90% of organic matter eliminated) for the bentonite membrane, between 1520 and 1123 mg/L (approximately 81% of organic matter eliminated) for the Aomar-clay membrane, and between 1320 and 798 mg/L (approximately 90% of organic matter eliminated) for the kaolin membrane. These values remain high following filtering by the two UF and MF procedures, indicating that the organic components in this cheese effluent were partly retained by all of the membranes tested. The results of our investigation of the COD show improved outcomes compared to previous research on the filtration of dairy effluent conducted over a one-month period at a sequencing-batch-reactor station [6]. Our results are even more favorable in comparison to the treatment of wastewater in a series of three microphyte-lagoon basins [63].
3.5.7. BOD5: Biological Oxygen Demand for 5 days
The findings in Table 4, Table 5 and Table 6 further demonstrate that the BOD5 value obtained for the effluent before filtering is extremely high (2400 mg/L), which explains why this effluent is so rich in biodegradable compounds. After filtering, the BOD5 in all permeates sampled at each applied pressure from 1 to 4 bars falls progressively across all membranes examined. This gradual drop when pressure is increased may be explained by the partial fouling of our membranes over time. It is critical to note that all BOD5 readings measured before and after filtering remain very high, and exceed the necessary level of 35 mg/L.
3.5.8. The Ratio of COD/BOD5
The COD/BOD5 ratio allows us to assess the biodegradability of contaminants and determine the purification chain of a given effluent. Wastewater rejected directly into receiving waters exhibits household-wastewater characteristics (COD/BOD5 < 3) [64].
This increasing ratio suggests an increase in non-biodegradable organic matter [57,59]. The COD/BOD5 ratio values obtained in Table 4, Table 5 and Table 6 for the permeate collected after filtering of our effluent on the three membranes investigated at varying pressures are significantly lower than 3 (between 0.74 and 0.94), indicating that these are readily biodegradable effluents [61]. The COD/BOD5 ratios of the effluent before filtering and the liquid retained in the feed tank, on the other hand, are between 2 and 3, indicating that they are moderately biodegradable effluents [57,58,59,61]; indeed, as stated by Mesrouk et al. [60] and Litébé et al. [65], an analysis of this ratio clearly highlights the biodegradability of wastewater. These findings indicate that all of our samples, both before and after membrane filtration, may be purified using biological treatment [58,61].
4. Conclusions
In the study conducted here, tubular supports were fabricated using three clays sourced from various regions in Algeria, with the intention of utilizing them in ultrafiltration and microfiltration processes. Results showed that the kaolin-based support had the highest water permeability. The developed membranes effectively clarified a local-cheese effluent and retained suspended particles and organic compounds at transmembrane pressures less than or equal to 3 bars. The ultrafiltration membranes based on bentonite and Aomar clay retained NH4+ ions, but this was not the case for the microfiltration based on kaolin, unlike the NO2− and NO3− ions which all three membranes tested retained. The study found that when the transmembrane pressure is greater than 3 bars, NH4+ and NO2− ions begin to cross the membranes and their retention is facilitated by ultrafiltration membranes based on bentonite and Aomar clay. The analysis showed that the permeates collected at pressures between 1 and 4 bars are readily biodegradable, and require biological treatment. The study concluded that COD and BOD5 are important for reducing organic matter and biodegradable-material loads, and that the permeates collected are fairly biodegradable and need biological treatment.
Author Contributions
Conceptualization, L.H. and D.E.A.; methodology, L.H., D.E.A. and A.H.; validation, L.M. and A.A.; formal analysis, L.H. and D.E.A., investigation, L.H.; resources, L.M. and A.A.; data curation, L.H. and D.E.A.; writing—original draft preparation, L.H.; writing—review and editing, A.H., L.M. and A.A.; visualization, L.M. and A.A.; supervision, L.M. and A.A.; project administration, L.M. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank all who assisted in conducting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mustapha, S.; Shuaib, D.T.; Ndamitso, M.M.; Etsuyankpa, M.B.; Sumaila, A.; Mohammed, U.M.; Nasirudeen, M.B. Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb(II), Cd(II), Zn(II) and Cu(II) ions from aqueous solutions using Albizia lebbeck pods. Appl. Water Sci. 2019, 9, 142. [Google Scholar] [CrossRef]
- Mustapha, S.; Dauda, B.; Ndamitso, M.; Mathew, J.; Bassey, U.; Muhammed, S. Biosorption of Copper from Aqueous Solutions by Raw and Activated Spines of Bombax Buonopozense: Equilibrium, Kinetics and Thermodynamic Studies. Int. J. Appl. Chem. 2014, 4, 887–903. [Google Scholar] [CrossRef]
- Crini, G.; Torri, G.; Lichtfouse, E.; Kyzas, G.Z.; Wilson, L.D.; Morin-Crini, N. Dye Removal by Biosorption Using Cross-Linked Chitosan-Based Hydrogels. Environ. Chem. Lett. 2019, 17, 1645–1666. [Google Scholar] [CrossRef]
- Sarkar, B.; Chakrabarti, P.; Vijaykumar, A.; Kale, V. Wastewater Treatment in Dairy Industries—Possibility of Reuse. Desalination 2006, 195, 141–152. [Google Scholar] [CrossRef]
- Sanja, P.; Dragičević, T.; Hren, M. The Improvement of Dairy Wastewater Treatment Efficiency By the Addition of Bioactivator. Mljekarstvo 2010, 60, 198–206. [Google Scholar]
- El Ghammat, A.; Riffi, K.; Zerrouk, M. A Study of the Performance of a Sequential Bioreactor Plant for the Treatment of Dairy Effluents. LARHYSS J. 2019, 37, 7–21. [Google Scholar]
- Saheed, M.; Dauda, B.E.N.; Iyaka, Y.; Tsado, M.J.; Aliyu, A.; Shaba, E.Y. Removal of Heavy Metals from Aqueous Solutions by Modified Activated Carbon from Bombax Buonopozense. Int. J. Eng. Sci. 2014, 3, 17–24. [Google Scholar]
- Zamouche, M.; Tahraoui, H.; Laggoun, Z.; Mechati, S.; Chemchmi, R.; Kanjal, M.I.; Amrane, A.; Hadadi, A.; Mouni, L. Optimization and Prediction of Stability of Emulsified Liquid Membrane (ELM): Artificial Neural Network. Processes 2023, 11, 364. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from Aqueous Solutions by Adsorption on Kaolin: Kinetic and Equilibrium Studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Bollinger, J.-C.; Belkhiri, L.; Tiri, A.; Bouzaza, A.; El Jery, A.; Assadi, A.; Amrane, A.; Mouni, L. Zeolite Waste Characterization and Use as Low-Cost, Ecofriendly, and Sustainable Material for Malachite Green and Methylene Blue Dyes Removal: Box–Behnken Design, Kinetics, and Thermodynamics. Appl. Sci. 2022, 12, 7587. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Hadadi, A.; Hamri, N.; Bollinger, J.-C.; Amrane, A.; Tahraoui, H.; Manseri, A.; Mouni, L. Adsorption Performance of Zeolite for the Removal of Congo Red Dye: Factorial Design Experiments, Kinetic, and Equilibrium Studies. Separations 2023, 10, 57. [Google Scholar] [CrossRef]
- Farch, S.; Yahoum, M.M.; Toumi, S.; Tahraoui, H.; Lefnaoui, S.; Kebir, M.; Zamouche, M.; Amrane, A.; Zhang, J.; Hadadi, A.; et al. Application of Walnut Shell Biowaste as an Inexpensive Adsorbent for Methylene Blue Dye: Isotherms, Kinetics, Thermodynamics, and Modeling. Separations 2023, 10, 60. [Google Scholar] [CrossRef]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.-C.; Assadi, A.A.; Amrane, A.; Mouni, L. Comparison of Four Plant-Based Bio-Coagulants Performances against Alum and Ferric Chloride in the Turbidity Improvement of Bentonite Synthetic Water. Water 2022, 14, 3324. [Google Scholar] [CrossRef]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.-C.; Cheikh, S.; Assadi, A.A.; Amrane, A.; Kebir, M.; Mouni, L. Parametrical Study for the Effective Removal of Mordant Black 11 from Synthetic Solutions: Moringa Oleifera Seeds’ Extracts Versus Alum. Water 2022, 14, 4109. [Google Scholar] [CrossRef]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.-C.; Bouzaza, A.; Amrane, A.; Tahraoui, H.; Mouni, L. Aleppo Pine Seeds (Pinus Halepensis Mill.) as a Promising Novel Green Coagulant for the Removal of Congo Red Dye: Optimization via Machine Learning Algorithm. J. Environ. Manag. 2023, 331, 117286. [Google Scholar] [CrossRef] [PubMed]
- Tahraoui, H.; Belhadj, A.-E.; Triki, Z.; Boudella, N.; Seder, S.; Amrane, A.; Zhang, J.; Moula, N.; Tifoura, A.; Ferhat, R.; et al. Mixed Coagulant-Flocculant Optimization for Pharmaceutical Effluent Pretreatment Using Response Surface Methodology and Gaussian Process Regression. Process Saf. Environ. Prot. 2023, 169, 909–927. [Google Scholar] [CrossRef]
- Cheikh, S.; Imessaoudene, A.; Bollinger, J.-C.; Hadadi, A.; Amar, M.; Bouzaza, A.; Assadi, A.A.; Amrane, A.; Zamouche, M.; El Jery, A.; et al. Complete Elimination of the Ciprofloxacin Antibiotic from Water by the Combination of Adsorption-Photocatalysis Process Using Natural Hydroxyapatite and TiO2. Catalysts 2023, 13, 336. [Google Scholar] [CrossRef]
- Joshiba, J.; Senthil Kumar, P.; Carolin, F.; Jayashree, E.; Ramamurthy, R.; Sivanesan, S. Critical Review on Biological Treatment Strategies of Dairy Wastewater. Desalination Water Treat. 2019, 160, 94–109. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammed, A.; Johnson, D.; Hilal, N. Forward Osmosis Research Trends in Desalination and Wastewater Treatment: A Review of Research Trends Over the Past Decade. J. Water Process Eng. 2019, 31, 100886. [Google Scholar] [CrossRef]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.; Bjarnadóttir, B.; Bjarnadóttir, M.; Axelsdóttir, S.; Wu, B. Direct Membrane Filtration for Wastewater Treatment and Resource Recovery: A Review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef]
- Ejraei, A.; Aroon, M.; Ziarati, A. Wastewater Treatment Using a Hybrid System Combining Adsorption, Photocatalytic Degradation and Membrane Filtration Processes. J. Water Process Eng. 2019, 28, 45–53. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pugazhenthi, G. Credibility of Polymeric and Ceramic Membrane Filtration in the Removal of Bacteria and Virus from Water: A Review. J. Environ. Manag. 2020, 268, 110583. [Google Scholar] [CrossRef] [PubMed]
- Khouni, I.; Louhichi, G.; Ghrabi, A.; MOULIN, P. Efficiency of a Coagulation/Flocculation–Membrane Filtration Hybrid Process for the Treatment of Vegetable Oil Refinery Wastewater for Safe Reuse and Recovery. Process Saf. Environ. Prot. 2020, 135, 323–341. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Vieira, W.; Farias, M.; Spaolonzi, M.; Silva, M.; Vieira, M. Removal of Endocrine Disruptors in Waters by Adsorption, Membrane Filtration and Biodegradation. A Review. Environ. Chem. Lett. 2020, 18, 1113–1143. [Google Scholar] [CrossRef]
- Alfonso, P.; Serna-Galvis, E.; Bussemaker, M.; Torres-Palma, R.; Lee, J. A Review on Pharmaceuticals Removal from Waters by Single and Combined Biological, Membrane Filtration and Ultrasound Systems. Ultrason. Sonochem. 2021, 76, 105656. [Google Scholar] [CrossRef]
- Fitobór, K.; Quant, B. Is the Microfiltration Process Suitable as a Method of Removing Suspended Solids from Rainwater? Resources 2021, 10, 21. [Google Scholar] [CrossRef]
- Jinlong, W.; Tang, X.; Liu, Y.; Xie, B.; Li, G.; Liang, H. Self-Sustained Ultrafiltration Coupling Vermifiltration for Decentralized Domestic Wastewater Treatment: Microbial Community and Mechanism. Resour. Conserv. Recycl. 2022, 177, 106008. [Google Scholar] [CrossRef]
- Bakhta, M.; Abderahmane, D.; Djafer, L.; Marin-Ayral, R.-M.; Ayral, A. Wastewater Treatment Using a Hybrid Process Coupling Adsorptionon Marl and Microfiltration. Membr. Water Treat. 2020, 11, 1–11. [Google Scholar]
- Rani, S.; Kumar, R. Insights on Applications of Low-Cost Ceramic Membranes in Wastewater Treatment: A Mini-Review. Case Stud. Chem. Environ. Eng. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic Membrane Technology for Water and Wastewater Treatment: A Critical Review of Performance, Full-Scale Applications, Membrane Fouling and Prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Samadi, A.; Gao, L.; Kong, L.; Orooji, Y.; Zhao, S. Waste-Derived Low-Cost Ceramic Membranes for Water Treatment: Opportunities, Challenges and Future Directions. Resour. Conserv. Recycl. 2022, 185, 106497. [Google Scholar] [CrossRef]
- Dong, B.; Wang, F.-H.; Yang, M.-Y.; Yu, J.-L.; Hao, L.-Y.; Xu, X.; Wang, G.; Agathopoulos, S. Polymer-Derived Porous SiOC Ceramic Membranes for Efficient Oil-Water Separation and Membrane Distillation. J. Membr. Sci. 2019, 579, 111–119. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.; Hanaor, D.; Gurlo, A. Materials and Applications for Low-Cost Ceramic Membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef]
- Aloulou, W.; Aloulou, H.; Khemakhem, M.; Duplay, J.; Daramola, M.O.; Amar, R. Synthesis and Characterization of Clay-Based Ultrafiltration Membranes Supported on Natural Zeolite for Removal of Heavy Metals from Wastewater. Environ. Technol. Innov. 2020, 18, 100794. [Google Scholar] [CrossRef]
- Iaich, S.; Lahcen, M. Development and Characterization of Inorganic Membranes for Micro-Filtration Deposited on Tubular Supports Ceramic Based on Natural Moroccan Clay. J. Mater. Environ. Sci. 2014, 5, 1808–1815. [Google Scholar]
- Khemakhem, M.; Khemakhem, S.; Ayedi, S.; Amar, R. Study of Ceramic Ultrafiltration Membrane Support Based on Phosphate Industry Subproduct: Application for the Cuttlefish Conditioning Effluents Treatment. Ceram. Int. 2011, 37, 3617–3625. [Google Scholar] [CrossRef]
- Boudaira, B.; Harabi, A.; Bouzerara, F.; Zenikheri, F.; Foughali, L.; Guechi, A. Preparation and characterization of membrane supports for microfiltration and ultrafiltration using kaolin (DD2) and CaCO3. Desalination Water Treat. 2015, 57, 5258–5265. [Google Scholar] [CrossRef]
- Harabi, A.; Bouzerara, F. Fabrication of Tubular Membrane Supports from Low Price Raw Materials, Using Both Centrifugal Casting and/or Extrusion Methods; INTECH Open Access Publisher: London, UK, 2011; ISBN 978-953-307-624-9. [Google Scholar]
- Jafari, B.; Abbasi, M.; Hashemifard, S.; Sillanpää, M. Elaboration and Characterization of Novel Two-Layer Tubular Ceramic Membranes by Coating Natural Zeolite and Activated Carbon on Mullite-Alumina-Zeolite Support: Application for Oily Wastewater Treatment. J. Asian Ceram. Soc. 2020, 8, 848–861. [Google Scholar] [CrossRef]
- Arzate, A. Procédés de Séparation Membranaire et Leur Application Dans l’industrie Alimentaire.Revue de Littérature; Entre de Recherche, de Développement et de Transfert Technologique Acéricole: Quebec, QC, Canada, 2008. [Google Scholar]
- Yang, G.; Tsai, C.-M. Effects of Starch Addition on Characteristics of Tubular Porous Ceramic Membrane Substrates. Desalination 2008, 233, 129–136. [Google Scholar] [CrossRef]
- Bouzerara, F.; Harabi, A.; Achour, S.; Larbot, A. Porous Ceramic Supports for Membranes Prepared from Kaolin and Doloma Mixtures. J. Eur. Ceram. Soc. 2006, 26, 1663–1671. [Google Scholar] [CrossRef]
- Zhu, B.; Morris, G.; Moon, I.; Gray, S.; Duke, M. Diffusion Behaviour of Multivalent Ions at Low PH through a MFI-Type Zeolite Membrane. Desalination 2018, 440, 88–98. [Google Scholar] [CrossRef]
- Harabi, A.; Guechi, A.; Condom, S. Production of Supports and Filtration Membranes from Algerian Kaolin and Limestone. Procedia Eng. 2012, 33, 220–224. [Google Scholar] [CrossRef]
- Jedidi, I.; Khemakhem, S.; Saidi, S.; Larbot, A.; Elloumi-Ammar, N.; Fourati, A.; Charfi, A.; Abdelhamid, B.S.; Amar, R. Preparation of a New Ceramic Microfiltration Membrane from Mineral Coal Fly Ash: Application to the Treatment of the Textile Dying Effluents. Powder Technol. 2011, 208, 427–432. [Google Scholar] [CrossRef]
- Daufin, G.; Aimar, P. Séparations Par Membrane Dans l’industrie Alimentaire; Techniques Ingénieur: Saint-denis, France, 2004. [Google Scholar]
- Mulyati, S.; Arahman, N.; Muchtar, S.; Yusuf, M. Removal of Metal Iron from Groundwater Using Aceh Natural Zeolite and Membrane Filtration. IOP Conf. Ser. Mater. Sci. Eng. 2017, 180, 012128. [Google Scholar] [CrossRef]
- Majouli, A.; Tahiri, S.; Younssi, S.; Loukili, H.; Albizane, A.A. Elaboration of New Tubular Ceramic Membrane from Local Moroccan Perlite for Microfiltration Process. Application to Treatment of Industrial Wastewaters. Ceram. Int. 2012, 38, 4295–4303. [Google Scholar] [CrossRef]
- Mustapha, s; Ndamitso, M.; Mohammed, U.M.; Adeosun, N.O.; Idris, M. Study on Activated from Melon (Citrullus lanatus) Husk as Natural Adsorbent for Removal of Hardness in Water. Adv. Anal. Chem. 2016, 6, 1–9. [Google Scholar]
- Demirel, B.; Yenigün, O.; Onay, T. Anaerobic Treatment of Dairy Wastewaters: A Review. Process Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Crini, G.; Montiel, A.J.; Badot, P.-M. Traitement et Épuration Des Eaux Industrielles Polluées: Procédés Membranaires, Bioadsorption et Oxydation Chimique; Presses Universitaires de Franche-Comté: Besançon, France, 2007; ISBN 2-84867-197-1. [Google Scholar]
- Mouiya, M.; Abourriche, A.; Bouazizi, A.; Benhammou, A.; El Hafiane, Y.; Abouliatim, Y.; Nibou, L.; Oumam, M.M.; Ouammou, M.; Smith, A.; et al. Flat Ceramic Microfiltration Membrane Based on Natural Clay and Moroccan Phosphate for Desalination and Industrial Wastewater Treatment. Desalination 2018, 427, 42–50. [Google Scholar] [CrossRef]
- Hamdani, A.; Ahmed, M.; Mountadar, M.; Assobhei, O. Évolution de La Qualité Physico-Chimique et Bactériologique d’un Effluent Laitier Sur Un Cycle Annuel. Déchets Sci. Tech. 2005, 8009. [Google Scholar] [CrossRef]
- Akil, A.; Hassan, T.; Lahcen, B.; Abderrahim, L. Etude de La Qualité Physico-Chimique et Contamination Métallique Des Eaux de Surface Du Bassin Versant de Guigou, Maroc. Eur. Sci. J. 2014, 10, 84–94. [Google Scholar]
- Cardot, C. Les Traitements de l’eau: Procédés Physico-Chimiques et Biologiques; Ellipses: Paris, France, 2010; ISBN 2-7298-6187-4. [Google Scholar]
- Belghyti, D.; El Guamri, Y.; Ztit, G.; Ouahidi, M.; Joti, M.; Harchrass, A.; Amghar, H.; Bouchouata, O.; El Kharrim, K.; Bounouira, H. Caractérisation Physico-Chimique Des Eaux Usées d’abattoir En Vue de La Mise En Œuvre d’un Traitement Adéquat: Cas de Kénitra Au Maroc. Afr. Sci. Rev. Int. Sci. Technol. 2009, 5, 5. [Google Scholar] [CrossRef]
- Maiga, A.; Konate, Y.; Wethe, J.; Denyigba, K.; Zoungrana, D.; Togola, L. Performances Épuratoires d’une Filière de Trois Étages de Bassins de Lagunage à Microphytes Sous Climat Sahélien: Cas de La Station de Traitement Des Eaux Usées de l’EIER. Sud Sci. Technol. 2006, 14, 4–12. [Google Scholar]
- Fathallah, Z.; Elkharrim, K.; Fath-allah, R.; Hbaiz, E.; Hamid, C.; Ayyach, A.; Elkhadmaoui, A.; Belghyti, D. Etude Physico-Chimique Des Eaux Usées de l’unité Industrielle Papetière (CDM) a Sidi Yahia El Gharb (Maroc). LARHYSS J. 2014. [Google Scholar]
- Mesrouk, H.; Hadj Mahammed, M.; Touil, Y.; Amrane, A. Physico-Chemical Characterization of Industrial Effluents from the Town of Ouargla (South East Algeria). Energy Procedia 2014, 50, 255–262. [Google Scholar] [CrossRef]
- Youssef, E.; Nahli, A.; Chlaida, M.; Kamal, C. Contribution A La Caractérisation Physico-Chimique Des Effluents De La Cosumar (Casablanca, Maroc) En Vue De Leur Traitement Approprié. Eur. Sci. J. 2018, 14, 212. [Google Scholar] [CrossRef][Green Version]
- Gnagne, Y.; Yapo, B.; Meite, L.; Kouame, V.; Gadji, A.; Mambo, V.; Houenou, P. Caractérisation Physico-Chimique et Bactériologique Des Eaux Usées Brutes Du Réseau d’égout de La Ville d’Abidjan. Int. J. Biol. Chem. Sci. 2015, 9, 1082. [Google Scholar] [CrossRef]
- Mustapha, S.; Oladejo, T.J.; Muhammed, N.M.; Saka, A.A.; Oluwabunmi, A.A.; Abdulkabir, M.; Joel, O.O. Fabrication of Porous Ceramic Pot Filters for Adsorptive Removal of Pollutants in Tannery Wastewater. Sci. Afr. 2021, 11, e00705. [Google Scholar] [CrossRef]
- Zegaoula, W.; Khellaf, N. Evaluation Du Degré de Pollution Des Rejets Liquides et Atmosphériques Du Complexe Fertial-Annaba (Algérie). LARHYSS J. 2014. [Google Scholar]
- Litébé, A.; Ngakegni-Limbili, A.; Mvouezolo, R.; Nkounkou Loumpangou, C.; Nzobadila, D.; Ouamba, J. Impact of Reject of Dairy Wastewater into the Aquatic Environment: Case of the Bayo Dairy Company (Brazzaville-Congo). Int. J. Environ. Clim. Chang. 2020, 1–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).